Abstract
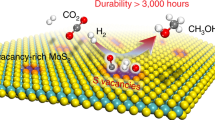
Conversion of methane to value-added chemicals at low temperature by directly using inexpensive O2 as oxidant offers an ideal route for methane utilization but remains a great challenge due to the chemical inertness of methane and the low activity of O2. Methane monooxygenase is the only known natural catalyst that can convert methane with O2 at room temperature. Here we report the realization of an artificial process for the direct methane conversion to C1 oxygenates with O2 on an edge-rich MoS2 catalyst at 25 °C, which delivers a remarkable methane conversion of 4.2% with >99% selectivity for C1 oxygenates. In situ spectroscopic and microscopic characterizations and theoretical calculations reveal that the binuclear molybdenum sites of sulfur vacancies at the MoS2 edge can directly dissociate O2 to form O=Mo=O* active species, which can activate the C–H bond and enable methane conversion at room temperature.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting this work are available in the article and its Supplementary Information or are available from the corresponding authors upon request. Source data are provided with this paper.
References
Guo, X. et al. Direct, non-oxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Wang, P., Zhao, G., Wang, Y. & Lu, Y. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3–Na2WO4/SiO2 catalyst. Sci. Adv. 3, e1603180 (2017).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Choudhary, V. R., Kinage, A. K. & Choudhary, T. V. Low-temperature non-oxidative activation of methane over H-galloaluminosilicate (MFI) zeolite. Science 275, 1286–1288 (1997).
Upham, D. C. et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 358, 917–920 (2017).
Morejudo, S. H. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Gao, J. et al. Identification of molybdenum oxide nanostructures on zeolites for natural gas conversion. Science 348, 686–690 (2015).
Song, Y. et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 367, 777–781 (2020).
Jones, C. et al. Selective oxidation of methane to methanol catalyzed, with C–H activation, by homogeneous, cationic gold. Angew. Chem. Int. Ed. 43, 4626–4629 (2004).
Periana, R. A., Mironov, O., Taube, D., Bhalla, G. & Jones, C. J. Catalytic, oxidative condensation of CH4 to CH3COOH in one step via CH activation. Science 301, 814–818 (2003).
Lin, M. & Sen, A. Direct catalytic conversion of methane to acetic acid in an aqueous medium. Nature 368, 613–615 (1994).
Periana, R. A. et al. Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science 280, 560–564 (1998).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Periana, R. A. et al. A mercury-catalyzed, high-yield system for the oxidation of methane to methanol. Science 259, 340–343 (1993).
Sen, A. Catalytic functionalization of carbon–hydrogen and carbon–carbon bonds in protic media. Acc. Chem. Res. 31, 550–557 (1998).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
An, Z., Pan, X., Liu, X., Han, X. & Bao, X. Combined redox couples for catalytic oxidation of methane by dioxygen at low temperatures. J. Am. Chem. Soc. 128, 16028–16029 (2006).
Paunovic, V., Zichittella, G., Moser, M., Amrute, A. P. & Perez-Ramirez, J. Catalyst design for natural-gas upgrading through oxybromination chemistry. Nat. Chem. 8, 803–809 (2016).
Hammond, C. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 51, 5129–5133 (2012).
Cui, X. et al. Room-temperature methane conversion by graphene-confined single iron atoms. Chem 4, 1902–1910 (2018).
Agarwal, N. et al. Aqueous Au–Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223–226 (2017).
Shen, Q. et al. Single chromium atoms supported on titanium dioxide nanoparticles for synergic catalytic methane conversion under mild conditions. Angew. Chem. Int. Ed. 59, 1216–1219 (2020).
Kwon, Y., Kim, T. Y., Kwon, G., Yi, J. & Lee, H. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 139, 17694–17699 (2017).
Balint, I., Miyazaki, A. & Aika, K. Methane reaction with NO over alumina-supported Ru nanoparticles. J. Catal. 207, 66–75 (2002).
Snyder, B. E. R. et al. The active site of low-temperature methane hydroxylation in iron-containing zeolites. Nature 536, 317–321 (2016).
Qi, G. et al. Au-ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2. Nat. Catal. 5, 45–54 (2022).
Liang, Z., Li, T., Kim, M., Asthagiri, A. & Weaver, J. F. Low-temperature activation of methane on the IrO2(110) surface. Science 356, 298–301 (2017).
Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–606 (2017).
Hutchings, G. J., Scurrell, M. S. & Woodhouse, J. R. Oxidative coupling of methane using oxide catalysts. Chem. Soc. Rev. 18, 251–283 (1989).
Grundner, S. et al. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat. Commun. 6, 7546 (2015).
Senanayake, S. D., Rodriguez, J. A. & Weaver, J. F. Low-temperature activation of methane on metal oxides and complex interfaces: insights from surface science. Acc. Chem. Res. 53, 1488–1497 (2020).
Latimer, A. A. et al. Understanding trends in C–H bond activation in heterogeneous catalysis. Nat. Mater. 16, 225–229 (2017).
Liu, Z. et al. Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2–Cu2O catalyst. Science 368, 513–517 (2020).
Ravi, M. et al. Misconceptions and challenges in methane-to-methanol over transition-metal-exchanged zeolites. Nat. Catal. 2, 485–494 (2019).
Tomkins, P., Ranocchiari, M. & van Bokhoven, J. A. Direct conversion of methane to methanol under mild conditions over Cu-zeolites and beyond. Acc. Chem. Res. 50, 418–425 (2017).
Narsimhan, K., Iyoki, K., Dinh, K. & Román-Leshkov, Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2, 424–429 (2016).
Yang, L. et al. Metal–organic framework-derived IrO2/CuO catalyst for selective oxidation of methane to methanol. ACS Energy Lett. 4, 2945–2951 (2019).
Dinh, K. T. et al. Continuous partial oxidation of methane to methanol catalyzed by diffusion-paired copper dimers in copper-exchanged zeolites. J. Am. Chem. Soc. 141, 11641–11650 (2019).
Groothaert, M. H., Smeets, P. J., Sels, B. F., Jacobs, P. A. & Schoonheydt, R. A. Selective oxidation of methane by the bis(μ-oxo) dicopper core stabilized on ZSM-5 and mordenite zeolites. J. Am. Chem. Soc. 127, 1394–1395 (2005).
Woertink, J. S. et al. A [Cu2O]2+ core in Cu-ZSM-5, the active site in the oxidation of methane to methanol. Proc. Natl Acad. Sci. USA 106, 18908–18913 (2009).
Alayon, E. M., Nachtegaal, M., Ranocchiari, M. & van Bokhoven, J. A. Catalytic conversion of methane to methanol over Cu-mordenite. Chem. Commun. 48, 404–406 (2012).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Tomkins, P. et al. Isothermal cyclic conversion of methane into methanol over copper-exchanged zeolite at low temperature. Angew. Chem. Int. Ed. 55, 5467–5471 (2016).
Lieberman, R. L. & Rosenzweig, A. C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434, 177–182 (2005).
Kim, H. J. et al. Biological conversion of methane to methanol through genetic reassembly of native catalytic domains. Nat. Catal. 2, 342–353 (2019).
Banerjee, R. & Lipscomb, J. D. Small-molecule tunnels in metalloenzymes viewed as extensions of the active site. Acc. Chem. Res. 54, 2185–2195 (2021).
Vrubel, H., Merki, D. & Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 5, 6136–6144 (2012).
Gao, M.-R. et al. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 6, 5982 (2015).
Hu, J. et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 4, 242–250 (2021).
Deng, J. et al. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nat. Commun. 8, 14430 (2017).
Weber, T., Muijsers, J. C., van Wolput, H., Verhagen, C. P. J. & Niemantsverdriet, J. W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2 as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem 100, 14144–14150 (1996).
Polyakov, M. et al. Hydrocarbon reactions on MoS2 revisited, I: activation of MoS2 and interaction with hydrogen studied by transient kinetic experiments. J. Catal. 256, 126–136 (2008).
Hong, Z. & Regalbuto, J. R. Nature of adsorption sites on sulfided Mo catalysts and their selectivity in chemisorption of probe molecules. J. Phys. Chem. 99, 9452–9457 (1995).
Chiu, N. S., Bauer, S. H. & Johnson, M. F. L. Co/Mo/Al2O3 catalyst structure determination by EXAFS. I. Mo K edge in the oxidized state. J. Catal. 89, 226–243 (1984).
Gaur, A. et al. Probing the active sites of MoS2-based hydrotreating catalysts using modulation excitation spectroscopy. ACS Catal. 9, 2568–2579 (2019).
Santos, V. P. et al. Mechanistic insight into the synthesis of higher alcohols from syngas: the role of K promotion on MoS2 catalysts. ACS Catal. 3, 1634–1637 (2013).
Koizumi, N., Bian, G., Murai, K., Ozaki, T. & Yamada, M. In situ DRIFT studies of sulfided K–Mo/γ-Al2O3 catalysts. J. Mol. Catal. 207, 173–182 (2004).
Grabow, L. C. & Mavrikakis, M. Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal. 1, 365–384 (2011).
Deng, J. et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 8, 1594–1601 (2015).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion-corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Wilson, J. A. & Yoffe, A. D. Transition metal dichalcogenides: discussion and interpretation of observed optical, electrical and structual properties. Adv. Phys. 18, 193–335 (1969).
Raybaud, P. et al. Ab initio study of the H2–H2S/MoS2 gas–solid interface: the nature of the catalytically active sites. J. Catal. 189, 129–146 (2000).
Tsai, C., Abild-Pedersen, F. & Norskov, J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Hjorth Larsen, A. et al. The Atomic Simulation Environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Acknowledgements
This work received financial support from the National Key R&D Program of China (2022YFA1504500 to D.D. and 2022YFA1503100 to X.C.), the National Natural Science Foundation of China (21988101, 21890753, 22225204 to D.D., 92145301 to L.Y. and 22272174 to X.C.), the Strategic Priority Research Program of the Chinese Academy of Science (XDB36030200 to D.D.), the Fundamental Research Funds for the Central Universities (20720220008 to D.D.) and the CAS Project for Young Scientists in Basic Research (YSBR-028 to X.C.). We thank the staff at XAFS beamline (BL14W1) and D-Line (BL05U) of the Shanghai Synchrotron Radiation Facilities for assistance with the EXAFS, XANES and ED-XAS measurements.
Author information
Authors and Affiliations
Contributions
D.D. conceived and designed the experiments. J.M. undertook the materials synthesis, characterization and performance testing. H.L., X.M. and L.Y. contributed to the DFT calculations. Y. Zhang performed the HAADF-STEM. Y. Zheng and M.C. assisted with the XPS. Y.P. assisted with the in situ synchrotron-based vacuum ultraviolet photoionization mass spectrometry test. X.C., Z.Z. and G.H. assisted with the NMR. J.H., Y.L., G.X. and R.H. assisted with data analysis and paper revision. J.M., H.L., X.C., L.Y. and D.D. co-wrote the paper. All the authors discussed and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Salvador Ordóñez, David Willock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figs. 1–27, Tables 1–4 and References.
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Supplementary Data 2
Statistical source data for supplementary figures.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source sata.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, J., Liu, H., Cui, X. et al. Direct conversion of methane with O2 at room temperature over edge-rich MoS2. Nat Catal 6, 1052–1061 (2023). https://doi.org/10.1038/s41929-023-01030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01030-2