Abstract
Nitroxides are widely used as probes and polarization transfer agents in spectroscopy and imaging. These applications require high stability towards reducing biological environments, as well as beneficial relaxation properties. While the latter is provided by spirocyclic groups on the nitroxide scaffold, such systems are not in themselves robust under reducing conditions. In this work, we introduce a strategy for stability enhancement through conformational tuning, where incorporating additional substituents on the nitroxide ring effects a shift towards highly stable closed spirocyclic conformations, as indicated by X-ray crystallography and density functional theory (DFT) calculations. Closed spirocyclohexyl nitroxides exhibit dramatically improved stability towards reduction by ascorbate, while maintaining long relaxation times in electron paramagnetic resonance (EPR) spectroscopy. These findings have important implications for the future design of new nitroxide-based spin labels and imaging agents.
Similar content being viewed by others
Introduction
Nitroxides (also known as aminoxyl or nitroxyl radicals) are suited for innumerable applications across the chemical sciences1,2. These stable organic radicals are, for example, used as oxidants in synthetic organic chemistry3, mediators for radical polymerization4,5,6,7, redox-active components for materials science8,9, and potential therapeutic agents10. Nitroxides have also found a unique place of significance in spectroscopy. Due to the localized radical distribution, small molecular size, and readily tunable properties through structural modification, nitroxides are highly suitable for use as observable probes in electron paramagnetic resonance (EPR) spectroscopy11,12,13 and EPR imaging14. Moreover, nitroxides are used in nuclear magnetic resonance (NMR) spectroscopy as dynamic nuclear polarization (DNP) transfer agents15,16, paramagnetic relaxation enhancement (PRE) probes17, and as contrast agents in magnetic resonance imaging (MRI)18,19.
Within the field of EPR spectroscopy, double electron-electron resonance spectroscopy (DEER), also known as pulsed electron double resonance (PELDOR), is a powerful tool for the investigation of biomolecular structure20. DEER experiments enable the measurement of nanometer-scale distances and angles between two spin centers (referred to as spin labels), which have been incorporated into biomolecular systems via site-directed spin labeling (SDSL)13,21,22. The resolution of DEER measurements is, among other things, influenced by the temperature-dependent relaxation parameter Tm, the phase memory time, which should be as high as possible22.
For imaging and for modern spectroscopy applications, moreover, nitroxides should possess a sufficiently high aqueous solubility and stability towards the reducing environments typically found within cells22. In the presence of reducing agents such as ascorbate or glutathione, nitroxides are known to undergo reduction to diamagnetic hydroxylamines, which are not detectable by EPR23. Several structural factors influence nitroxide stability towards reduction (Fig. 1a). The size and saturation of the nitroxide ring has a significant impact: saturated five-membered pyrrolidinyl nitroxides are more stable than the unsaturated five-membered pyrrolinyl nitroxides, while six-membered piperidinyl nitroxides are more prone to reduction22. Electronic factors also influence the reduction resistance of nitroxides: electron-donating substituents enhance stability, while electron-withdrawing moieties decrease it24. Finally, the α-substituents shielding the paramagnetic center are a crucial factor. Replacing the most common methyl substituents with spirocyclohexyl groups results in a slight increase in the stability in reducing environments24. However, stability is significantly enhanced by more conformationally mobile ethyl substituents, which effectively occupy a larger spatial volume surrounding the spin center25,26.
Beyond their impact on reduction stability, α-substituents also have a critical influence on Tm. The rotation of methyl groups is a significant contributor to the relaxation of nitroxides, especially at temperatures above 70 K27. Thus, the commonly used tetramethyl-substituted nitroxides require the use of cryogenic temperatures for DEER. This effect is also present in tetraethyl-substituted nitroxides, where a lower energy barrier for methyl rotation leads to increased relaxation rates at even lower temperatures28. In spirocyclic systems, however, the lack of methyl groups significantly enhances Tm, even at higher temperatures29,30. Thus, the design of nitroxides for in-cell applications is often a compromise between high reduction stability and long Tm.
The conformation of spirocyclohexyl nitroxides is an important consideration: it has been proposed that such scaffolds exist as an equilibrium between open and closed conformations, where the former is preferred in solution (Fig. 1b)30,31. Closed conformations are, however, expected to have higher stability in reducing environments due to more effective steric shielding of the nitroxide. Previous investigations have demonstrated that disubstituted five-membered pyrrolidinyl nitroxides (e.g., 1, Fig. 1c) can adopt semi-open conformations with improved reduction stability32. Moreover, six-membered spirocyclohexyl nitroxides can be forced to adopt more stable semi-open or even closed conformations by the introduction of substituents on the cyclohexane rings (e.g., 2)31,33,34.
Here, we report that structural modification of the nitroxide rings itself can cause a conformational shift to provide nitroxides in fully closed conformations (Fig. 1d). The additional steric shielding obtained through this strategy results in a dramatic increase in the stability of these nitroxides towards reduction by ascorbate. Moreover, the beneficial Tm of spirocyclic systems is largely preserved, resulting in a scaffold that finds a balance between improved stability and beneficial relaxation properties. These findings are of great significance for the future design of new nitroxide-based spin labels and imaging agents.
Results and discussion
Synthesis, X-ray crystallography, and conformational analysis
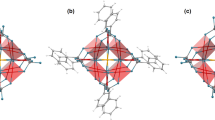
Our initial strategy for the synthesis of substituted spirocyclohexyl piperidine nitroxides relied on classical enolate chemistry (Fig. 2a). Spirocyclohexyl piperidinone 3 was prepared from the commercially available 2,2,6,6-tetramethylpiperidinone according to a literature procedure35. Deprotonation of 3 with lithium diisopropylamide (LDA), followed by alkylation with methyl iodide, resulted in the mono-methylated product 4 in moderate yield. Subsequent oxidation with m-chloroperbenzoic acid (m-CPBA) provided nitroxide radical 5 in 84% yield. As radical 5 is crystalline, we investigated its three-dimensional structure by X-ray crystallography to reveal the conformational effect of a single substituent on the nitroxide ring. The solid-state structure (Fig. 2b) indicates that the methyl group adopts an axial orientation, with the nitroxide ring preferring a twist-boat conformation. Indeed, twist-boat conformations have also previously been observed in crystal structures of spirocyclic piperidinyl nitroxides29,36. The twist-boat conformation permits the spirocyclic scaffold to maintain an overall open arrangement, despite the presence of the additional methyl substituent.
We next aimed to force the spirocyclohexyl moieties into closed conformations by further increasing the steric pressure through additional α-functionalization of ketoamine 4. However, this highly sterically hindered substrate proved unreactive towards further alkylation, either upon deprotonation with LDA and treatment with various alkylating agents (e.g., MeI, Me2SO4), or under Stork enamine alkylation conditions. Conversely, starting from ketoamine 3, two methylene substituents were directly incorporated through condensation with formaldehyde (Fig. 2a)37. Although dienone 6 could be isolated, it polymerized in a matter of hours. Thus, it was immediately subjected to hydrogenation to afford dimethyl spirocyclohexyl ketoamine 7 in excellent yield as a mixture of diastereomers (dr 1:1). Subsequent oxidation with m-CPBA resulted in two diastereomers of dimethylated nitroxide 8, which was recrystallized to enable structural investigation by X-ray crystallography. We obtained a solid-state structure for the trans-diastereomer of 8, where the nitroxide ring adopts a chair conformation with one methyl substituent in an axial and the other in an equatorial orientation. The equatorial methyl group induces a conformational change of the proximal spirocyclohexyl moiety, resulting in a semi-open arrangement.
The two diastereomers of 8 were not readily separable by chromatography; moreover, we were concerned about possible diastereomeric interconversion via keto-enol tautomeric equilibria. Thus, we proceeded to reduce ketone 8 to alcohol 9, which was obtained in excellent yield as a mixture of two diastereomers (dr 1:1), which were separable by column chromatography. The cis,cis-diastereomer of 9 was a crystalline solid, with the relative stereochemistry confirmed by X-ray crystallography (Fig. 2b). To our delight, cis,cis-9 exhibits a closed conformation, presumably due to the combined steric pressure exerted by the two equatorial methyl substituents. The nitroxide ring adopted a chair conformation, placing the alcohol functionality in an axial position.
Kinetic studies—stability toward reduction
The stability of nitroxides towards reduction was measured by continuous-wave (CW) X-band EPR spectroscopy as a function of decay of the characteristic triplet signal in the presence of sodium ascorbate (1.4 equivalents). These conditions permitted the observation of meaningful decay rates at nitroxide concentrations high enough to be reliably measured on a benchtop EPR spectrometer. The time-dependent decay curves of the novel nitroxides 5, 8, and cis,cis-9 are shown in Fig. 3a. To confirm our hypothesis, these substituted nitroxides were compared with the non-methylated spirocyclohexyl nitroxides 10 and 11. The reduction kinetics of the well-established tetramethyl-substituted TEMPOL (12) and tetraethyl-substituted TEEPOL (13), were included as benchmarks for the piperidinyl nitroxides that are least and most resistant towards reduction, respectively25. Second-order rate constants k were derived by fitting decay data to a second-order kinetic rate law (see the SI for more details), and are included in Fig. 3c. Deviations from ideal second-order behavior were apparent, especially for rapidly reducing nitroxides, for which reliable rate constants could not be extracted. The non-ideal reduction kinetics are consistent with previous findings that the mechanism of nitroxide reduction by ascorbate is reversible and proceeds through a complex mechanism23.
a Second-order kinetic reduction curves of nitroxides (2 mM) with sodium ascorbate (1.4 eq.) in PBS buffer/DMSO (1:1) at 291 K. Data points are the average of three runs, with error bars representing 2 × standard deviation. b Structures of nitroxides for kinetic studies. c Second-order rate constants k [M−1 s−1] for 2 mM piperidinyl nitroxides, derived from decay curves by fitting to a second-order kinetic rate law. See the SI, Figs. S11, S12, and Table S1 for further details.
The presence of functional groups is known to influence the reduction rate of nitroxides25. In line with previous studies, the presence of an alcohol vs a ketone functionality has a negligible impact on the rate of reduction for the conditions and nitroxide scaffolds examined in this work. It is known that spirocyclohexyl-substituted piperidinyl nitroxides show slightly higher stability towards reduction than tetramethyl-substituted nitroxides, but not higher than tetraethyl-substituted radicals26,38,39, a trend that is also clear from the decay curves in Fig. 3a. However, we were pleased to observe a significant decrease of almost one order of magnitude in the rate of reduction of dimethylated spirocyclohexyl nitroxide cis,cis-9 compared to non-methylated nitroxide 11. This effect can be ascribed to the conformational effect on the spirocyclohexyl rings caused by the additional steric pressure from the two methyl substituents in 9, causing the compound to prefer a closed conformation, similar to that observed in the solid-state X-ray crystal structure (Fig. 2b), that more effectively shields the radical from reducing agents. The reduction rate of mono-methylated ketone nitroxide 5, however, does not differ significantly from that of the non-methylated version 10. This is also in agreement with the open conformation adopted in the crystal structure of this compound (Fig. 2b). Surprisingly, the dimethylated ketone 8, which was used as a mixture of diastereomers, is reduced more rapidly than all other spirocyclohexyl nitroxides. While the trans-diastereomer of 8 adopts a semi-open conformation in the solid state (Fig. 2b), the preferred conformation of the other diastereomer is unknown. Thus, at this point, the conformation of 8 cannot be related to its reactivity.
Relaxation time measurements
For potential applications as spin labels for DEER or polarization transfer agents in DNP NMR, nitroxides should also ideally exhibit sufficiently long relaxation times (Tm) to enable precise measurement of long distances, and efficient polarization transfer for signal enhancement, respectively20,40. It is well-known that the presence of methyl groups shortens Tm due to methyl rotation, especially at temperatures above 70 K27. Although the requirement for using cryogenic temperatures that rely on liquid helium increases the operating costs of EPR spectrometers, contemporary instruments now minimize liquid helium consumption by the use of circulating liquid helium pumps41. Nevertheless, for reasons of cost and sustainability, it is desirable to preserve extended Tm into temperatures that can be acquired using liquid nitrogen, especially for DNP NMR applications42.
For Tm measurements, nitroxides were dissolved in 1:1 PBS buffer/glycerol, in which concentrations of 50 µM were easily achieved. After flash freezing in liquid nitrogen, samples were cooled to 50 K and spin echo decay curves were recorded using pulsed Q-band EPR spectroscopy. The spin echo decays of nitroxide cis,cis-9, 11, 12 and 13 at this temperature are shown in Fig. 4a. Dimethylated spirocyclohexyl nitroxide cis,cis-9 has a similar echo decay to the non-methylated spirocyclohexyl nitroxide 11, which exhibits the longest Tm. Tetramethyl nitroxide 12 has a slightly shorter Tm even at 50 K, but tetraethyl nitroxide 13 shows significantly more rapid relaxation. This has previously been ascribed to an increased local proton concentration in the tetraethyl substituents43. However, we note that spirocyclohexyl nitroxides also have an increased local proton concentration, and that the line shape of the echo decay of 13 suggests there are multiple mechanisms of spin echo dephasing in operation for the tetraethyl system at 50 K.
a Spin echo decay curves for nitroxides cis,cis-9, 11, 12, and 13 at 50 and 100 K. b Temperature-dependent Tm values for nitroxides cis,cis-9, 11, 12, and 13 measured by spin echo decay. The method for the extraction of Tm values is described in the SI, Fig. S13.
We next investigated Tm across a range of temperatures. A plot of Tm as a function of temperature (Fig. 4b) clearly shows how methyl rotation significantly increases relaxation in tetramethyl nitroxide 12 (from 80 K) and tetraethyl nitroxide 13 (from 60 K). The earlier onset of methyl rotation in tetraethyl nitroxides is explained by a lower energy barrier of methyl rotation in these systems44. It is not clear, however, why the Tm apparently increases again, and becomes longer than the tetramethyl nitroxide 12 as the temperature rises above ca. 120 K. This unexpected behavior is in line with observations in our previous work, and suggests further complications connected with the use of tetraethyl nitroxides45. The origins of this effect will be further investigated in the future. Conversely, spirocyclohexyl nitroxides cis,cis-9 and 11 exhibit low Tm dephasing rates even at higher temperatures, with dimethylated spirocyclohexyl nitroxide cis,cis-9 having relaxation rates similar to the non-methylated analog 11 up to ca. 100 K. Additional dephasing beyond this temperature is presumably driven by rotation of the methyl substituents on the nitroxide ring. The spin echo decay curves at 100 K (Fig. 4a) illustrate how distinct behavior of different methyl environments have a critical impact on Tm at this temperature, which can be easily achieved using liquid nitrogen cooling. The diversity of the impact of methyl substituents on Tm between cis,cis-9 and 12 may be a function of the distance between the paramagnetic center and the methyl groups, or different rates of methyl rotation44. The alignment of the group with the anisotropic spin tensor of the electron may be an additional contributing factor.
DFT calculations
To assess the impact of the additional nitroxide ring substitution across the conformational equilibrium, a computational analysis using density functional theory (DFT, PBE0-D3BJ/def2-TZVPP[IEFPCM])46,47,48,49,50,51,52,53,54,55,56 was performed. The robustness of computed results was checked by applying B3LYP-D3 for comparison (SI, Fig. S14). First, Gibbs free energies and Boltzmann distributions were computed for open, semi-open, and closed conformations of alcohol nitroxides 11 and 9 (Fig. 5 and SI, Fig. S15). Conformers were identified where both the central nitroxide-containing ring and spirocyclohexyl substituents adopt chair conformations. Interconversion between these conformers corresponds to a ring-flip of either the nitroxide-containing heterocycle or the spirocyclohexyl rings, via intermediate twist-boat and chair structures. We expect the identified conformations to be freely interconverting in solution at room temperature; as the nitroxide functionality is approximately planar, nitroxides 11 and 9 are structurally comparable to highly sterically crowded cyclohexanones, which have an estimated energy barrier for conformational interconversion of 16.7 kcal/mol57. For the non-methylated nitroxide 11 (Fig. 5a), the open conformation with an equatorial hydroxyl group is favored over the corresponding closed conformation by 2.2 kcal/mol, in line with the expectation that this compound favors an open conformation. This contrasts with the dimethylated analog 9, which can exist as the diastereomers cis,cis-9, trans,trans-9, and cis,trans-9. For cis,cis-9 there are two low-energy conformations: a closed structure with equatorial methyl groups (as also observed in the solid-state crystal structure, Fig. 2b), and an open conformation with both methyl groups in axial positions (Fig. 5b). These structures are the predominantly populated conformations in solution, in an approximate ratio of 1:2. Notably, the two most stable conformers have very similar energies, resulting in a change in their order of relative stability when using B3LYP-D3 instead of PBE0-D3BJ (see SI, Fig. S14). Thus, the calculations support our hypothesis that methyl substituents at the 3- and 5-positions of the nitroxide ring exert a steric pressure that can increase the preference for the closed conformation.
Distribution of calculated conformers in open, semi-open, and closed conformations for nitroxides 11 (a), cis,cis-9 (b), cis,trans-9 (c), and trans,trans-9 (d). The most highly populated conformers are shown for each nitroxide, along with the calculated Gibbs free energies (in kcal/mol, relative to the most stable conformation) and Boltzmann distribution (parentheses, in percentage) (298 K, PBE0-D3BJ/def2-TZVPP[IEFPCM, water])46,47,48,49,50,51,52,53,54,55,56. See the SI, Fig. S15, for all calculated conformations and energies.
The calculations also show that the relative stereochemical configuration of the ring substituents is important. The diastereomer cis,trans-9 (Fig. 5c) mostly populates semi-open and closed conformations, with the former, lowest-energy structure demonstrating that an equatorial methyl group is more effective at forcing the spirocyclohexyl ring to adopt a closed position than an axial group, for nitroxide rings in a chair conformation. In line with this, the last possible diastereomer, trans,trans-9, displays a strong preference for the closed over the open conformation, by 5.0 kcal/mol, if all nitroxide ring substituents are equatorial and 2.7 kcal/mol if substituents are axial (Fig. 5d and SI, Fig. S15). The increased preference for closed conformations of these other diastereomers of 9 suggests that they may be even more stable towards reduction than cis,cis-9.
The calculated conformational energies and Boltzmann distributions of ketone nitroxides 10, 5, and 8 present a more complicated picture (Fig. 6 and SI, Fig. S16). Due to reduced torsional strain, six-membered rings with two sp2-hybridized centers in a 1,4-relationship can readily access low-energy twist-boat conformations58. Reduced strain in the transition states reported for conformational interconversion further indicates that such systems will rapidly interconvert at room temperature59. Indeed, the non-methylated ketone nitroxide 10 preferentially adopts an open twist-boat conformation, which is favored over a chair conformation by 1.7 kcal/mol (Fig. 6a and SI, Fig. S16). In mono-methylated nitroxide 5 (Fig. 6b), a small energetic preference is found for an open twist-boat conformation that places the methyl substituent in an axial orientation, as observed in the solid-state crystal structure of this compound (Fig. 2b). Nitroxide 5, however, also populates several other low-energy open and semi-open structures, but the steric pressure exerted by a single methyl substituent is apparently insufficient to induce any significant population of closed conformations. The trans-diastereomer of dimethylated nitroxide 8 prefers a semi-open chair conformation, as observed in the X-ray structure (Fig. 2b), but can also access two closed structures only 0.5 kcal/mol higher in energy (Fig. 6c). For the cis-8 diastereomer, a preference is found for a semi-open twist-boat conformation, but this compound also significantly populates a closed chair conformation that is only 0.2 kcal/mol higher in energy (Fig. 6d). Overall, the calculated conformational energies for nitroxides 5, 8, and 10 indicate that the introduction of methyl substituents does increase the occupancy of semi-open and closed conformations also for ketone spirocyclohexyl nitroxides, although this does not translate into increased stability towards reduction (cf. Fig. 3a). A possible explanation may be found in a higher thermodynamic electron affinity of 5 and 8 (vide infra).
Distribution of calculated conformers in open, semi-open, and closed conformations for nitroxides 10 (a), 5 (b), trans-8 (c), and cis-8 (d). The most highly populated conformers are shown for each nitroxide, along with the calculated Gibbs free energies (in kcal/mol, relative to the most stable conformation) and Boltzmann distribution (parentheses, in percentage) (298 K, PBE0-D3BJ/def2-TZVPP[IEFPCM, water])46,47,48,49,50,51,52,53,54,55,56. See the SI, Fig. S16, for all calculated conformations and energies.
To assess any thermodynamic contribution to reduction stability, we next calculated nitroxide electron affinities (EAs). Energies were calculated for optimized geometries of all possible conformations of the hydroxylamine anions formed by single-electron reduction of nitroxide radicals (SI, Fig. S17), and the adiabatic EA was taken as the energy difference between the most stable conformation of the nitroxide (Figs. 5, 6) and the most stable conformation of the hydroxylamine anion (SI, Fig. S17) for each compound. The EAs (Fig. 7) are generally higher for ketone nitroxides compared to the alcohols. This may be due to an electronic contribution from the electron-withdrawing carbonyl groups. For the alcohol nitroxides, dimethylated spirocyclohexyl nitroxide cis,cis-9, and the non-methylated analog 11 have identical EAs, which supports our assumption that the stabilization achieved by the introduction of methyl groups is a kinetic effect. By comparison, tetramethyl-substituted nitroxide 12 has a slightly higher apparent EA, while the EA of tetraethyl analog 13 is considerably lower. We ascribe this trend to the electronic repulsion between the hydroxylamine anion and the α-substituents, which is reduced in 12 and increased in 13, and constitutes a thermodynamic factor that disfavors the reduction of nitroxides. The ketone nitroxide cis-8, however, has a significantly higher EA than all other nitroxides, which counteracts the presumed stability in more highly populated semi-open and closed conformations. This can explain the experimental finding (Fig. 3a) that nitroxide 8 (as a mixture of both diastereomers) is reduced more rapidly than the other spirocyclohexyl nitroxides.
Calculated adiabatic electron affinities (EAs) for nitroxides 5, 8, cis,cis-9, and 10–13 (298 K, PBE0-D3BJ/def2-TZVPP[IEFPCM, water])46,47,48,49,50,51,52,53,54,55,56. EAs were taken as the energy difference between the most stable nitroxide conformer and the most stable hydroxylamine anion conformer for each compound.
Conclusion
In this work, we have successfully developed a new and reliable synthetic route to obtain a series of novel 3,5-substituted spirocyclohexyl piperidinyl nitroxides. Investigation of the conformations of these compounds by X-ray crystallography and DFT calculations confirmed our hypothesis that the introduction of additional substituents on the nitroxide ring can induce spirocyclohexyl nitroxides to favor closed conformations. Our results indicate that only methyl substituents in an equatorial position on chair conformations of the central nitroxide ring can maximize the steric pressure to fully enforce a closed conformation of the nearby spirocyclohexyl groups. As indicated by kinetic decay studies with CW-EPR, this conformational biasing significantly increases the stability of spirocyclohexyl nitroxides towards reduction by ascorbate. Moreover, the beneficial extended phase memory times (Tm) exhibited by spirocyclohexyl nitroxides in pulsed EPR are generally preserved despite the introduction of methyl substituents on the nitroxide ring, thereby striking an excellent compromise between reduction stability and extended Tm in these scaffolds. These balanced properties are of great potential utility in nitroxides intended for applications such as biocompatible spin labels, imaging probes, or DNP NMR polarization transfer agents.
Methods
General methods and materials are described in the Supplementary Information (Page 2, SI).
Chemical synthesis
Detailed synthetic procedures and characterization data for all nitroxides and intermediates are described in the Supplementary Information.
X-ray crystallography
Crystals of compounds 5, 8, and cis,cis-9 were grown from diethyl ether by the slow evaporation method. Crystals were glued to glass fibers and mounted on the goniometer. Data were collected using a D8 Venture system with a Cu-anode d (λ = 1.54178 Å). See the SI, Figs. S1–S3 for the ellipsoids.
Electron paramagnetic resonance (EPR) spectroscopy
Room temperature CW-EPR measurements were acquired using an X-band Affirmo microESR Benchtop EPR Spectrometer (now marketed as the Bruker microESR). See the SI, Figures S4-S10 for X-band CW-EPR spectra of the nitroxides. Bruker Elexsys E580 with high powered (150 W) Q-band (34 GHz) and an ER 5106QT-2w cylindrical resonator was used for relaxation parameter investigations. Detailed parameters and procedures are described in the Supplementary Information.
Kinetic studies
Sodium ascorbate solution in PBS buffer/DMSO (1:1 v/v) was used as a reduction agent. Ascorbate solution was added to the nitroxide solution and the peak height of the low-field line of the nitroxide triplet CW-EPR spectrum was measured as a function of time. Each kinetic run was repeated three times on the same day using the same ascorbate solution. Detailed procedures and data fitting are described in the Supplementary Information.
DFT calculations
All calculations were performed on complete molecular systems without any truncations using Gaussian16 (Revision B.01)56. The systems were fully relaxed, and no symmetry constraints were imposed. For the geometry optimizations, the DFT hybrid functional PBE049,50, which contains 25 percent Hartree–Fock exchange, was used along with the D3BJ55 dispersion correction (additional calculations with B3LYP47-D3 provide similar trends, see Figure S14). The basis set used for geometry optimizations and frequency calculations was def2-TZVPP46,48,52. Solvation effects were included using the polarizable continuum model (IEFPCM)54 with the parameters of water (ε = 80). Reported Gibbs free energies include thermal corrections computed at 298 K. See the SI, Fig. S18, for the optimized geometries. Hartree energies of all calculated conformers are shown in Table S2.
Data availability
Copies of the NMR spectra are included in Supplementary Data 1 (Figs. S19–S46). The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2214625, 2214626, and 2214627. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Moreover, CIF files and SCXRD reports for nitroxides 5, trans-8, and cis,cis-9 are included in Supplementary Data 2–7. Coordinates of all optimized geometries and their energies are given in Supplementary Data 8 (XYZ file). Additional raw data is available through the DataverseNO repository at https://doi.org/10.18710/UQMMZE60.
References
Karoui, H., Moigne, F. L., Ouari, O. & Tordo, P. in Stable Radicals (ed. Hicks, R. G.) Ch. 5 (Wiley, 2010).
Bagryanskaya, E. G. & Marque, S. R. A. Scavenging of organic C-centered radicals by nitroxides. Chem. Rev. 114, 5011–5056 (2014).
Brückner, C. in Stable Radicals (ed. Hicks, R. G.) Ch. 12 (Wiley, 2010).
Studer, A. & Schulte, T. Nitroxide-mediated radical processes. Chem. Rec. 5, 27–35 (2005).
Lamontagne, H. R. & Lessard, B. H. Nitroxide-mediated polymerization: a versatile tool for the engineering of next generation materials. ACS Appl. Polym. Mater. 2, 5327–5344 (2020).
Tebben, L. & Studer, A. Nitroxides: applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. 50, 5034–5068 (2011).
Hawker, C. J., Bosman, A. W. & Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 101, 3661–3688 (2001).
Hansen, K.-A. & Blinco, J. P. Nitroxide radical polymers – a versatile material class for high-tech applications. Polym. Chem. 9, 1479–1516 (2018).
Ratera, I. & Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 41, 303–349 (2012).
Lewandowski, M. & Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 18, 2490 (2017).
Keana, J. F. W. Newer aspects of the synthesis and chemistry of nitroxide spin labels. Chem. Rev. 78, 37–64 (1978).
Haugland, M. M., Anderson, E. A. & Lovett, J. E. Electron Paramagnetic Resonance Vol. 25 (Royal Society of Chemistry, 2016).
Hubbell, W. L., Cafiso, D. S. & Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 7, 735–739 (2000).
Hyodo, F. et al. Pulsed EPR imaging of nitroxides in mice. J. Magn. Reson. 197, 181–185 (2009).
Kubicki, D. J. et al. Rational design of dinitroxide biradicals for efficient cross-effect dynamic nuclear polarization. Chem. Sci. 7, 550–558 (2016).
Levien, M., Hiller, M., Tkach, I., Bennati, M. & Orlando, T. Nitroxide derivatives for dynamic nuclear polarization in liquids: the role of rotational diffusion. J. Phys. Chem. Lett. 11, 1629–1635 (2020).
Clore, G. M. & Iwahara, J. Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem. Rev. 109, 4108–4139 (2009).
Soule, B. et al. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 42, 1632–1650 (2007).
Shah, S. A. et al. Nitroxide‐enhanced MRI of cardiovascular oxidative stress. NMR Biomed. https://doi.org/10.1002/nbm.4359 (2020).
El Mkami, H. & Norman, D. G. EPR distance measurements in deuterated proteins. Methods Enzymol. 564, 125–152 (2015).
Shelke, S. A. & Sigurdsson, S. T. Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences (eds Timmel, C. R. & Harmer, J. R.), chapters: 2.2, 3.2, 3.3 (Springer, 2011).
Haugland, M. M., Lovett, J. E. & Anderson, E. A. Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling. Chem. Soc. Rev. 47, 668–680 (2018).
Bobko, A. A., Kirilyuk, I. A., Grigor’Ev, I. A., Zweier, J. L. & Khramtsov, V. V. Reversible reduction of nitroxides to hydroxylamines: roles for ascorbate and glutathione. Free Radic. Biol. Med. 42, 404–412 (2007).
Yamasaki, T. et al. Structure−reactivity relationship of piperidine nitroxide: electrochemical, ESR and computational studies. J. Org. Chem. 76, 435–440 (2011).
Jagtap, A. P. et al. Sterically shielded spin labels for in-cell EPR spectroscopy: analysis of stability in reducing environment. Free Radic. Res. 49, 78–85 (2015).
Paletta, J. T., Pink, M., Foley, B., Rajca, S. & Rajca, A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org. Lett. 14, 5322–5325 (2012).
Nakagawa, K., Candelaria, M. B., Chik, W. W. C., Eaton, S. S. & Eaton, G. R. Electron-spin relaxation times of chromium(V). J. Magn. Reson. 98, 81–91 (1992).
Sato, H. et al. Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K. Mol. Phys. 105, 2137–2151 (2007).
Rajca, A. et al. A spirocyclohexyl nitroxide amino acid spin label for pulsed EPR spectroscopy distance measurements. Chem. Eur. J. 16, 5778–5782 (2010).
Zaytseva, E. V. & Mazhukin, D. G. Spirocyclic nitroxides as versatile tools in modern natural sciences: from synthesis to applications. Part I. Old and new synthetic approaches to spirocyclic nitroxyl radicals. Molecules 26, 677 (2021).
Stevanato, G. et al. Open and closed radicals: local geometry around unpaired electrons governs magic-angle spinning dynamic nuclear polarization performance. J. Am. Chem. Soc. 142, 16587–16599 (2020).
Kirilyuk, I. A. et al. Synthesis of 2,5-Bis(spirocyclohexane)-substituted nitroxides of pyrroline and pyrrolidine series, including thiol-specific spin label: an analogue of MTSSL with long relaxation time. J. Org. Chem. 77, 8016–8027 (2012).
Jing, Y., Mardyukov, A., Bergander, K., Daniliuc, C. G. & Studer, A. Synthesis of bulky nitroxides, characterization, and their application in controlled radical polymerization. Macromolecules 47, 3595–3602 (2014).
Jing, Y., Tesch, M., Wang, L., Daniliuc, C. G. & Studer, A. Synthesis of a bulky nitroxide and its application in the nitroxide-mediated radical polymerization. Tetrahedron 72, 7665–7671 (2016).
Sakai, K. et al. Effective 2,6-substitution of piperidine nitroxyl radical by carbonyl compound. Tetrahedron 66, 2311–2315 (2010).
Kiesewetter, M. K., Corzilius, B., Smith, A. A., Griffin, R. G. & Swager, T. M. Dynamic nuclear polarization with a water-soluble rigid biradical. J. Am. Chem. Soc. 134, 4537–4540 (2012).
Malievskii, A. D., Shapiro, A. B., Yakovleva, I. V. & Koroteev, S. V. Quantitative study of nucleophilic addition of secondary amines to 3,5-dimethylene-2,2,6,6-tetramethyl-4-oxopiperidine-1-oxyl. Bull. Acad. Sci. USSR Div. Chem. Sci. 37, 542–548 (1988).
Wang, Y. et al. Synthesis of unnatural amino acids functionalized with sterically shielded pyrroline nitroxides. Org. Lett. 16, 5298–5300 (2014).
Okazaki, S. et al. Enzymatic reduction-resistant nitroxyl spin probes with spirocyclohexyl rings. Free Radic. Res. 41, 1069–1077 (2007).
Zagdoun, A. et al. Large molecular weight nitroxide biradicals providing efficient dynamic nuclear polarization at temperatures up to 200 K. J. Am. Chem. Soc. 135, 12790–12797 (2013).
Zhang, Y., Zhou, F., Li, J., Kang, J. & Zhang, Q. Application and research of new energy-efficiency technology for liquid ring vacuum pump based on turbulent drag reduction theory. Vacuum 172, 109076 (2020).
Ni, Q. Z. et al. Peptide and protein dynamics and low-temperature/DNP magic angle spinning NMR. J. Phys. Chem. B 121, 4997–5006 (2017).
Huber, M. et al. Phase memory relaxation times of spin labels in human carbonic anhydrase II: pulsed EPR to determine spin label location. Biophys. Chem. 94, 245–256 (2001).
Huang, S. et al. Synthesis and electron spin relaxation of tetracarboxylate pyrroline nitroxides. J. Org. Chem. 82, 1538–1544 (2017).
Haugland, M. M. et al. 2′-Alkynylnucleotides: a sequence- and spin label-flexible strategy for EPR spectroscopy in DNA. J. Am. Chem. Soc. 138, 9069–9072 (2016).
Schäfer, A., Horn, H. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 97, 2571–2577 (1992).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 78, 1396–1396 (1997).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297 (2005).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057 (2006).
Scalmani, G. & Frisch, M. J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 132, 114110 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Frisch, M. J. et al. Gaussian, Inc., (2016).
Fitjer, L., Scheuermann, H.-J. & Wehle, D. Permethylcyclohexane. Tetrahedron Lett. 25, 2329–2332 (1984).
Allinger, N. L., Blatter, H. M., Freiberg, L. A. & Karkowski, F. M. Conformational analysis. LI. The conformations of cyclohexanone rings in simple molecules1-3. J. Am. Chem. Soc. 88, 2999–3011 (1966).
St-Jacques, M. & Bernard, M. The barrier to the conformational interconversion of 1,4-dimethylenecyclohexane and related compounds. Can. J. Chem. 47, 2911–2913 (1969).
Sowiński, M. P. et al. Conformational tuning improves the stability of spirocyclic nitroxides with long paramagnetic relaxation times. https://doi.org/10.18710/UQMMZE (2023).
Acknowledgements
The authors thank Dr. D. G. Mazhukin for helpful suggestions for the preprint version of this work. M.M.H. and A.-L.W. thank the Tromsø Research Foundation and UiT Centre for New Antibacterial Strategies (CANS) for a start-up grant (TFS project ID: 18_CANS). K.H.H. and S.G. thank the Research Council of Norway (No. 300769) and Sigma2 (No. nn9330k and nn4654k), and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 859910. J.E.L. thanks Drs Hassane El Mkami and Robert I. Hunter for technical assistance, the BBSRC (BB/T017740/1) and the Wellcome Trust (099149/Z/12/Z) for the Q-band EPR spectrometer, the Royal Society for a University Research Fellowship and Research Grant RG120645 for the benchtop spectrometer. B.A.L. thanks The Research Council of Norway for the Centre of Excellence and project grants (Grant Nos. 262695 and 274858).
Funding
Open access funding provided by UiT The Arctic University of Norway (incl University Hospital of North Norway).
Author information
Authors and Affiliations
Contributions
In descending order of degree of contribution. Conceptualization: M.M.H.; funding acquisition: M.M.H., J.E.L., and K.H.H.; methodology: M.M.H., J.E.L., K.H.H., S.G., and M.P.S.; investigation: M.P.S., S.G., A.-L.W., B.A.L., and J.E.L.; formal analysis: M.P.S., S.G., B.A.L., A.-L.W., M.M.H., J.E.L., K.H.H.; software: S.G., K.H.H., and J.E.L.; validation: M.P.S., S.G., B.A.L., M.M.H., K.H.H., and J.E.L.; data curation: M.P.S., S.G., and B.A.L.; visualization: M.P.S., M.M.H., and S.G.; writing—original draft: M.P.S., M.M.H., and S.G.; writing—review & editing: K.H.H., A.-L.W., J.E.L., and B.A.L.; project administration: M.M.H.; resources: M.M.H., J.E.L., and K.H.H.; supervision: M.M.H., K.H.H., and J.E.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sowiński, M.P., Gahlawat, S., Lund, B.A. et al. Conformational tuning improves the stability of spirocyclic nitroxides with long paramagnetic relaxation times. Commun Chem 6, 111 (2023). https://doi.org/10.1038/s42004-023-00912-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00912-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.