Abstract
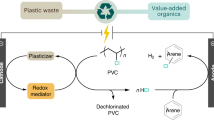
Catalytic upcycling of plastic wastes to valuable chemicals offers the opportunity to simultaneously address the enormous environmental problems associated with plastics and achieve the circular economy. However, the upcycling of plastic wastes containing polyvinyl chloride (PVC) is particularly challenging due to the interference of chlorine, which can be released during PVC depolymerization and deactivate the catalyst. Here we present a catalytic process for the co-upcycling of PVC and polyethylene terephthalate (PET). By using a chlorine-containing ionic liquid as the catalyst/solvent and ZnCl2 as Lewis acid catalyst, with in situ utilization of PVC-released chlorine, we successfully converted PET into terephthalic acid and 1,2-dichloroethane with high yields. The results reveal that chlorine from PVC, previously considered detrimental to the transformation of other polymers and the cause of catalyst poisoning, can actually have a positive role in the upcycling of plastic wastes. This work can incentivize further progress in plastics upcycling and pave the way to sustainable plastic wastes management, moving societies one step forward towards realizing the circular economy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Geyer, R. in Plastic Waste and Recycling (ed Trevor M. Letcher) Ch. 2 (Academic Press, 2020).
Li, H. et al. Expanding plastics recycling technologies: chemical aspects, technology status and challenges. Green Chem. 24, 8899–9002 (2022).
Martín, A. J., Mondelli, C., Jaydev, S. D. & Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 7, 1487–1533 (2021).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Roy, P. S., Garnier, G., Allais, F. & Saito, K. Strategic approach towards plastic waste valorization: challenges and promising chemical upcycling possibilities. ChemSusChem 14, 4007–4027 (2021).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Lee, K., Jing, Y., Wang, Y. & Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 6, 635–652 (2022).
Hu, H. et al. Recycling and upgrading utilization of polymer plastics. Chin. J. Chem. 41, 469–480 (2023).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Conk, R. J. et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561–1566 (2022).
Zhang, W. et al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 379, 807–811 (2023).
Wang, N. M. et al. Chemical recycling of polyethylene by tandem catalytic conversion to propylene. J. Am. Chem. Soc. 144, 18526–18531 (2022).
Kots, P. A., Vance, B. C. & Vlachos, D. G. Polyolefin plastic waste hydroconversion to fuels, lubricants, and waxes: a comparative study. React. Chem. Eng. 7, 41–54 (2022).
Yeung, C. W. S., Teo, J. Y. Q., Loh, X. J. & Lim, J. Y. C. Polyolefins and polystyrene as chemical resources for a sustainable future: challenges, advances, and prospects. ACS Mater. Lett. 3, 1660–1676 (2021).
Huang, Z. et al. Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J. Am. Chem. Soc. 144, 6532–6542 (2022).
Oh, S. & Stache, E. E. Chemical upcycling of commercial polystyrene via catalyst-controlled photooxidation. J. Am. Chem. Soc. 144, 5745–5749 (2022).
Cao, R. et al. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. 13, 4809 (2022).
Xu, Z. et al. Cascade degradation and upcycling of polystyrene waste to high-value chemicals. Proc. Natl Acad. Sci. USA 119, e2203346119 (2022).
Payne, J. & Jones, M. D. The chemical recycling of polyesters for a circular plastics economy: challenges and emerging opportunities. ChemSusChem 14, 4041–4070 (2021).
Tian, S. et al. Catalytic amination of polylactic ccid to alanine. J. Am. Chem. Soc. 143, 16358–16363 (2021).
Sun, B. et al. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustain https://doi.org/10.1038/s41893-023-01082-z (2023).
Kratish, Y. & Marks, T. J. Efficient polyester hydrogenolytic deconstruction via tandem catalysis. Angew. Chem. Int. Ed. 61, e202112576 (2021).
Li, Y. et al. Catalytic transformation of PET and CO2 into high‐value chemicals. Angew. Chem. Int. Ed. 61, e202117205 (2022).
Lu, S. et al. H2‐free plastic conversion: converting PET back to BTX by unlocking hidden hydrogen. ChemSusChem 14, 4242–4250 (2021).
Hofmann, M., Sundermeier, J., Alberti, C. & Enthaler, S. Zinc(II) acetate catalyzed depolymerization of poly(ethylene terephthalate). ChemistrySelect 5, 10010–10014 (2020).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016).
Tournier, V. et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219 (2020).
Kim, D. H. et al. One-pot chemo-bioprocess of PET depolymerization and recycling enabled by a biocompatible catalyst, betaine. ACS Catal. 11, 3996–4008 (2021).
Jiao, Y., Wang, M. & Ma, D. Catalytic cracking of polylactic acid to acrylic acid. Chin. J. Chem. 41, 2071–2076 (2023).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 378, 207–211 (2022).
Jing, Y. et al. Towards the circular economy: converting aromatic plastic waste back to arenes over a Ru/Nb2O5 catalyst. Angew. Chem. Int. Ed. 60, 5527–5535 (2021).
Miskolczi, N., Bartha, L. & Angyal, A. Pyrolysis of polyvinyl chloride (PVC)-containing mixed plastic wastes for recovery of hydrocarbons. Energy Fuels 23, 2743–2749 (2009).
Paci, M. & La Mantia, F. P. Influence of small amounts of polyvinylchloride on the recycling of polyethyleneterephthalate. Polym. Degrad. Stab. 63, 11–14 (1999).
Bhaskar, T., Negoro, R., Muto, A. & Sakata, Y. Prevention of chlorinated hydrocarbons formation during pyrolysis of PVC or PVDC mixed plastics. Green Chem. 8, 697–700 (2006).
Yu, J., Sun, L., Ma, C., Qiao, Y. & Yao, H. Thermal degradation of PVC: a review. Waste Manage. 48, 300–314 (2016).
Meng, T.-T., Zhang, H., Lü, F., Shao, L.-M. & He, P.-J. Comparing the effects of different metal oxides on low temperature decomposition of PVC. J. Anal. Appl. Pyrolysis 159, 105312 (2021).
Ling, M. et al. Hydrothermal treatment of polyvinyl chloride: reactors, dechlorination chemistry, application, and challenges. Chemosphere 316, 137718 (2023).
Lin, R., Amrute, A. P. & Pérez-Ramírez, J. Halogen-mediated conversion of hydrocarbons to commodities. Chem. Rev. 117, 4182–4247 (2017).
Fagnani, D. E., Kim, D., Camarero, S. I., Alfaro, J. F. & McNeil, A. J. Using waste poly(vinyl chloride) to synthesize chloroarenes by plasticizer-mediated electro(de)chlorination. Nat. Chem. 15, 222–229 (2022).
Feng, B., Jing, Y., Liu, X., Guo, Y. & Wang, Y. Waste PVC upcycling: transferring unmanageable Cl species into value-added Cl-containing chemicals. Appl. Catal. B 331, 122671 (2023).
Zhang, Y., Jiang, H., Wang, K., Wang, H. & Wang, C. Green flotation of polyethylene terephthalate and polyvinyl chloride assisted by surface modification of selective CaCO3 coating. J. Clean. Prod. 242, 118441 (2020).
Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 23, 2399–2407 (2001).
Xiao, Q. et al. Tuning the basicity for highly efficient and reversible hydrogen chloride absorption to develop a green acid scavenger. ACS Sustain. Chem. Eng. 10, 2289–2293 (2022).
Glas, D., Hulsbosch, J., Dubois, P., Binnemans, K. & De Vos, D. E. End-of-life treatment of poly(vinyl chloride) and chlorinated polyethylene by dehydrochlorination in ionic liquids. ChemSusChem 7, 610–617 (2014).
Lorenzetti, A., Choi, S. Y., Roso, M., Modesti, M. & McNally, T. Effect of dual functional ionic liquids on the thermal degradation of poly(vinyl chloride). Polym. Degrad. Stabil. 129, 12–18 (2016).
Oster, K. et al. Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids. Green. Chem. 22, 5132–5142 (2020).
Dong, N., Hui, H., Li, S. & Du, L. Study on preparation of aromatic-rich oil by thermal dechlorination and fast pyrolysis of PVC. J. Anal. Appl. Pyrolysis 169, 105817 (2023).
Chang, Y. et al. Converting polyvinyl chloride plastic wastes to carbonaceous materials via room-temperature dehalogenation for high-performance supercapacitor. ACS Appl. Energy Mater. 1, 5685–5693 (2018).
Wang, J. et al. Polyvinyl chloride-derived carbon spheres for CO2 adsorption. ChemSusChem 13, 6426–6432 (2020).
O’Rourke, G. et al. Catalytic tandem dehydrochlorination-hydrogenation of PVC towards valorisation of chlorinated plastic waste. Chem. Sci. 14, 4401–4412 (2023).
Acknowledgements
This work received financial support from the Natural Science Foundation of China (22072002, 21725301, 22232001, 21932002, 21821004), China National Petroleum Corporation–Peking University Strategic Cooperation Project of Fundamental Research, National Key R&D Program of China (2022YFA1504800, 2021YFA1501102) and the New Cornerstone Science Foundation. D.M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
D.M. and M.W. conceived the project. R.C., M.-Q.Z, Y.J. and Y.L. performed most of the reactions. R.C., M.-Q.Z, Y.J., B.S., D.X., M.W. and D.M. analysed the data and wrote the paper. All authors contributed to the discussion and revision of the paper. We appreciate the help of Analytical Instrumentation Center of Peking University.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Bert M. Weckhuysen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1–7.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, R., Zhang, MQ., Jiao, Y. et al. Co-upcycling of polyvinyl chloride and polyesters. Nat Sustain 6, 1685–1692 (2023). https://doi.org/10.1038/s41893-023-01234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01234-1
This article is cited by
-
A Unified View of Carbon Neutrality: Solar-Driven Selective Upcycling of Waste Plastics
Transactions of Tianjin University (2024)