Abstract
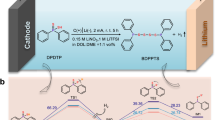
The synthesis of N-containing chemicals directly from N2 is highly desirable in chemistry but has been challenged by inert N2 molecules and limited product scope. Herein we propose a cascade electrosynthesis strategy for the facile reduction of N2 and subsequent conversion to various N-containing chemicals via a looped Li–N2 battery. The electrosynthesis of lithium bis(trifluoromethanesulfonyl)imide is illustrated as a prototype using cascade reactions involving electrocatalytic N2 reduction to Li3N on discharge, a relay reaction of Li3N acylation to lithium bis(trifluoromethanesulfonyl)imide and finally the elimination of the LiCl by-product on charge to complete the synthesis loop. This strategy provides direct access to analogues with different N–X bonds (X = S, C and so on) and metal cations (Li+, Zn2+ and so on) by extending the substrate scope, and can be scaled up for improved energy efficiency and atom economy. This work provides a general electrosynthesis protocol for the practical production of N-containing chemicals and has potential implications for a wider field of sustainable chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the article and its Supplementary Information. Data are also available from the corresponding author upon request. Source data are provided with this paper.
References
Foster, S. L. et al. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1, 490–500 (2018).
Deng, J., Iñiguez, J. A. & Liu, C. Electrocatalytic nitrogen reduction at low temperature. Joule 2, 846–856 (2018).
Chen, G. F. et al. Saving the energy loss in lithium-mediated nitrogen fixation by using a highly reactive Li3N intermediate for C−N coupling reactions. Angew. Chem. Int. Ed. 134, e202203170 (2022).
Yang, J.-H. et al. Fixation of N2 into value-added organic chemicals. ACS Catal. 12, 2898–2906 (2022).
Chen, J. G. et al. Beyond fossil fuel–driven nitrogen transformations. Science 360, eaar6611 (2018).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
Lv, Z. J. et al. Direct transformation of dinitrogen: synthesis of N-containing organic compounds via N−C bond formation. Natl Sci. Rev. 7, 1564–1583 (2020).
Bezdek, M. J. & Chirik, P. J. Expanding boundaries: N2 cleavage and functionalization beyond early transition metals. Angew. Chem. Int. Ed. 55, 7892–7896 (2016).
Li, L., Tang, C., Jin, H., Davey, K. & Qiao, S.-Z. Main-group elements boost electrochemical nitrogen fixation. Chem 7, 3232–3255 (2021).
Kim, S., Loose, F. & Chirik, P. J. Beyond ammonia: nitrogen-element bond forming reactions with coordinated dinitrogen. Chem. Rev. 120, 5637–5681 (2020).
Iriawan, H. et al. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Primers 1, 56 (2021).
Qing, G. et al. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 120, 5437–5516 (2020).
Fu, X. et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023).
Du, H. L. et al. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 609, 722–727 (2022).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Zhang, X. et al. Identifying and tailoring C–N coupling site for efficient urea synthesis over diatomic Fe–Ni catalyst. Nat. Commun. 13, 5337 (2022).
Maurel, A. et al. Poly(ethylene oxide)–LiTFSI solid polymer electrolyte filaments for fused deposition modeling three-dimensional printing. J. Electrochem. Soc. 167, 070536 (2020).
Conte, L., Gambaretto, G., Caporiccio, G., Alessandrini, F. & Passerini, S. Perfluoroalkanesulfonylimides and their lithium salts: synthesis and characterisation of intermediates and target compounds. J. Fluor. Chem. 125, 243–252 (2004).
Ma, J., Bao, D., Shi, M., Yan, J. & Zhang, X. Reversible nitrogen fixation based on a rechargeable lithium-nitrogen battery for energy storage. Chem 2, 525–532 (2017).
Zhang, Z. et al. Li-N2 batteries: a reversible energy storage system? Angew. Chem. Int. Ed. 58, 17782–17787 (2019).
Meng, F., Xiong, X., He, S., Liu, Y. & Hu, R. Post nitrogen electrocatalysis era from Li–N2 batteries to Zn–N2 batteries. Adv. Energy Mater. 13, 2300269 (2023).
Feng, Y., Yan, S., Zhang, X. & Wang, Y. Development, essence, and application of a metal-catalysis battery. Acc. Chem. Res. 56, 1645–1655 (2023).
Li, J. et al. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 12, 3502 (2021).
Liu, Y. et al. Unveiling the protonation kinetics-dependent selectivity in nitrogen electroreduction: achieving 75.05% selectivity. Angew. Chem. Int. Ed. 61, e202209555 (2022).
Heligman, B. T. & Manthiram, A. Elemental foil anodes for lithium-ion batteries. ACS Energy Lett. 6, 2666–2672 (2021).
Westhead, O. et al. Near ambient N2 fixation on solid electrodes versus enzymes and homogeneous catalysts. Nat. Rev. Chem. 7, 184–201 (2023).
Steinberg, K. et al. Imaging of nitrogen fixation at lithium solid electrolyte interphases via cryo-electron microscopy. Nat. Energy 8, 138–148 (2023).
Tsuneto, A., Kudo, A. & Sakata, T. Efficient electrochemical reduction of N2 to NH3 catalyzed by lithium. Chem. Lett. 22, 851–854 (1993).
Tsuneto, A., Kudo, A. & Sakata, T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 367, 183–188 (1994).
Lazouski, N., Schiffer, Z. J., Williams, K. & Manthiram, K. Understanding continuous lithium-mediated electrochemical nitrogen reduction. Joule 3, 1127–1139 (2019).
Andersen, S. Z. et al. Increasing stability, efficiency, and fundamental understanding of lithium-mediated electrochemical nitrogen reduction. Energy Environ. Sci. 13, 4291–4300 (2020).
Feng, Y. et al. Production of high-energy 6-Ah-level Li || LiNi0.83Co0.11Mn0.06O2 multi-layer pouch cells via negative electrode protective layer coating strategy. Nat. Commun. 14, 3639 (2023).
Li, S. et al. Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid-electrolyte interphase. Joule 6, 2083–2101 (2022).
Braun, A., Wang, H., Shim, J., Lee, S. S. & Cairns, E. J. Lithium K(1s) synchrotron NEXAFS spectra of lithium-ion battery cathode, anode and electrolyte materials. J. Power Sources 170, 173–178 (2007).
Cheng, D. et al. Unveiling the stable nature of the solid electrolyte interphase between lithium metal and LiPON via cryogenic electron microscopy. Joule 4, 2484–2500 (2020).
Zhang, X. et al. Photoelectrochemical N2-to-NH3 fixation with high efficiency and rates via optimized Si-based system at positive potential versus Li0/+. Adv. Mater. 35, 2211894 (2023).
Cui, C. et al. Unlocking the in situ Li plating dynamics and evolution mediated by diverse metallic substrates in all-solid-state batteries. Sci. Adv. 8, eadd2000 (2022).
Du, H.-L. et al. The chemistry of proton carriers in high-performance lithium mediated ammonia electrosynthesis. Energy Environ. Sci. 16, 1082–1090 (2023).
Yi, S., Liu, G., Liu, Z., Hu, W. & Deng, H. Theoretical insights into nitrogen fixation on Ti2C and Ti2CO2 in a lithium–nitrogen battery. J. Mater. Chem. A 7, 19950–19960 (2019).
Cui, Y. et al. Improved performance using a plasticized polymer electrolyte for quasi-solid state dye-sensitized solar cells. Electrochim. Acta 74, 194–200 (2012).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Lazouski, N., Chung, M., Williams, K., Gala, M. L. & Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 3, 463–469 (2020).
Li, K. et al. Increasing current density of Li-mediated ammonia synthesis with high surface area copper electrodes. ACS Energy Lett. 7, 36–41 (2021).
Chen, Y. et al. An AB alternating diblock single ion conducting polymer electrolyte membrane for all-solid-state lithium metal secondary batteries. J. Membr. Sci. 566, 181–189 (2018).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994).
Mao, C. et al. Hydrogen spillover to oxygen vacancy of TiO2–xHy/Fe: breaking the scaling relationship of ammonia synthesis. J. Am. Chem. Soc. 142, 17403–17412 (2020).
Hammer, B., Morikawa, Y. & Norskov, J. K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 76, 2141–2144 (1996).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Acknowledgements
We acknowledge support from the National Natural Science Foundation of China (grant nos 22022110 (Y.W.), 22279141 (Y.W.), 22205238 (X.Z.) and 22209182 (Y.F.)), the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (no. ZDBS-LY-SLH028 (Y.W.)) and the National Key Research & Development Program of China (no. 2021YFA1501500 (Y.W.)). We gratefully thank, for the financial support, the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (nos 2021ZZ106 (Y.W.) and 2021ZR123 (J.L.)), Fujian Provincial Science and Technology Service Network Initiative programme supporting project of the Chinese Academy of Sciences (no. 2022T3001 (M.W.)) and the Nature Science Foundation of Fujian Province (nos 2023I0033 (X.Z.) and 2022J05094 (J.L.)). This work was also supported by Fujian Shaowu Chuangxin New Materials Co., Ltd. (no. HX-JS-2023-007 (Y.W.)).
Author information
Authors and Affiliations
Contributions
Y.W. conceived the idea and supervised the project. X.Z. designed the experiments. W.X., T.W., E.C. and X.Z. carried out the electrochemical measurements and characterization. L.H. assisted with the experiments. J.L. performed the computational calculation. X.Z. wrote the paper. Y.W., Y.F. and M.W. revised the paper, with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36, Tables 1 and 2, Notes 1 and 2 and references.
Supplementary Data 1
Atomic coordinates of optimized models.
Source data
Source Data Fig. 2
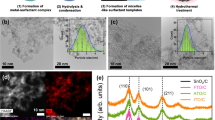
Electrocatalytic N2 reduction to Li3N.
Source Data Fig. 3
S–N acylation reaction.
Source Data Fig. 4
Cascade electrosynthesis and performance metrics.
Source Data Fig. 5
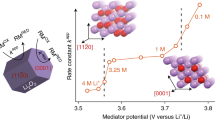
Extensibility verification.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Xiong, W., Wang, T. et al. Cascade electrosynthesis of LiTFSI and N-containing analogues via a looped Li–N2 battery. Nat Catal 7, 55–64 (2024). https://doi.org/10.1038/s41929-023-01067-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01067-3