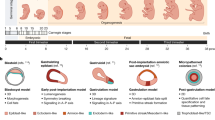
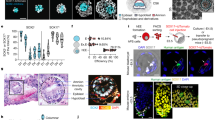
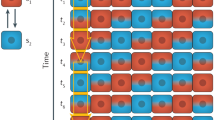
Abstract
Morphogenetic events during development shape the body plan and establish structural foundations for tissue forms and functions. Acquiring spatiotemporal information of development, especially for humans, is limited by technical and ethical constraints. Thus, both stem cell-based, in vitro development models and theoretical models have been constructed to recapitulate morphogenetic events during development. These in vitro experimental and theoretical models offer accessibility, efficiency and modulability. However, their physiological relevance often remains obscure, owing to their simplistic nature, which obstructs their applicability as faithful and predictive models of natural development. We examine existing in vitro experimental and theoretical models of various developmental events and compare them with the current knowledge of natural development, with particular considerations of biomechanical driving forces and stereotypic morphogenetic features. We highlight state-of-the-art methods used to construct these in vitro models and emphasize the biomechanical and biophysical principles these models have helped unveil. We also discuss challenges faced by the current in vitro experimental and theoretical models and propose how theoretical modelling and in vitro experimental models should be combined with in vivo studies to advance fundamental understanding of development.
Key points
-
Pluripotent stem cell-based in vitro models and theoretical models can effectively recapitulate mammalian development, including those of topological and conformational morphogenesis.
-
Pluripotent stem cell-based in vitro models can reconstitute essential aspects governing tissue morphogenesis, such as endogenous scales, exogenous stimuli and boundary conditions, and thereby provide mechanistic insights.
-
Driven by state-of-art engineering tools, the geometry, stimuli and extracellular microenvironment of in vitro morphogenesis models can be modulated with heightened precision and specificity.
-
Through combining in vitro and theoretical approaches, high-order complexity underlying morphogenetic dynamics can be decoupled and quantitatively studied.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fu, J., Warmflash, A. & Lutolf, M. P. Stem-cell-based embryo models for fundamental research and translation. Nat. Mater. 20, 132–144 (2021).
Shao, Y. & Fu, J. Engineering multiscale structural orders for high-fidelity embryoids and organoids. Cell Stem Cell 29, 722–743 (2022).
Yin, X. et al. Engineering stem cell organoids. Cell Stem Cell 18, 25–38 (2016).
Brassard, J. A. & Lutolf, M. P. Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24, 860–876 (2019).
Torres-Sanchez, A., Kerr Winter, M. & Salbreux, G. Tissue hydraulics: physics of lumen formation and interaction. Cell Dev. 168, 203724 (2021).
Dumortier, J. G. et al. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science 365, 465–468 (2019).
Harrington, M. J., Hong, E. & Brewster, R. Comparative analysis of neurulation: first impressions do not count. Mol. Reprod. Dev. 76, 954–965 (2009).
Bedzhov, I. & Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032–1044 (2014).
Kim, Y. S. et al. Deciphering epiblast lumenogenesis reveals proamniotic cavity control of embryo growth and patterning. Sci. Adv. 7, eabe1640 (2021).
Molè, M. A. et al. Integrin β1 coordinates survival and morphogenesis of the embryonic lineage upon implantation and pluripotency transition. Cell Rep. 34, 108834 (2021).
Orietti, L. C. et al. Embryo size regulates the timing and mechanism of pluripotent tissue morphogenesis. Stem Cell Rep. 16, 1182–1196 (2021).
Taniguchi, K. et al. Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Rep. 5, 954–962 (2015).
Taniguchi, K. et al. An apicosome initiates self-organizing morphogenesis of human pluripotent stem cells. J. Cell Biol. 216, 3981–3990 (2017).
Wang, S. et al. Spatially resolved cell polarity proteomics of a human epiblast model. Sci. Adv. 7, eabd8407 (2021).
Shao, Y. et al. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 8, 208 (2017).
Shao, Y. et al. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 16, 419–425 (2017).
Indana, D., Agarwal, P., Bhutani, N. & Chaudhuri, O. Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv. Mater. 33, e2101966 (2021).
Dasgupta, S., Gupta, K., Zhang, Y., Viasnoff, V. & Prost, J. Physics of lumen growth. Proc. Natl Acad. Sci. USA 115, E4751–E4757 (2018).
Dokmegang, J., Yap, M. H., Han, L., Cavaliere, M. & Doursat, R. Computational modelling unveils how epiblast remodelling and positioning rely on trophectoderm morphogenesis during mouse implantation. PLoS ONE 16, e0254763 (2021).
Burgess, R., Rawls, A., Brown, D., Bradley, A. & Olson, E. N. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature 384, 570–573 (1996).
Rowton, M. et al. Regulation of mesenchymal-to-epithelial transition by PARAXIS during somitogenesis. Dev. Dyn. 242, 1332–1344 (2013).
Wiggan, O., Fadel, M. P. & Hamel, P. A. Pax3 induces cell aggregation and regulates phenotypic mesenchymal-epithelial interconversion. J. Cell Sci. 115, 517–529 (2002).
Nakaya, Y., Kuroda, S., Katagiri, Y. T., Kaibuchi, K. & Takahashi, Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev. Cell 7, 425–438 (2004).
Takahashi, Y., Sato, Y., Suetsugu, R. & Nakaya, Y. Mesenchymal-to-epithelial transition during somitic segmentation: a novel approach to studying the roles of Rho family GTPases in morphogenesis. Cell Tissues Organs 179, 36–42 (2005).
Kulesa, P. M. & Fraser, S. E. Cell dynamics during somite boundary formation revealed by time-lapse analysis. Science 298, 991–995 (2002).
Cooke, J. & Zeeman, E. C. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J. Theor. Biol. 58, 455–476 (1976).
Dubrulle, J., McGrew, M. J. & Pourquié, O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106, 219–232 (2001).
Dubrulle, J. & Pourquie, O. From head to tail: links between the segmentation clock and antero-posterior patterning of the embryo. Curr. Opin. Genet. Dev. 12, 519–523 (2002).
Simsek, M. F. et al. Periodic inhibition of Erk activity drives sequential somite segmentation. Nature 613, 153–159 (2023).
Nelemans, B. K. A., Schmitz, M., Tahir, H., Merks, R. M. H. & Smit, T. H. Somite division and new boundary formation by mechanical strain. iScience 23, 100976 (2020).
Naganathan, S. R., Popovic, M. & Oates, A. C. Left-right symmetry of zebrafish embryos requires somite surface tension. Nature 605, 516–521 (2022).
Richardson, M. K., Allen, S. P., Wright, G. M., Raynaud, A. & Hanken, J. Somite number and vertebrate evolution. Development 125, 151–160 (1998).
Gomez, C. et al. Control of segment number in vertebrate embryos. Nature 454, 335–339 (2008).
Eckalbar, W. L., Fisher, R. E., Rawls, A. & Kusumi, K. Scoliosis and segmentation defects of the vertebrae. Wiley Interdiscip. Rev. Dev. Biol. 1, 401–423 (2012).
Budjan, C. et al. Paraxial mesoderm organoids model development of human somites. eLife 11, e68925 (2022).
Sanaki-Matsumiya, M. et al. Periodic formation of epithelial somites from human pluripotent stem cells. Nat. Commun. 13, 2325 (2022).
Miao, Y. et al. Reconstruction and deconstruction of human somitogenesis in vitro. Nature 614, 500–508 (2022).
Yamanaka, Y. et al. Reconstituting human somitogenesis in vitro. Nature 614, 509–520 (2022).
Yaman, Y. I. & Ramanathan, S.Controlling human organoid symmetry breaking reveals signaling gradients drive segmentation clock waves. Cell 186, 513–527.e19 (2023).
Morelli, L. G. et al. Delayed coupling theory of vertebrate segmentation. HFSP J. 3, 55–66 (2009).
Hubaud, A., Regev, I., Mahadevan, L. & Pourquie, O. Excitable dynamics and Yap-dependent mechanical cues drive the segmentation clock. Cell 171, 668–682.e11 (2017).
Baker, R. E., Schnell, S. & Maini, P. K. A clock and wavefront mechanism for somite formation. Dev. Biol. 293, 116–126 (2006).
Hester, S. D., Belmonte, J. M., Gens, J. S., Clendenon, S. G. & Glazier, J. A. A multi-cell, multi-scale model of vertebrate segmentation and somite formation. PLoS Comput. Biol. 7, e1002155 (2011).
Adhyapok, P. et al. A mechanical model of early somite segmentation. iScience 24, 102317 (2021).
Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D. & Copp, A. J. Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144, 552–566 (2017).
Morriss-Kay, G. M. Growth and development of pattern in the cranial neural epithelium of rat embryos during neurulation. J. Embryol. Exp. Morphol. 65, 225–241 (1981).
Sawyer, J. M. et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5–19 (2010).
Botto, L. D., Moore, C. A., Khoury, M. J. & Erickson, J. D. Neural-tube defects. N. Engl. J. Med. 341, 1509–1519 (1999).
Karzbrun, E. et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272 (2021).
Sahni, G. et al. A micropatterned human-specific neuroepithelial tissue for modeling gene and drug-induced neurodevelopmental defects. Adv. Sci. 8, e2101786 (2021).
Jacobson, A. G. & Gordon, R. Changes in the shape of the developing vertebrate nervous system analyzed experimentally, mathematically and by computer simulation. J. Exp. Zool. 197, 191–246 (1976).
Odell, G. M., Oster, G., Alberch, P. & Burnside, B. The mechanical basis of morphogenesis: I. Epithelial folding and invagination. Dev. Biol. 85, 446–462 (1981).
Inoue, Y. et al. Mechanical roles of apical constriction, cell elongation, and cell migration during neural tube formation in Xenopus. Biomech. Model. Mechanobiol. 15, 1733–1746 (2016).
Fletcher, A. G., Osterfield, M., Baker, R. E. & Shvartsman, S. Y. Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291–2304 (2014).
de Goederen, V., Vetter, R., McDole, K. & Iber, D. Hinge point emergence in mammalian spinal neurulation. Proc. Natl Acad. Sci. USA 119, e2117075119 (2022).
Chen, X. & Brodland, G. W. Multi-scale finite element modeling allows the mechanics of amphibian neurulation to be elucidated. Phys. Biol. 5, 015003 (2008).
Taber, L. A. Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487–545 (1995).
Brodland, G. W., Chen, X., Lee, P. & Marsden, M. From genes to neural tube defects (NTDs): insights from multiscale computational modeling. HFSP J. 4, 142–152 (2010).
Helander, H. F. & Fandriks, L. Surface area of the digestive tract — revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Shyer, A. E. et al. Villification: how the gut gets its villi. Science 342, 212–218 (2013).
Shyer, A. E., Huycke, T. R., Lee, C., Mahadevan, L. & Tabin, C. J. Bending gradients: how the intestinal stem cell gets its home. Cell 161, 569–580 (2015).
Walton, K. D. et al. Villification in the mouse: Bmp signals control intestinal villus patterning. Development 143, 427–436 (2016).
Mao, J., Kim, B. M., Rajurkar, M., Shivdasani, R. A. & McMahon, A. P. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137, 1721–1729 (2010).
Rao-Bhatia, A. et al. Hedgehog-activated Fat4 and PCP pathways mediate mesenchymal cell clustering and villus formation in gut development. Dev. Cell 52, 647–658.e6 (2020).
Karlsson, L., Lindahl, P., Heath, J. K. & Betsholtz, C. Abnormal gastrointestinal development in PDGF-A and PDGFR-α deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127, 3457–3466 (2000).
Chin, A. M., Hill, D. R., Aurora, M. & Spence, J. R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 66, 81–93 (2017).
Walton, K. D., Freddo, A. M., Wang, S. & Gumucio, D. L. Generation of intestinal surface: an absorbing tale. Development 143, 2261–2272 (2016).
Sumigray, K. D., Terwilliger, M. & Lechler, T. Morphogenesis and compartmentalization of the intestinal crypt. Dev. Cell 45, 183–197.e5 (2018).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Mithal, A. et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 11, 215 (2020).
Workman, M. J. et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 23, 49–59 (2017).
Yang, Q. et al. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat. Cell Biol. 23, 733–744 (2021).
Perez-Gonzalez, C. et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 23, 745–757 (2021).
Hartl, L., Huelsz-Prince, G., van Zon, J. & Tans, S. J. Apical constriction is necessary for crypt formation in small intestinal organoids. Dev. Biol. 450, 76–81 (2019).
Serra, D. et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72 (2019).
Krndija, D. et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365, 705–710 (2019).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Balbi, V. & Ciarletta, P. Morpho-elasticity of intestinal villi. J. R. Soc. Interface 10, 20130109 (2013).
Hannezo, E., Prost, J. & Joanny, J.-F. Instabilities of monolayered epithelia: shape and structure of villi and crypts. Phys. Rev. Lett. 107, 078104 (2011).
Ben Amar, M. & Jia, F. Anisotropic growth shapes intestinal tissues during embryogenesis. Proc. Natl Acad. Sci. USA 110, 10525–10530 (2013).
Freddo, A. M. et al. Coordination of signaling and tissue mechanics during morphogenesis of murine intestinal villi: a role for mitotic cell rounding. Integr. Biol. 8, 918–928 (2016).
Thalheim, T. et al. Stem cell competition in the gut: insights from multi-scale computational modelling. J. R. Soc. Interface 13, 20160218 (2016).
Buske, P. et al. On the biomechanics of stem cell niche formation in the gut — modelling growing organoids. FEBS J. 279, 3475–3487 (2012).
Itzkovitz, S., Blat, I. C., Jacks, T., Clevers, H. & van Oudenaarden, A. Optimality in the development of intestinal crypts. Cell 148, 608–619 (2012).
Almet, A. A., Maini, P. K., Moulton, D. E. & Byrne, H. M. Modeling perspectives on the intestinal crypt, a canonical system for growth, mechanics, and remodeling. Curr. Opin. Biomed. Eng. 15, 32–39 (2020).
Schoenwolf, G. C., Bleyl, S. B., Brauer, P. R. & Francis-West, P. H. Larsen’s Human Embryology (Elsevier Health Sciences, 2014).
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453, 745–750 (2008).
Swarr, D. T. & Morrisey, E. E. Lung endoderm morphogenesis: gasping for form and function. Annu. Rev. Cell Dev. Biol. 31, 553–573 (2015).
Whitsett, J. A., Kalin, T. V., Xu, Y. & Kalinichenko, V. V. Building and regenerating the lung cell by cell. Physiol. Rev. 99, 513–554 (2019).
Goodwin, K. & Nelson, C. M. Branching morphogenesis. Development 147, dev184499 (2020).
Lang, C., Conrad, L. & Iber, D. Organ-specific branching morphogenesis. Front. Cell Dev. Biol. 9, 671402 (2021).
Varner, V. D. & Nelson, C. M. Cellular and physical mechanisms of branching morphogenesis. Development 141, 2750–2759 (2014).
Goodwin, K. et al. Smooth muscle differentiation shapes domain branches during mouse lung development. Development 146, dev181172 (2019).
Kim, H. Y. et al. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev. Cell 34, 719–726 (2015).
Bellusci, S., Grindley, J., Emoto, H., Itoh, N. & Hogan, B. L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867–4878 (1997).
Volckaert, T. et al. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 140, 3731–3742 (2013).
Chen, Y. W. et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 19, 542–549 (2017).
Miller, A. J. et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119 (2018).
Strikoudis, A. et al. Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep. 27, 3709–3723.e5 (2019).
Tian, L. et al. Human pluripotent stem cell-derived lung organoids: potential applications in development and disease modeling. Wiley Interdiscip. Rev. Dev. Biol. 10, e399 (2021).
Dye, B. R. et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4, e05098s (2015).
McCauley, K. B. et al. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20, 844–857.e6 (2017).
Konishi, S. et al. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Rep. 6, 18–25 (2016).
Menshykau, D., Blanc, P., Unal, E., Sapin, V. & Iber, D. An interplay of geometry and signaling enables robust lung branching morphogenesis. Development 141, 4526–4536 (2014).
Menshykau, D., Kraemer, C. & Iber, D. Branch mode selection during early lung development. PLoS Comput. Biol. 8, e1002377 (2012).
Varner, V. D., Gleghorn, J. P., Miller, E., Radisky, D. C. & Nelson, C. M. Mechanically patterning the embryonic airway epithelium. Proc. Natl Acad. Sci. USA 112, 9230–9235 (2015).
Hannezo, E. et al. A unifying theory of branching morphogenesis. Cell 171, 242–255.e27 (2017).
Benazeraf, B. & Pourquie, O. Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 29, 1–26 (2013).
Takada, S. et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 8, 174–189 (1994).
Bertrand, N., Medevielle, F. & Pituello, F. FGF signalling controls the timing of Pax6 activation in the neural tube. Development 127, 4837–4843 (2000).
Gouti, M. et al. A gene regulatory network balances neural and mesoderm specification during vertebrate trunk development. Dev. Cell 41, 243–261.e7 (2017).
Koch, F. et al. Antagonistic activities of Sox2 and Brachyury control the fate choice of neuro-mesodermal progenitors. Dev. Cell 42, 514–526.e7 (2017).
Olivera-Martinez, I., Harada, H., Halley, P. A. & Storey, K. G. Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 10, e1001415 (2012).
Boulet, A. M. & Capecchi, M. R. Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo. Dev. Biol. 371, 235–245 (2012).
Benazeraf, B. et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248–252 (2010).
Mongera, A. et al. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 561, 401–405 (2018).
Xiong, F., Ma, W., Benazeraf, B., Mahadevan, L. & Pourquie, O. Mechanical coupling coordinates the co-elongation of axial and paraxial tissues in avian embryos. Dev. Cell 55, 354–366.e5 (2020).
Beccari, L. et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276 (2018).
van den Brink, S. C. et al. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409 (2020).
Moris, N. et al. An in vitro model of early anteroposterior organization during human development. Nature 582, 410–415 (2020).
Veenvliet, J. V. et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 370, eaba4937 (2020).
Anand, G. M. et al. Controlling organoid symmetry breaking uncovers an excitable system underlying human axial elongation. Cell 186, 497–512.e23 (2023).
Libby, A. R. G. et al. Axial elongation of caudalized human organoids mimics aspects of neural tube development. Development 148, dev198275 (2021).
Pennimpede, T. et al. In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev. Biol. 372, 55–67 (2012).
Amin, S. et al. Cdx and T Brachyury co-activate growth signaling in the embryonic axial progenitor niche. Cell Rep. 17, 3165–3177 (2016).
Regev, I., Guevorkian, K., Gupta, A., Pourquié, O. & Mahadevan, L. Rectified random cell motility as a mechanism for embryo elongation. Development 149, dev199423 (2022).
Lawton, A. K. et al. Regulated tissue fluidity steers zebrafish body elongation. Development 140, 573–582 (2013).
Valet, M., Siggia, E. D. & Brivanlou, A. H. Mechanical regulation of early vertebrate embryogenesis. Nat. Rev. Mol. Cell Biol. 23, 169–184 (2022).
Turing, A. M. The chemical basis of morphogenesis. 1953. Bull. Math. Biol. 52, 153–197 (1990).
Kondo, S. & Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Howard, J., Grill, S. W. & Bois, J. S. Turing’s next steps: the mechanochemical basis of morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 392–398 (2011).
Almuedo-Castillo, M. et al. Scale-invariant patterning by size-dependent inhibition of Nodal signalling. Nat. Cell Biol. 20, 1032–1042 (2018).
Werner, S. et al. Scaling and regeneration of self-organized patterns. Phys. Rev. Lett. 114, 138101 (2015).
Recho, P., Hallou, A. & Hannezo, E. Theory of mechanochemical patterning in biphasic biological tissues. Proc. Natl Acad. Sci. USA 116, 5344–5349 (2019).
Meinhardt, H. The Algorithmic Beauty of Sea Shells (Springer Science & Business Media, 2009).
Watanabe, M. & Kondo, S. Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet. 31, 88–96 (2015).
Glover, J. D. et al. The developmental basis of fingerprint pattern formation and variation. Cell 186, 940–956 (2023).
Iber, D. & Menshykau, D. The control of branching morphogenesis. Open. Biol. 3, 130088 (2013).
Zhang, Z., Zwick, S., Loew, E., Grimley, J. S. & Ramanathan, S. Mouse embryo geometry drives formation of robust signaling gradients through receptor localization. Nat. Commun. 10, 4516 (2019).
Gao, H., Ji, B., Jäger, I. L., Arzt, E. & Fratzl, P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc. Natl Acad. Sci. USA 100, 5597–5600 (2003).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011).
Bauwens, C. L. et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cell 26, 2300–2310 (2008).
Tewary, M. et al. A stepwise model of reaction-diffusion and positional information governs self-organized human peri-gastrulation-like patterning. Development 144, 4298–4312 (2017).
Etoc, F. et al. A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell 39, 302–315 (2016).
Xue, X. et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 17, 633–641 (2018).
Diaz-Cuadros, M. et al. Metabolic regulation of species-specific developmental rates. Nature 613, 550–557 (2023).
Lázaro, J. et al. A stem cell zoo uncovers intracellular scaling of developmental tempo across mammals. Cell Stem Cell, https://doi.org/10.1016/j.stem.2023.05.014 (2023).
Matsuda, M. et al. Species-specific segmentation clock periods are due to differential biochemical reaction speeds. Science 369, 1450–1455 (2020).
Matsuda, M. et al. Recapitulating the human segmentation clock with pluripotent stem cells. Nature 580, 124–129 (2020).
Diaz-Cuadros, M. et al. In vitro characterization of the human segmentation clock. Nature 580, 113–118 (2020).
Rogers, K. W. & Schier, A. F. Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377–407 (2011).
Stapornwongkul, K. S. & Vincent, J. P. Generation of extracellular morphogen gradients: the case for diffusion. Nat. Rev. Genet. 22, 393–411 (2021).
Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 (1969).
Nordstrom, U., Jessell, T. M. & Edlund, T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525–532 (2002).
Swindell, E. C. et al. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 216, 282–296 (1999).
Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008).
Towers, M. & Tickle, C. Growing models of vertebrate limb development. Development 136, 179–190 (2009).
Tickle, C., Summerbell, D. & Wolpert, L. Positional signalling and specification of digits in chick limb morphogenesis. Nature 254, 199–202 (1975).
Barriga, E. H., Franze, K., Charras, G. & Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527 (2018).
Zhu, M. et al. Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc. Natl Acad. Sci. USA 117, 4781–4791 (2020).
Shellard, A. & Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 600, 690–694 (2021).
Zheng, Y. et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425 (2019).
Manfrin, A. et al. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 16, 640–648 (2019).
Rifes, P. et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 38, 1265–1273 (2020).
Muncie, J. M. et al. Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev. Cell 55, 679–694.e11 (2020).
Saadaoui, M., Rocancourt, D., Roussel, J., Corson, F. & Gros, J. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science 367, 453–458 (2020).
Liu, X. et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591, 627–632 (2021).
Yu, L. et al. Blastocyst-like structures generated from human pluripotent stem cells. Nature 591, 620–626 (2021).
Kagawa, H. et al. Human blastoids model blastocyst development and implantation. Nature 601, 600–605 (2022).
Zheng, Y. et al. Dorsal-ventral patterned neural cyst from human pluripotent stem cells in a neurogenic niche. Sci. Adv. 5, eaax5933 (2019).
Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. & Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, eaal1810 (2017).
Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335–373 (2001).
Xia, Y. & Whitesides, G. M. Soft lithography. Annu. Rev. Mater. Sci. 28, 153–184 (1998).
Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. & Brivanlou, A. H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854 (2014).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010).
Alom Ruiz, S. & Chen, C. S. Microcontact printing: a tool to pattern. Soft Matter 3, 168–177 (2007).
Bernard, A., Renault, J. P., Michel, B., Bosshard, H. R. & Delamarche, E. Microcontact printing of proteins. Adv. Mater. 12, 1067–1070 (2000).
Karp, J. M. et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7, 786–794 (2007).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Dahlmann, J. et al. The use of agarose microwells for scalable embryoid body formation and cardiac differentiation of human and murine pluripotent stem cells. Biomaterials 34, 2463–2471 (2013).
Chen, K. et al. Branching development of early post-implantation human embryonic-like tissues in 3D stem cell culture. Biomaterials 275, 120898 (2021).
Ma, Z. et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 6, 7413 (2015).
Gjorevski, N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021 (2022).
Nelson, C. M., Inman, J. L. & Bissell, M. J. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat. Protoc. 3, 674–678 (2008).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020).
Brassard, J. A., Nikolaev, M., Hübscher, T., Hofer, M. & Lutolf, M. P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 20, 22–29 (2021).
Bratt-Leal, A. M., Nguyen, A. H., Hammersmith, K. A., Singh, A. & McDevitt, T. C. A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 34, 7227–7235 (2013).
Suri, S. et al. Microfluidic-based patterning of embryonic stem cells for in vitro development studies. Lab Chip 13, 4617–4624 (2013).
O’Grady, B. et al. Spatiotemporal control and modeling of morphogen delivery to induce gradient patterning of stem cell differentiation using fluidic channels. Biomater. Sci. 7, 1358–1371 (2019).
Demers, C. J. et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development 143, 1884–1892 (2016).
Samal, P., van Blitterswijk, C., Truckenmüller, R. & Giselbrecht, S. Grow with the flow: when morphogenesis meets microfluidics. Adv. Mater. 31, 1805764 (2019).
Martinez-Ara, G. et al. Optogenetic control of apical constriction induces synthetic morphogenesis in mammalian tissues. Nat. Commun. 13, 5400 (2022).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Vining, K. H. & Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017).
Abdel Fattah, A. R. et al. Actuation enhances patterning in human neural tube organoids. Nat. Commun. 12, 3192 (2021).
Li, Y. et al. Volumetric compression induces intracellular crowding to control intestinal organoid growth via Wnt/β-catenin signaling. Cell Stem Cell 28, 63–78.e7 (2021).
Pelham, R. J. Jr. & Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA 94, 13661–13665 (1997).
Sun, Y. et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 13, 599–604 (2014).
Fu, J. et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7, 733–736 (2010).
Riehl, B. D., Park, J. H., Kwon, I. K. & Lim, J. Y. Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs. Tissue Eng. Part B Rev. 18, 288–300 (2012).
Moraes, C., Wang, G., Sun, Y. & Simmons, C. A. A microfabricated platform for high-throughput unconfined compression of micropatterned biomaterial arrays. Biomaterials 31, 577–584 (2010).
Sakthivel, K. et al. High throughput screening of cell mechanical response using a stretchable 3D cellular microarray platform. Small 16, e2000941 (2020).
Hsieh, H. Y. et al. Gradient static-strain stimulation in a microfluidic chip for 3D cellular alignment. Lab Chip 14, 482–493 (2014).
Liu, H., Usprech, J. F., Parameshwar, P. K., Sun, Y. & Simmons, C. A. Combinatorial screen of dynamic mechanical stimuli for predictive control of MSC mechano-responsiveness. Sci. Adv. 7, eabe7204 (2021).
Shemesh, J. et al. Flow-induced stress on adherent cells in microfluidic devices. Lab Chip 15, 4114–4127 (2015).
Yu, W. et al. A microfluidic-based multi-shear device for investigating the effects of low fluid-induced stresses on osteoblasts. PLoS ONE 9, e89966 (2014).
Mandrycky, C., Hadland, B. & Zheng, Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci. Adv. 6, eabb3629 (2020).
Topal, T. et al. Acoustic tweezing cytometry induces rapid initiation of human embryonic stem cell differentiation. Sci. Rep. 8, 12977 (2018).
Chen, D. et al. Two-bubble acoustic tweezing cytometry for biomechanical probing and stimulation of cells. Biophys. J. 108, 32–42 (2015).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Mitchell, A. C., Briquez, P. S., Hubbell, J. A. & Cochran, J. R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 30, 1–12 (2016).
Gelmi, A. & Schutt, C. E. Stimuli-responsive biomaterials: scaffolds for stem cell control. Adv. Healthc. Mater. 10, e2001125 (2021).
Yavitt, F. M. et al. In situ modulation of intestinal organoid epithelial curvature through photoinduced viscoelasticity directs crypt morphogenesis. Sci. Adv. 9, eadd5668 (2023).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Flaim, C. J., Chien, S. & Bhatia, S. N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2, 119–125 (2005).
Zustiak, S. P. & Leach, J. B. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 11, 1348–1357 (2010).
Kozlowski, M. T., Crook, C. J. & Ku, H. T. Towards organoid culture without Matrigel. Commun. Biol. 4, 1387 (2021).
Acknowledgements
Studies in the Fu Research Group are supported by the Michigan-Cambridge Collaboration Initiative, University of Michigan Mcubed Fund, University of Michigan Mid-career Biosciences Faculty Achievement Recognition Award, National Science Foundation (PFI-TT 2213845, I-Corps 2112458, CMMI 1917304, CBET 1901718 and CMMI 2325361) and National Institutes of Health (R21 NS113518, R21 HD100931, R21 HD105126, R21 NS127983, R21 HD109635, R21 HD105192, R33 CA261696, R01 GM134535 and R01 NS129850). The authors apologize to colleagues whose work they could not cite owing to space restrictions.
Author information
Authors and Affiliations
Contributions
Y.L. and J.F. wrote the article text. Y.L. generated the figures. All authors contributed to the conceptualization of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Physics thanks Carl-Philipp Heisenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Alveoli
-
Hollow, distensible cavities in lungs in which the exchange of oxygen and carbon dioxide occurs.
- Anencephaly
-
A congenital defect in the formation of neural tube, in which a baby is born without parts of the brain and skull.
- Blastocoel
-
A fluid-filled cavity inside pre-implantation embryos called blastocysts.
- Caudal
-
Towards the tail.
- Cleavage furrow
-
The indentation of the surface of a cell that begins the progression of membrane separation during cell division.
- Dorsal
-
Towards the back.
- Epiblast
-
Composed of pluripotent cells derived from inner cell mass in a blastocyst. It is located between hypoblast and trophoblast and gives rise to three definitive germ layers.
- Gastrulation
-
A morphogenetic process through which epiblast cells reorganize, differentiate and ultimately form three spatially organized germ layers, namely (dorsal to ventral) ectoderm, mesoderm and endoderm.
- Lateral
-
Away from the body midline.
- Lumen
-
A cavity or inner space enclosed by cells or tissues.
- Medial
-
Towards the body midline.
- Mesenchymal-to-epithelial transition
-
A biological process during which loosely connected mesenchymal cells reorganize, establish apical-basal polarity and transition into an assembly of closely packed epithelial cells. Its reverse process is called epithelial-to-mesenchymal transition.
- Neural plate
-
A region of ectoderm which contains a flat layer of columnar neuroepithelial cells.
- Neural tube
-
A tubular neural tissue and the precursor of the central nervous system.
- Neuromesodermal progenitor
-
A population of bipotent progenitor cells in the caudal region of the embryo. It contributes to both spinal cord and presomitic mesoderm development.
- Neurulation
-
Formation of neural tube, which involves two different morphogenetic processes. In primary neurulation, the neural plate folds inward until opposing edges come into contact, fuse and give rise to the neural tube. In secondary neurulation, cavities form in caudal neural precursors and later merge with the neural tube formed by primary neurulation.
- Respiratory diverticulum
-
A ventral outpouching structure that develops from the endodermal foregut and bifurcates into left and right lung buds. Lung buds are rudiments of two lungs and the left and right primary bronchi, and the diverticulum stem forms the trachea and larynx.
- Rostral
-
Towards the head.
- Somite
-
Segmented, block-like structures flanking the neural tube. They are the precursors to vertebrae, part of occipital bones of the skull, skeletal muscles, dermis, cartilage and tendons.
- Ventral
-
Towards the front.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Xue, X., Sun, S. et al. Morphogenesis beyond in vivo. Nat Rev Phys 6, 28–44 (2024). https://doi.org/10.1038/s42254-023-00669-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42254-023-00669-x