Abstract
The thyroid functions as an apex endocrine organ that controls growth, differentiation and metabolism1, and thyroid diseases comprise the most common endocrine disorders2. Nevertheless, high-resolution views of the cellular composition and signals that govern the thyroid have been lacking3,4. Here, we show that Notch signalling controls homeostasis and thermoregulation in adult mammals through a mitochondria-based mechanism in a subset of thyrocytes. We discover two thyrocyte subtypes in mouse and human thyroids, identified in single-cell analyses by different levels of metabolic activity and Notch signalling. Therapeutic antibody blockade of Notch in adult mice inhibits a thyrocyte-specific transcriptional program and induces thyrocyte defects due to decreased mitochondrial activity and ROS production. Thus, disrupting Notch signalling in adult mice causes hypothyroidism, characterized by reduced levels of circulating thyroid hormone and dysregulation of whole-body thermoregulation. Inducible genetic deletion of Notch1 and 2 in thyrocytes phenocopies this antibody-induced hypothyroidism, establishing a direct role for Notch in adult murine thyrocytes. We confirm that hypothyroidism is enriched in children with Alagille syndrome, a genetic disorder marked by Notch mutations, suggesting that these findings translate to humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All RNA-seq data and single-cell RNA-seq data are publicly available as of the date of this publication. The datasets produced in this study are available in the following databases: SuperSeries: Notch Signaling in the Thyroid is Essential for Adult Homeostasis GSE200268. SubSeries: Notch Signaling in the Thyroid is Essential for Adult Homeostasis (RNA-seq thyroid) GSE200265. SubSeries: Notch Signaling in the Thyroid is Essential for Adult Homeostasis (RNA-seq BAT) GSE200266. SubSeries: Notch Signaling in the Thyroid is Essential for Adult Homeostasis (scRNA-seq thyroid) GSE200267. The primary human single-cell sequencing is deposited in the Sequence Read Archive under BioProject ID PRJNA1006400. The reference genome used in this study was the Mus musculus (GRCm38) RefSeq assembly and the Homo sapiens (GRCh38) RefSeq assembly, deposited and publicly available in the NCBI BioProject databank under accession code GCF_000001635.20 and GCF_000001405.26. All raw data in this manuscript are available from the corresponding author upon reasonable request.
Code availability
This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the corresponding author upon request.
References
Brent, G. A. Mechanisms of thyroid hormone action. J. Clin. Invest. 9, 3035–3043 (2012).
Iwen, K. A., Oelkrug, R. & Brabant, G. Effects of thyroid hormones on thermogenesis and energy partitioning. J. Mol. Endocrinol. 60, R157–R170 (2018).
Biondi, B. & Wartofsky, L. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? J. Clin. Endocrinol. Metab. 7, 2256–2271 (2012).
Han, X. et al. Mapping the mouse cell atlas by microwell-seq. Cell 172, 1091–1107 (2018).
Schaum, N. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Lafkas, D. et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 528, 127–131 (2015).
Tran, I. T. et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J. Clin. Invest. 123, 1590–1604 (2013).
Wu, Y. et al. Therapeutic antibody targeting of individual Notch receptors. Nature 464, 1052–1057 (2010).
Yu, J., Siebel, C. W., Schilling, L. & Canalis, E. An antibody to Notch3 reverses the skeletal phenotype of lateral meningocele syndrome in male mice. J. Cell. Physiol. 235, 210–220 (2020).
Amson, R., Pece, S., Marine, J. C., Fiore, P. P. D. & Telerman, A. TPT1/ TCTP-regulated pathways in phenotypic reprogramming. Trends Cell Biol. 23, 37–46 (2013).
Tan, Y. & Cahan, P. SingleCellNet: a computational tool to classify single cell RNA-seq data across platforms and across species. Cell Syst. 9, 207–213 (2019).
Siebel, C. & Lendahl, U. Notch signaling in development, tissue homeostasis, and disease. Physiol. Rev. 97, 1235–1294 (2017).
Gordon, W. R. et al. Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev. Cell 33, 729–736 (2015).
Strooper, B. D. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Stoeck, A. et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 4, 1154–1167 (2014).
Morgan, S. M., Samulowitz, U., Darley, L., Simmons, D. L. & Vestweber, D. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood 93, 165–175 (1999).
Kaspari, R. R. et al. The paradoxical lean phenotype of hypothyroid mice is marked by increased adaptive thermogenesis in the skeletal muscle. Proc. Natl Acad. Sci. USA 117, 22544–22551 (2020).
Mack, J. J. & Iruela-Arispe, M. L. Notch regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 25, 212–218 (2018).
Wang, Q., Zhao, N., Kennard, S. & Lilly, B. Notch2 and Notch3 function together to regulate vascular smooth muscle development. PLoS ONE 7, e37365 (2012).
Maier, M. M. & Gessler, M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new notch target genes. Biochem. Biophys. Res. Commun. 275, 652–660 (2000).
Pirot, P., van Grunsven, L. A., Marine, J.-C., Huylebroeck, D. & Bellefroid, E. J. Direct regulation of the Nrarp gene promoter by the Notch signaling pathway. Biochem. Biophys. Res. Commun. 322, 526–534 (2004).
Ambesi-Impiombato, F. S., Parks, L. A. & Coon, H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc. Natl Acad. Sci. USA 77, 3455–3459 (1980).
Saito, Y. et al. Development of a functional thyroid model based on an organoid culture system. Biochem. Biophys. Res. Commun. 497, 783–789 (2018).
Dey, K. K., Hsiao, C. J. & Stephens, M. Visualizing the structure of RNA-seq expression data using grade of membership models. PLoS Genet. 13, e1006599 (2017).
Szanto, I., Pusztaszeri, M. & Mavromati, M. H2O2 metabolism in normal thyroid cells and in thyroid tumorigenesis: focus on NADPH oxidases. Antioxidants 8, 126 (2019).
Gerencser, A. A. et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 590, 2845–2871 (2012).
Reguera, D. P. et al. Cytochrome c oxidase subunit 4 isoform exchange results in modulation of oxygen affinity. Cells https://doi.org/10.3390/cells9020443 (2020).
Maharjan, S., Oku, M., Tsuda, M., Hoseki, J. & Sakai, Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 4, 5896 (2014).
Lee, J. et al. Morphological and functional changes in the thyroid follicles of the aged murine and humans. J. Pathol. Transl. Med. 50, 426–435 (2016).
Sheehan, M. T. Biochemical testing of the thyroid: tsh is the best and, oftentimes, only test needed - a review for primary care. Clin. Med. Res. 14, 83–92 (2016).
Warner, A. et al. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor α1. Proc. Natl Acad. Sci. USA 110, 16241–16246 (2013).
Weiner, J. et al. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep. 6, 38124 (2016).
Ueta, C. B., Olivares, E. L. & Bianco, A. C. Responsiveness to thyroid hormone and to ambient temperature underlies differences between brown adipose tissue and skeletal muscle thermogenesis in a mouse model of diet-induced obesity. Endocrinology 152, 3571–3581 (2011).
Riccio, O. et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 9, 377–383 (2008).
Weiner, J. et al. Leptin counteracts hypothermia in hypothyroidism through its pyrexic effects and by stabilizing serum thyroid hormone levels. Mol. Metab. 54, 101348 (2021).
Ring, G. The importance of the thyroid in maintaining an adequate production of heat during exposure to cold. Am. J. Physiol. 137, 582–588 (1942).
Sanchez-Gurmaches, J., Hung, C. M. & Guertin, D. A. Emerging complexities in adipocyte origins and identity. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2016.01.004 (2016).
Maliszewska, K. & Kretowski, A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int. J. Mol. Sci. 22, 1530 (2021).
Undeutsch, H., Löf, C., Offermanns, S. & Kero, J. A mouse model with tamoxifen-inducible thyrocyte-specific cre recombinase activity. Genesis 52, 333–340 (2014).
McCright, B., Lozier, J. & Gridley, T. Generation of new Notch2 mutant alleles. Genesis 44, 29–33 (2006).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Samuels, M. H. et al. Thyroid function variation in the normal range, energy expenditure, and body composition in L-T4–treated subjects. J. Clin. Endocrinol. Metab. 102, 2533–2542 (2017).
Filippis, Tde et al. A frequent oligogenic involvement in congenital hypothyroidism. Hum. Mol. Genet. 26, 2507–2514 (2017).
Filippis, Tde et al. JAG1 loss-of-function variations as a novel predisposing event in the pathogenesis of congenital thyroid defects. J. Clin. Endocrinol. Metab. 101, 861–870 (2016).
Marelli, F. & Persani, L. Role of jagged1-Notch pathway in thyroid development. J. Endocrinol. Invest. 41, 75–81 (2018).
Kamath, B. M., Baker, A., Houwen, R., Todorova, L. & Kerkar, N. Systematic review: the epidemiology, natural history, and burden of Alagille syndrome. J. Pediatr. Gastroenterol. Nutr. 67, 148–156 (2018).
Hunter, I., Greene, S. A., MacDonald, T. M. & Morris, A. D. Prevalence and aetiology of hypothyroidism in the young. Arch. Dis. Child 83, 207 (2000).
Huber, G. et al. Generation of intercellular heterogeneity of growth and function in cloned rat thyroid cells (Frtl-5). Endocrinology 126, 1639–1645 (1990).
Roger, P. P., Baptist, M. & Dumont, J. E. A mechanism generating heterogeneity in thyroid epithelial cells: suppression of the thyrotropin/cAMP-dependent mitogenic pathway after cell division induced by cAMP-independent factors. J. Cell Biol. 117, 383–393 (1992).
Studer, H., Peter, H. J. & Gerber, H. Natural heterogeneity of thyroid cells: The basis for understanding thyroid function and nodular goiter growth. Endocr. Rev. 10, 125–135 (1989).
Fernández, L. P., López-M´arquez, A. & Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 11, 29–42 (2015).
Damante, G. & Lauro, R. D. Thyroid-specific gene expression. Biochim. Biophys. Acta 1218, 255–266 (1994).
SUZUKI, K. et al. In vivo expression of thyroid transcription factor-1 RNA and its relation to thyroid function and follicular heterogeneity: identification of follicular thyroglobulin as a feedback suppressor of thyroid transcription factor-1 RNA levels and thyroglobulin synthesis. Thyroid 9, 319–331 (1999).
Ulianich, L. et al. Follicular thyroglobulin (TG) suppression of thyroid-restricted genes involves the apical membrane asialoglycoprotein receptor and TG phosphorylation. J. Biol. Chem. 274, 25099–25107 (1999).
Romitti, M. et al. Single-cell trajectory inference guided enhancement of thyroid maturation in vitro using TGF-β inhibition. Front. Endocrinol. 12, 657195 (2021).
Vaart, Jvander et al. Adult mouse and human organoids derived from thyroid follicular cells and modeling of Graves’ hyperthyroidism. Proc. Natl Acad. Sci. USA 118, e2117017118 (2021).
Han, X. et al. Construction of a human cell landscape at single-cell level. Nature 581, 303–309 (2020).
Gillotay, P. et al. Single-cell transcriptome analysis reveals thyrocyte diversity in the zebrafish thyroid gland. EMBO Rep. 21, e50612 (2020).
Porazzi, P. et al. Disruptions of global and Jagged1-mediated notch signaling affect thyroid morphogenesis in the zebrafish. Endocrinology 153, 5645–5658 (2012).
Ferretti, E. et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J. Clin. Endocrinol. Metab. 93, 4080–4087 (2008).
Kessler, M. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 8989 (2015).
Jayasena, C. S., Ohyama, T., Segil, N. & Groves, A. K. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135, 2251–2261 (2008).
Xu, J. et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J. Clin. Invest. 125, 1579–1590 (2015).
Landor, S. K. J. et al. Hypo- and hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proc. Natl Acad. Sci. USA 108, 18814–18819 (2011).
Basak, N. P., Roy, A. & Banerjee, S. Alteration of mitochondrial proteome due to activation of Notch1 signaling pathway. J. Biol. Chem. 289, 7320–7334 (2014).
Döhla, J. et al. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat. Cell Biol. 24, 148–154 (2022).
Ludikhuize, M. C. et al. Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch Axis. Cell Metab. 32, 889–900 (2020).
Allen, F. & Maillard, I. Therapeutic targeting of Notch signaling: from cancer to inflammatory disorders. Front. Cell Dev. Biol. 9, 649205 (2021).
Aster, J. C., Pear, W. S. & Blacklow, S. C. The varied roles of Notch in cancer. Annu Rev. Pathol. Mech. Dis. 12, 245–275 (2016).
Xu, H. & Wang, L. The role of Notch signaling pathway in non-alcoholic fatty liver disease. Front. Mol. Biosci. 8, 792667 (2021).
Krop, I. et al. Phase I pharmacologic and pharmacodynamic study of the γ secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 30, 2307–2313 (2012).
Taylor, P. N. et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. https://doi.org/10.1038/nrendo.2018.18 (2018).
Roberts, C. G. & Ladenson, P. W. Hypothyroidism. Lancet 363, 793–803 (2004).
Karnovsky, M. J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy.J. Cell Biol. 27, 1A–149A (1965).
Wu, T. D. & Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26, 873–881 (2010).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Srinivasan, K. et al. Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat. Commun. 7, 11295 (2016).
Long, J. E. et al. Therapeutic resistance and susceptibility is shaped by cooperative multi-compartment tumor adaptation. Cell Death Differ. 26, 2416–2429 (2019).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17, 137–145 (2020).
Germain, P.-L., Lun, A., Meixide, C. G., Macnair, W. & Robinson, M. D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Research 10, 979 (2021).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Xiao, Y. et al. A novel significance score for gene selection and ranking. Bioinformatics 30, 801–807 (2014).
Tan, B., Lu, Z., Dong, S., Zhao, G. & Kuo, M.-S. Derivatization of the tricarboxylic acid intermediates with O-benzylhydroxylamine for liquid chromatography–tandem mass spectrometry detection. Anal. Biochem. 465, 134–147 (2014).
Jaochico, A., Sangaraju, D. & Shahidi-Latham, S. K. A rapid derivatization based LC–MS/MS method for quantitation of short chain fatty acids in human plasma and urine. Bioanalysis 11, 741–753 (2019).
Dunn, W. B. et al. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 9, 44–66 (2013).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–423 (2015).
Acknowledgements
We are grateful to K. Seidel, C. Cottonham, P. Himmels and A. Lun for experimental support and discussion, M. Chen and M. Stolzenberg for helping score BAT activity. We thank the Genentech Research Pathology, Necropsy, Histology and Next-Generation Sequencing laboratories for their experimental contributions and B. Bolon and D. Wilson (GEMpath) for their assessment of thyroid histology. We thank S. Kajimura, A. Zorzano and N. Chandel for sharing methods and for expert feedback on metabolism and mitochondria results. We thank E. Andersson (Karolinska Institutet) and N. Van Hul (Karolinska Institutet) for providing serum samples from Jag1-mutated mice (data not included). We appreciate the insightful feedback and comments on the paper from F. de Sousa e Melo, C. Metcalfe, W. Ye and H. Jasper. The scRNA-seq work in Barcelona was funded by Instituto de Salud Carlos III, Madrid, Spain, grant PI1700324 and co-financed by the European Regional Development Fund.
Author information
Authors and Affiliations
Contributions
L.M. designed and performed the experiments described below, analyzed and discussed data throughout the manuscript and co-wrote the manuscript. T.T.N. and R.P. analyzed and discussed the mouse and human scRNA-seq and bulk RNA-seq data. S.H. provided experimental assistance with the genetic mouse model. D.A.-S. and R.P.-B. provided the human thyroids sequencing dataset. M.R. performed the cryo-EM experiments and analysis. S.M.V. and B.M.K. collected the thyroid data from ALGS patients. Z.L., F.K.C. and D.S. performed the thyroid metabolomics experiment and analysis. A.S. provided histopathological analysis and discussion. C.W.S. supervised the study, provided feedback on experimental design and data interpretation and co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
L.M., T.T.T.N., S.H., M.R., Z.L., F.K.C., D.S., A.S., R.P. and C.W.S. are or were employed by Genentech, which has commercial interest in some of the molecules described. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Luca Persani, Sabine Costagliola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson, Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mouse and human thyroids are composed of a heterogeneous population of thyrocytes.
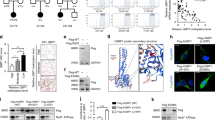
A. Schematic representation of the mouse scRNA sequencing. Dissected thyroids were combined into a pool of six per sample and digested into single cells. Libraries were prepared using the Chromium platform and sequenced in single lanes using the HiSeq400 (Illumina). Sample demultiplexing, barcode processing and single cell 3’ gene counting were performed using 10x CellRanger. After assessing the percentages of mitochondrial reads and doublets, only cells with a mitochondrial UMI fraction <0.25 were analyzed. Dataset alignment, cell clustering, visualization and differential expression analysis were performed according to “Orchestrating Single-Cell Analysis with Bioconductor” (OSCA, (Amezquita et al., 2020)). The pipeline merged single-cell data generated from multiple batches, and batch effects (observed in two processing dates) were corrected. B. Heatmap of the z-scores of the expression of published cell-specific markers used for cluster identification and annotation in mouse single cell sequencing analysis. The cluster identity is shown on the X axis and the gene names on the Y axis. C. Uniform Manifold Approximation and Projection for dimension reduction (UMAP) plot corresponding to Fig. 1a. Colors highlight the 16 clusters identified: endothelial cells, EC; Thyroid Follicular Cells or thyrocytes, TFC; erythrocytes, Eryth; myeloid cells, M; T cells, T; fibroblasts, F; smooth muscle cells, S; parathyroid cells, P; Schwann cells, Sw. D. t-distributed stochastic neighbor embedding (t-SNE) plot for the mTFC and mTFC2 clusters, with blue intensities showing expression of the indicated genes. n = 3 samples. E. Representative immunofluorescent and -histochemical staining images of PAX8 and NKX2-1 in thyroid sections. 40X objective; scale bars = 20 μm; n = 5 mice. F. Heatmap of the z-scores of the expression of published cell-specific markers used for cluster identification and annotation in human single cell sequencing analysis. The cluster identity is shown on the X axis and the gene names on the Y axis. G. UMAP plot for all sequenced cells isolated from the thyroid of patient samples. Colors highlight the 7 clusters identified: endothelial cells, EC and Thyroid Follicular Cells or thyrocytes, TFC. H. Single cell net mapping of mouse TFC1 and TFC2 (yellow and blue, respectively) signatures onto the UMAP of human single cell thyroid data. Graphs indicate the number of human cells onto which the mTFC1 and mTFC2 cells mapped. Extended Data Fig. 1a reprinted from BioRender.com (2023). https://app.biorender.com/biorender-templates.
Extended Data Fig. 2 TFC1 express Notch ligands, receptors and target genes.
A. t-SNE plots for the mouse TFC clusters (top) and human TFC clusters (bottom) showing the expression levels (blue intensities) of the indicated Notch ligands and receptors. n = 3 mice and n = 4 human. B. t-SNE plots for the mouse TFC clusters (top) and human TFC clusters (bottom) showing the expression levels (blue intensities) of the indicated Notch target genes. n = 3 mice and n = 4 human. C. Expression of Jag1, Jag2, Notch1 and Notch2 mRNAs detected by RNAscope (amplified in situ hybridization) in control thyroid sections, n = 3 mice. Scale bars = 100μm.
Extended Data Fig. 3 N2 activity is detected in thyrocytes.
A. Representative double immunofluorescent detection of gamma-secretase-cleaved (active) NICD1* plus PAX8 or NICD1* plus CALCITONIN in thyroid sections of control mice (n = 5). 40X objective; n = 5 mice. B. Representative images of immunohistochemistry or -fluorescent detection of NICD1*, NICD1* plus the endothelial cell marker ENDOMUCIN, NOTCH2 (N2), and cleaved (active) NICD2*. 40X objective; scale bars = 100μm; n = 5 mice. C. Representative image of dual staining using amplified in situ hybridization (RNAscope) and immunostaining to detect Notch2 mRNA (red, Opal 690) and NKX2-1 protein (yellow, Opal 570), respectively. n = 3 mice. White arrows: thyrocytes co-stained for Notch2 mRNA and NKX2-1 protein; orange arrows: thyrocytes negative for NKX2-1 protein with low or no Notch2 mRNA. Scale bars = 50 μm.
Extended Data Fig. 4 Notch inhibition decreases the expression thyrocyte markers.
A. NICD2*, in thyroid sections from mice (n = 6 and 9 controls, n = 9 aJ12) treated as in Fig. 2a. 40X objective. Mean ± s.d. B. Quantification of Heyl, Hey1 and Nrarp mRNA expression detected using RNAscope probes in thyroid sections from mice (n = 4) treated as in Fig. 2b. Percentage of positive cells and H score. H score is calculated by totaling the percentage of cells in each bin, according to the number of dots/cell (0, 0 dots; 1, 1-3 dots; 2, 4-9 dots; 3, 10-15 dots and 4, >15 dots). Mean ± s.d. C. Notch3 mRNA expression detected using RNAscope in thyroid sections from mice (n = 3) treated as in (B), showing decreased levels in aJ12 treated thyroids. Scale bars = 100μm. Mean H score ± s.d. D. Normalized Enrichment Score (NES) of a Notch target gene set (Hey1, Nrarp, HeyL, Notch3) from thyroids isolated at the indicated times after treating mice (n = 5) as in (A). E. Top 30 pathways downregulated by aJ12 treatment (relative to aRW), identified using GSEA of differentially expressed genes in total thyroids (bulk RNA-seq) from mice treated as in (A) (n = 5 mice). P values were derived by comparing calculated ES to ES values calculated from 10000 permutations of the gene rank list followed by Benjamini-Hochberg correction. F. Fold change of thyrocyte and parafollicular (PFC) marker gene expression induced by aJ12 treatment and determined by bulk RNA sequencing of thyroids isolated from mice (n = 5 thyroids) treated as in (A). y axis, nRPKM fold change relative to aRW isotype control. Mean ± s.d. G. Fold change of mRNA expression of thyrocyte and parafollicular (PFC) marker genes and Notch target genes (normalized to actin mRNA expression), induced by aJ12 treatment and determined by qRT-PCR of thyroid mRNA isolated from mice (n = 8 aRW thyroids and 6 aJ12) treated as in (A) for 5 days. y axis, fold change relative to aRW isotype control. Mean ± s.d.
Extended Data Fig. 5 Notch inhibition induces defects in thyrocytes.
A. Representative immunohistochemical detection of CALCITONIN and calcitonin gene-related protein (CGRP) in thyroid sections from mice (n = 5) treated as in (A) for 5 days. Scale bars = 100μm. B. Percentage of PAX8+ cells assessed by immunofluorescence in the thyroids of mice (n = 8 aRW and n = 10 aJ12) treated as in (A). Mean ± s.d. C. Distribution of expression of the indicated genes in mTFC2 cells from mice (n = 3) treated as in (B). Boxes represent interquartile ranges and medians are shown as lines. Whiskers above and below the boxes are the lowest and highest values within 1.5 times IQR. Outliers are shown as circles beyond the whiskers. D. Representative immunofluorescent detection of PAX8 in FRTL5 cells (n = 4 wells) treated for 2 days with aRW (25 μg/ml), aJ12 (25 μg/ml each) or aN123 (25 μg/ml each). DAPI was used for nuclear staining. 20X objective. E. Representative immunofluorescent images of the thyroid organoids derived from thyrocytes isolated from mice treated as in (A) and stained for NKX2-1 (red), CALCITONIN (green) and PAX8 (white). Scale bars = 100 μm. F. Number of organoids generated from 10000 single cells isolated from thyroids of mice treated as in (A). Single cells were either isolated from 7 thyroids, digested in a pool, and plated into 3 wells, before counting organoids/well on day 6 (D6) or isolated from 8 thyroids, pooled into 2 thyroids/pool, and plated into single wells before counting organoids/well on day 7 (D7). Mean ± s.d.; n = 3 wells. G. Characterization of organoids formed 10 days after their isolation from mice treated in vivo. Size (small<20 cells/organoid≤big); development (developed, > 1 follicle formed; underdeveloped, no follicles, unorganized cell mass). n = 49 aRW and 33 aJ12 organoids. H. Top 200 pathways (gray dots) identified using Gene Set Enrichment Analysis (GSEA) that are deregulated following treatment as in Fig. 1b with aN12 (Y axis) and aJ12 (X axis) in mTFC2 cells. Yellow dots represent pathways associated with endoplasmic reticulum. I. Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) statistics per number of topics represented in the single cell data.Y axis= AIC and BIC scores; X axis= number of topics. Seven topics yielded a low AIC score and was the smallest number that minimized the BIC score, which otherwise increased with topic numbers > 8 because of over-fitting. J. Boxplots showing the topic scores in each treatment group. Boxes represent interquartile ranges and medians are shown as lines. Whiskers above and below the boxes are the lowest and highest values within 1.5 times IQR. Outliers are shown as circles beyond the whiskers. n = 3 thyroids/group. Statistical significance was assessed using the unpaired two-tailed Student’s t-test with Welch’s correction: p < 0.05, *; p < 0.01, **; p < 0.001, ***; p < 0.0001, ****.
Extended Data Fig. 6 Notch blockade induces mitochondrial defects in thyrocytes.
A. Distribution of expression of the indicated mitochondrial genes in mTFC1 cells isolated from mice (n = 3) treated for 7 days with aRW (40 mg/kg), aJ12 (20 mg/kg each) or aN12 (5 and 10 mg/kg, respectively), assessed by scRNA-seq (Fig. 2b). Boxes represent interquartile ranges and medians are shown as lines. Whiskers above and below the boxes are the lowest and highest values within 1.5 times IQR. Outliers are shown as circles beyond the whiskers. B. Representative electron microscopy images of mitochondria from non-thyrocyte cells from thyroids of mice (n = 3) treated as in (A). Scale bars = 100 nm. C. Fluorescent intensity (Y axis) of TMRM in primary thyrocytes from mice (n = 5) treated as in (A). TMRM fluorescence intensity decreases after aJ12 (red) or aN12 treatment (blue), compared to control aRW (gray dotted). D. Quantification of COX staining using the VitroViewTM kit in thyroid cryosections of mice from Fig. 3c (n = 6 aRW; n = 8 aJ12; n = 6 aN12) treated as in (A). Mean ± s.d. E. Cytochrome c oxidation kinetics of primary thyrocytes isolated from mice (n = 5) treated as in (A). Mean ± s.d. F. Ratio of mitochondrial DNA (mDNA, Nd1 and 16 S) versus genomic DNA (gDNA, HK and 18 S) in thyroids from mice (n = 9) treated as in (A). Mean ± s.d. G. Seahorse assay of ATP production rates in the FRTL5 thyrocyte cell line cultured for 2 days as in Fig. 3e. Mean ± s.d.; n = 13 wells. H. Gating strategy used in Fig. 3f. Data represents one aRW sample and the same gates were applied to all samples.
Extended Data Fig. 7 Notch inhibition decreases mitochondrial activity.
A. Percentages of thyrocytes with high ( ≥ 2500 MFI); low mitoTracker ( < 2500 MFI) intensities isolated from mice treated for 7 days with aRW (40 mg/kg), aJ12 (20 mg/kg each) or aN12 (5 and 10 mg/kg, respectively). Percentages of mitoSOX positive thyrocytes ( ≥ 450 MFI) in the same samples. Mean fluorescence intensities (MFI) of mitoTracker and mitosox in cells with low mitoTracker staining. Mean ± s.d.; n = 5 mice. B. Mean fluorescence intensity following DCFDA staining to measure total ROS levels as in (A). C. Principal component analysis (PCA) of thyroid metabolites from mice (n = 4) treated as in (A). Samples group together according to their treatments. D. Quantification of glycolysis and TCA cycle metabolites of samples in (C). Succinate, Citrate, Lactate and Pyruvate were quantified using targeted analysis (ng/g), and glucose-6-phosphate levels are reported as MS peak area units. Mean ± s.d.; n = 4 thyroids. E. Quantification in MS peak area units of urea cycle and glutamine cycle metabolites of samples in (C). Mean ± s.d.; n = 4 thyroids. F. Quantification of ketogenesis metabolites of samples in (C). 3-OH-butyrate and 2-OH-3-methylbutyric acid were quantified using targeted analysis or with MS peak area units, respectively. Mean ± s.d.; n = 4 thyroids. G. Quantification of catalase activity in thyrocytes isolated from mice (n = 5) treated as in (A). Mean ± s.d. H. Quantification of glutathione disulfide (GSSG) in thyrocytes isolated from mice (n = 5) treated as in (A). Mean ± s.d. Statistical significance was assessed using the unpaired two-tailed Student’s t-test with Welch’s correction: p < 0.05, *; p < 0.01, **; p < 0.001, ***; p < 0.0001, ****.
Extended Data Fig. 8 Notch inhibition induces hypothyroidism.
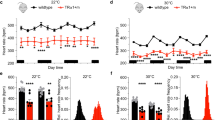
A. Quantification of thyroid histological changes from male (n = 4) and female (n = 5) mice treated as in Fig. 4a. Thyroid sections estimated w > 40% of peripheral follicles inactive ( < 4 vacuoles) were interpreted as having reduced activity. Flattening epithelium in follicles: 0% = normal, <10% = ‘minimal’, 10-40%= ‘mild’, 40-75% = ‘moderate’ and >80% follicles = ‘marked’. Percentage of follicles with decreased thyroid activity ( < 4 resorption vacuoles) or lined with cuboidal epithelium (as indicated) in thyroids from different female mice (dots). B. Thyroxine (T4) serum levels from female mice (n = 5) treated as in (A) for 7 days and measured by ELISA. Mean ± s.d. C. T4 and triiodothyronine (T3) in male mice (n = 6) treated as in (A) over the indicated time course. Mean ± s.d. n = 11 aRW and n = 6 aJ12 mice at day 1; n = 7 mice at day 5; n = 10 mice at day 7. D. Serum levels of thyroxine (T4) measured at 7 days post treatment. Male mice were treated with a single i.p. dose of aRW (40 mg/kg); aJ12 (20 mg/kg each); aN123 (5, 10 and 20 mg/kg); aN1 (5 mg/kg); aN2 (10 mg/kg); aN3 (20 mg/kg); aJ1 (20 mg/kg) or aJ2 (20 mg/kg); aN12 (5,10 mg/kg), aN23 (10, 20 mg/kg, aN3 twice a week) and aN13 (5, 20 mg/kg, aN3 injected twice a week). Mean ± s.d; n ≥ 5 mice. E. Serum levels of Thyrotropin Releasing Hormone (TRH), secreted by the hypothalamus, and Thyrotropin Secreting Hormone (TSH), secreted by the pituitary gland, in male mice treated as in (A) for the indicated days. Mean ± s.d.; n = 6 mice. F. Serum levels of calcitonin in male mice treated as in (A) for 7 days. Mean ± s.d.; n = 11 aRW treated mice; n = 11 aJ12 treated mice and n = 3 aN123 treated mice. G. Body temperatures measured using a rectal probe at the same time on each of the indicated days in male mice treated as in (A) and housed at 22 °C. Data from 5 experiments. Mean ± s.d.; n = 22 mice (D1-7) and n = 6 (D12−16). H. Body temperatures measured using a rectal probe at the same time on each of the indicated days in female mice treated as in (A) and housed at 22 °C. Mean ± s.d.; n = 5 aRW, 4 aJ12, 5 aN12 mice. I. T4 serum levels assessed by ELISA in Ob/Ob or WT male mice housed at the indicated temperatures and treated for 7 days as in (A). Mean ± s.d.; n = 6 Ob/Ob mice; n = 5 mice at 4 °C; n = 6 mice at 20 °C.
Extended Data Fig. 9 Exogenous administration of thyroid hormones rescues the hypothyroidism effects induced by Notch blockade.
A. T4 levels following T3 and T4 administration (1 µg/g and 0.05 µg/g, respectively) according to the schematic. T3 and T4 administration significantly increase the levels of T4 in serum. Mean ± s.d.; n = 4 mice. B. Body temperatures measured using a rectal probe at the same time on each of the indicated days in male mice treated as in (A) and housed at 22 °C or at 4 °C. The dotted line indicates when the T3T4 treatment starts. Mean ± s.d.; n = 5 mice. C. Electron microscopy images of mitochondria of thyrocytes from male mice treated as in (A) for 7 days, n = 3 mice. Arrows = aberrant mitochondria. Scale bars = 400 nm. D. Representation of the temperature dissipated from the tail measured by infrared thermography in female and male mice treated as in (A) during 6 days. Mean ± s.d.; n = 5 mice. E. Score of the BAT activity, assessed blindly in the BAT sections from male mice treated as in (A). Score of 1 is white adipose tissue phenotype and 10, BAT at its maximum activity. Mean ± s.d.; n = 5 mice. Representative picture of the interscapular brown adipose tissue (BAT) of male mice treated with aRW or aJ12 antibodies. n = 5 mice. F. Wet weight of brown adipose tissue (BAT) in female mice treated as in (A), normalized to the average wet weight measured in the control group. Scoring done as in (E). Mean ± s.d.; n = 5 mice. G. RNA sequencing (nRPKM fold compared to aRW) of the indicated genes in the BAT of male mice treated as in (A). Mean ± s.d.; n = 5 mice. H. Representative images of immunohistochemistry against UCP1 in BAT from male mice treated for 5 days as in (A). n = 5 mice. Scale bars = 100μm. I. BAT temperature in male mice treated as in (A). Temperature was measured by IPT transponders implanted in the interscapular region. Data were collected from 3 independent experiments. Mean ± s.d.; n = 12 aRW mice; n = 12 aJ12 mice; n = 10; aRW+T3T4-treated mice; aJ12 + T3T4-treated mice. Statistical significance was assessed using the unpaired two-tailed Student’s t-test with Welch’s correction: p < 0.05, *; p < 0.01, **; p < 0.001, ***; p < 0.0001, ****.
Extended Data Fig. 10 Genetic deletion of Notch1 and Notch2 in the thyroid induces hypothyroidism.
A. Serum levels of glucose, cholesterol, triglycerides and NEFAs of male mice treated for 7 days after a single i.p. injection of aRW (40 mg/kg), aJ12 (20 mg/kg each) or aN12 (5 and 10 mg/kg, respectively) and daily dose of T4 and T3 (1 and 0.05 µg/g, respectively). Mean ± s.d.; n = 5 mice. B. Energy expenditure in mice (n = 5) treated as in (A). Black and white bars represent daily night and day cycles, respectively. Oxygen and carbon dioxide levels were measured every 15 min for 3 days before and 11 days after treatment, as indicated. Mean ± s.d. C. Scheme of tamoxifen administration in N1fl/fl/N2fl/fl; Tg-CreERT2pos (+/+) mice and the respective Tg-CreERT2neg (-/-) controls. Analysis started at day 7 after the first tamoxifen administration and tissues were collected at day 14. Agarose gel electrophoresis showing PCR products of genomic DNA isolated from the thyroids and amplified using primers to detect recombined Notch1 (478 bp band), WT Notch2 (1485 bp band) or recombined Notch2 (480 bp band). The experiment was performed 2 times. Immunohistochemistry against CRE (upper) or NOTCH2 (middle) or dual RNAscope (bottom) against Notch1 (red) and Notch2 (green) on thyroid sections, n > 8 mice. Scale bars = 50μm. D. Infrared thermography pictures of mice treated as in (C) and measured at day 13 after tamoxifen, showing increased heat dissipation in N1fl/fl/N2fl/fl; Tg-CreERT2pos (+/+) mice compared to their Tg-CreERT2pos (-/-) counterparts. The bar on the right shows the maximum and minimum temperature registered in each picture with the corresponding gradient of colors, n = 5 mice. E. Score of the BAT activity, assessed blindly in the BAT sections from mice treated as in (C), showing increased BAT activity in N1fl/fl/N2fl/fl; Tg-CreERT2pos (+/+) thyroids compared to their Tg-CreERT2pos (-/-) counterparts. Score of 1 is white adipose tissue phenotype and 10, BAT at its maximum activity. F. Serum levels of glucose, cholesterol and triglycerides of mice treated as in (C). Mean ± s.d.; n = 8 mice. Statistical significance was assessed using the unpaired two-tailed Student’s t-test with Welch’s correction: p < 0.05, *; p < 0.01, **; p < 0.001, ***; p < 0.0001, ****. Extended Data Fig. 10c reprinted from BioRender.com (2023). https://app.biorender.com/biorender-templates.
Supplementary information
Supplementary information
Supplementary Fig. 1 (gating strategy) and Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mosteiro, L., Nguyen, T.T.T., Hankeova, S. et al. Notch signaling in thyrocytes is essential for adult thyroid function and mammalian homeostasis. Nat Metab 5, 2094–2110 (2023). https://doi.org/10.1038/s42255-023-00937-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00937-1