Abstract
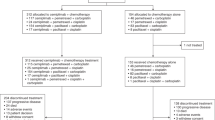
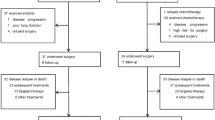
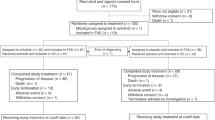
The randomized, double-blinded, multi-center, phase III GEMSTONE-302 (NCT03789604) study evaluated the efficacy and safety of sugemalimab versus placebo in combination with chemotherapy as first-line treatment for metastatic non-small-cell lung cancer (NSCLC). In this study, 479 treatment-naive patients with stage IV squamous or non-squamous NSCLC without known EGFR sensitizing mutations, ALK, ROS1 or RET fusions were randomized (2:1) to receive 1,200 mg of sugemalimab (n = 320) or placebo (n = 159) every 3 weeks in combination with platinum-based chemotherapy for up to four cycles, followed by maintenance therapy with sugemalimab or placebo for squamous NSCLC and sugemalimab or placebo plus pemetrexed for non-squamous NSCLC. Placebo-treated patients could cross over to receive sugemalimab monotherapy on disease progression. The primary endpoint was investigator-assessed progression-free survival (PFS) and the secondary endpoints included overall survival (OS) and objective response rate. Sugemalimab plus chemotherapy has demonstrated significant PFS prolongation in the primary analysis as reported previously. As of 22 November 2021, the prespecified interim OS analysis showed significant improvement with the addition of sugemalimab to chemotherapy (median OS = 25.4 versus 16.9 months; hazard ratio = 0.65; 95% confidence interval = 0.50–0.84; P = 0.0008). Sugemalimab plus chemotherapy provided superior PFS and OS compared to placebo plus chemotherapy, supporting the use of sugemalimab as a first-line treatment option for metastatic NSCLC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are not publicly available due to restrictions regarding patient privacy but are available upon reasonable request. Data will be available to evaluate this paper, verify its contents and for research use. All requests for clinical data will be reviewed by the sponsor (CStone Pharmaceuticals) to verify if the request is subject to any intellectual property or confidentiality obligations. Source data are provided with this paper.
Change history
05 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s43018-023-00719-4
References
Rodríguez-Abreu, D. et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 32, 881–895 (2021).
Paz-Ares, L. et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J. Thorac. Oncol. 15, 1657–1669 (2020).
Nishio, M. et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J. Thorac. Oncol. 16, 653–664 (2021).
Jotte, R. et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J. Thorac. Oncol. 15, 1351–1360 (2020).
Johnson, M. et al. PL02.01 durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. J. Thorac. Oncol. 16, S844 (2021).
Baggstrom, M. Q. et al. Maintenance sunitinib following initial platinum-based combination chemotherapy in advanced-stage IIIB/IV non-small cell lung cancer: a randomized, double-blind, placebo-controlled phase III study—CALGB 30607 (Alliance). J. Thorac. Oncol. 12, 843–849 (2017).
Wang, J. et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 7, 709–717 (2021).
Ren, S. et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J. Thorac. Oncol. 17, 544–557 (2022).
Gong, J. et al. Safety, antitumor activity and biomarkers of sugemalimab in Chinese patients with advanced solid tumors or lymphomas: results from the first-in-human phase 1 trial. Cancer Immunol. Immunother. 71, 1897–1908 (2022).
Zhou, C. et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 23, 220–233 (2022).
CStone Pharmaceuticals. CStone announces the GEMSTONE-302 Study of Sugemalimab Met the Endpoint of Overall Survival in the First-Line Treatment of Metastatic Non-Small Cell Lung Cancer Patients (2022); https://www.cstonepharma.com/en/html/news/2738.html
CStone Pharmaceuticals. Cejemly (sugemalimab) injection, for intravenous use [in Chinese] (2022); https://www.cstonepharma.com/uploads/2022/06/165450667353203.pdf
Paz-Ares, L. G. et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 31, 2895–2902 (2013).
Scagliotti, G. et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 6, 64–70 (2011).
Gadgeel, S. et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 38, 1505–1517 (2020).
Qu, J. et al. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther. Adv. Med. Oncol. 13, 1758835921992968 (2021).
U.S. Department of Health and Human Services. Cancer Clinical Trial Eligibility Criteria: Brain Metastases. Guidance for Industry (2020); https://www.fda.gov/media/121317/download
Tan, A. C. et al. Clinical trial eligibility criteria and recently approved cancer therapies for patients with brain metastases. Front. Oncol. 11, 780379 (2022).
Lim, J. H. & Um, S.-W. The risk factors for brain metastases in patients with non-small cell lung cancer. Ann. Transl. Med. 6, S66 (2018).
Rangachari, D. et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88, 108–111 (2015).
Waqar, S. N. et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin. Lung Cancer 19, e373–e379 (2018).
Yang, X. et al. Efficacy of PD-1/PD-L1 inhibitors versus chemotherapy in lung cancer with brain metastases: a systematic review and meta-analysis. J. Immunol. Res. 2022, 4518898 (2022).
Powell, S. F. et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: pooled analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. 16, 1883–1892 (2021).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
U.S. Department of Health and Human Services, National Institutes of Health & National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03 (2010); https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Chen, L. M., Ibrahim, J. G. & Chu, H. Flexible stopping boundaries when changing primary endpoints after unblinded interim analyses. J. Biopharm. Stat. 24, 817–833 (2014).
Acknowledgements
The study was supported by CStone Pharmaceuticals. The funders had a role in the conceptualization, study design, data collection, data analysis and manuscript preparation. The authors thank all patients and their families for participating in the study, as well as the study investigators and other staff. We also thank S. Chi from CStone Pharmaceuticals and X. Wang from IQVIA (funded by CStone Pharmaceuticals) for their assistance with the statistical analysis. Medical writing assistance was provided by J. Kolston and S. Neville of Parexel (funded by CStone Pharmaceuticals), and M. Zhao and A. Wu of CStone Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
C.Z., Z.W., Y.L., Z.M., H.S., J.W. and J.Y. provided substantial contributions to the conception and design of the study. C.Z., Z.W., M.S., L.C., Z.M., R.W., Y.Y., W.Y., S.S., J. Che, W.Z., J. Cu, X.C., Y. Lu, H.S., C.H., J. Liu, Y. Liu, M.W., X.L., P.S., Y.S., J.Z., J. Li, K.G., C.W., H.Z., Y.Z. and C.L. enrolled and treated the patients. H.W. carried out the statistical analyses and generated the data. C.Z., J.W., R.C., M.Q. and J.Y. analyzed and interpreted the data. C.Z., J.W., R.C., M.Q. and J.Y. drafted the manuscript and provided critical revision of the manuscript. C.Z., J.W., R.C., M.Q. and H.W. verified the data. All authors had full access to the study data, reviewed the data, contributed to the development of the manuscript, approved the final version and had final responsibility for the decision to submit it for publication.
Corresponding author
Ethics declarations
Competing interests
C.Z. reports speaker honoraria from CStone Pharmaceuticals during the course of this study and, outside this study, reports speaker honoraria from Lilly China, Sanofi, Boehringer Ingelheim, Roche, MSD, Qilu Pharmaceutical, Jiangsu Hengrui Therapeutics, Innovent Bio, Luye Pharma Group, TopAlliance Biosciences and Amoy Diagnostics; he is an adviser for Innovent Bio, Jiangsu Hengrui Therapeutics, Qilu Pharmaceutical and TopAlliance Biosciences. J.W., R.C., M.Q., H.W. and J.Y. are paid employees of CStone Pharmaceuticals. J.W. and J.Y. declare stock ownership in the company. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Aaron Mansfield, Zhuoxin Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Supplementary progression-free survival in patients in the double-blind intention-to-treat population grouped according to tumor PD-L1 expression level.
a, All patients. Sugemalimab group, N = 320 patients; Placebo group, N = 159 patients. b, Patients with PD-L1 ≥ 1%. Sugemalimab group, N = 196 patients; Placebo group, N = 96 patients.c, Patients with PD-L1 < 1%. Sugemalimab group, N = 124 patients; Placebo group, N = 63 patients. d, Patients with PD-L1 1–49%. Sugemalimab group, N = 92 patients; Placebo group, N = 48 patients.e, Patients with PD-L1 ≥ 50%. Sugemalimab group, N = 104 patients; Placebo group, N = 47 patients. CI, confidence interval; HR, hazard ratio; PD-L1, programmed death ligand 1.
Extended Data Fig. 2 Waterfall plots of best percentage change from baseline in sum of diameters assessed by investigator in double-blind phase.
a, Sugemalimab plus platinum-based chemotherapy. N = 203 patients. b, Placebo plus platinum-based chemotherapy. N = 64 patients.
Extended Data Fig. 3 Duration of response (double-blind ITT population).
Kaplan–Meier estimates of duration of response (investigator assessed) in patients with metastatic NSCLC without known sensitising mutations, treated with sugemalimab plus chemotherapy (sugemalimab group, N = 203 patients) or placebo plus chemotherapy (placebo group, N = 64 patients). CI, confidence interval; NSCLC, non-small-cell lung cancer.
Extended Data Fig. 4 progression-free survival and overall survival in crossover arm.
N = 45 patients in crossover arm. a, progression-free survival and b, overall survival in patients with metastatic NSCLC without known sensitizing mutations who were treated with 2 L sugemalimab monotherapy in the crossover arm. 2 L, second-line; CI, confidence interval; NSCLC, non-small cell lung cancer.
Supplementary information
Supplementary Information
Supplementary Tables 1–16.
Supplementary Information
Clinical trial protocol, statistical analysis plan and CONSORT checklist.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Table 1
Statistical source data.
Source Data Table 2
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, C., Wang, Z., Sun, M. et al. Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC. Nat Cancer 4, 860–871 (2023). https://doi.org/10.1038/s43018-023-00578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00578-z
This article is cited by
-
Mechanisms of immune checkpoint inhibitors: insights into the regulation of circular RNAS involved in cancer hallmarks
Cell Death & Disease (2024)
-
Prediction uncertainty estimates elucidate the limitation of current NSCLC subtype classification in representing mutational heterogeneity
Scientific Reports (2024)
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis
Clinical and Translational Oncology (2024)