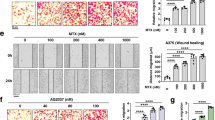
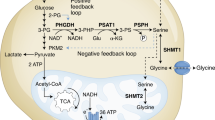
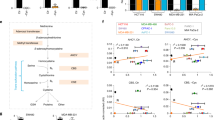
Abstract
The de novo serine synthesis pathway is upregulated in many cancers. However, even cancer cells with increased serine synthesis take up large amounts of serine from the environment1, and we confirm that exogenous serine is needed for maximal proliferation of these cells. Here we show that even when enzymes in the serine synthesis pathway are genetically upregulated, the demand for oxidized NAD+ constrains serine synthesis, rendering serine-deprived cells sensitive to conditions that decrease the cellular NAD+/NADH ratio. Further, purine depletion is a major consequence of reduced intracellular serine availability, particularly when NAD+ regeneration is impaired. Thus, cells rely on exogenous serine consumption to maintain purine biosynthesis. In support of this explanation, providing exogenous purine nucleobases, or increasing NAD+ availability to facilitate de novo serine and purine synthesis, rescues maximal proliferation even in the absence of extracellular serine. Together, these data indicate that NAD+ is an endogenous limitation for cancer cells to synthesize the serine needed for purine production to support rapid proliferation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hosios, A. M. et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36, 540–549 (2016).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Newman, A. C. & Maddocks, O. D. K. One-carbon metabolism in cancer. Br. J. Cancer 116, 1499–1504 (2017).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Sullivan, M. R. et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, P1410–1421 (2019).
Mattaini, K. R., Sullivan, M. R. & Heiden, M. G. V. The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 47, 1475–1481 (2015).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Labuschagne, C. F., van den Broek, N. J. F., Mackay, G. M., Vousden, K. H. & Maddocks, O. D. K. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and 1-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Gui, D. Y. et al. environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab. 24, 716–727 (2016).
Gravel, S.-P. et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 74, 7521–7533 (2014).
Murphy, J. P. et al. The NAD+ Salvage Pathway Supports PHGDH-driven serine biosynthesis. Cell Rep. 24, 2381–2391.e5 (2018).
Bao, X. R. et al. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. eLife 5, (2016).
Hosios, A. M. & Heiden, M. G. V. The redox requirements of proliferating mammalian cells. J. Biol. Chem. 293, 7490–7498 (2018).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Alkan, H. F. et al. Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab. 28, 706–720.e6 (2018).
Mayers, J. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Muir, A. et al. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife 6, e27713 (2017).
Sullivan, L. B. et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 20, 782–788 (2018).
Acknowledgements
We thank members of the Vander Heiden Lab for helpful discussions. We thank H. Furkan Alkan for providing the A549 shAGC1 cells. F.F.D. acknowledges support from the NIH (F31CA236036). M.G.V.H. acknowledges support from a Faculty Scholar grant from the Howard Hughes Medical Institute, SU2C, a division of the Entertainment Industry Foundation, the Lustgarten Foundation, the MIT Center for Precision Cancer Medicine, the Ludwig Center at MIT and the NIH (R01CA201276, R01CA168653 and P30CA14051).
Author information
Authors and Affiliations
Contributions
F.F.D. performed most experiments. F.F.D. and C.A.L. performed metabolite tracing experiments and LCMS data analysis. B.P.F. and C.A.L. performed the [6-13C]glucose and [3-13C]serine tracing experiment. F.F.D. and M.G.V.H. designed the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are aware of no direct conflicts with the topic of the paper; however M.G.V.H. discloses that he is a scientific advisor and/or shareholder of Agios Pharmaceuticals, Aeglea Biotherapeutics and Auron Therapeutics. B.P.F. discloses he is a founder of Mythic Therapeutics.
Additional information
Peer review information Primary Handling Editor: Ana Mateus.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Table 1
Rights and permissions
About this article
Cite this article
Diehl, F.F., Lewis, C.A., Fiske, B.P. et al. Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat Metab 1, 861–867 (2019). https://doi.org/10.1038/s42255-019-0108-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0108-x
This article is cited by
-
Epithelial and stromal co-evolution and complicity in pancreatic cancer
Nature Reviews Cancer (2023)
-
Phosphoserine phosphatase as a prognostic biomarker in patients with gastric cancer and its potential association with immune cells
BMC Gastroenterology (2022)
-
Mitochondria preserve an autarkic one-carbon cycle to confer growth-independent cancer cell migration and metastasis
Nature Communications (2022)
-
Nucleotide imbalance decouples cell growth from cell proliferation
Nature Cell Biology (2022)
-
Dietary supplementation with inosine-5′-monophosphate improves the functional, energetic, and antioxidant status of liver and muscle growth in pigs
Scientific Reports (2022)