Abstract
The evidence of the impact of traditional statistical (TS) and artificial intelligence (AI) tool interventions in clinical practice was limited. This study aimed to investigate the clinical impact and quality of randomized controlled trials (RCTs) involving interventions evaluating TS, machine learning (ML), and deep learning (DL) prediction tools. A systematic review on PubMed was conducted to identify RCTs involving TS/ML/DL tool interventions in the past decade. A total of 65 RCTs from 26,082 records were included. A majority of them had model development studies and generally good performance was achieved. The function of TS and ML tools in the RCTs mainly included assistive treatment decisions, assistive diagnosis, and risk stratification, but DL trials were only conducted for assistive diagnosis. Nearly two-fifths of the trial interventions showed no clinical benefit compared to standard care. Though DL and ML interventions achieved higher rates of positive results than TS in the RCTs, in trials with low risk of bias (17/65) the advantage of DL to TS was reduced while the advantage of ML to TS disappeared. The current applications of DL were not yet fully spread performed in medicine. It is predictable that DL will integrate more complex clinical problems than ML and TS tools in the future. Therefore, rigorous studies are required before the clinical application of these tools.
Similar content being viewed by others
Introduction
An abundance of prediction tools in medicine has been developed and validated to support health decision-making. Prediction tools usually use several predictors to estimate the probability of individuals’ present disease or predict specific situations or events in the future1,2,3. Conventionally, prediction tools are constructed by statistical regression models based on structured patients’ clinical data4,5. The recent development of computer technology facilitates the application of machine learning (ML) and even deep learning (DL) algorithms which is a subset of ML in the establishment of prediction tools6,7. In contrast to conventional prediction tools, ML- or DL-based prediction tools which are both subsets of artificial intelligence (AI) technology usually use data with high-dimensional features, medical images, or even videos to develop models8,9,10,11,12,13. Many observational studies of model development and validation showed that ML prediction tools performed better than traditional statistical (TS) models in the prediction of disease diagnosis and prognosis by showing higher values of area under the receiver operating characteristic curve (AUC) or accuracy14,15,16,17. Others found that DL models outperformed standard ML18,19. Some of the DL prediction tools have achieved expertise level of diagnostic accuracy in several aspects of diseases20,21. Many reports claimed that a well-developed AI prediction tool with adequate performance could assist or even replace clinicians in treatment strategy making for patients' care11.
However, some observational studies found that ML prediction algorithms did not outperform TS models for binary outcomes22,23,24, while DL did not always perform better than ML in model development and/or validation studies25. More importantly, the clinical effectiveness of these prediction tools based on both traditional and advanced technology for clinical application remains controversial2,11,22,26,27. Randomized controlled trials (RCTs) are considered to be the gold standard to establish whether using a prediction tool provides an improvement in the management of patients compared to not using the tool26,28,29,30,31. This kind of design played an important role in providing high-quality evidence in evidence-based medicine in the past decades32. In prediction model research, more and more RCTs involving interventions evaluating TS, ML, and DL tools were published to evaluate the efficacy of a prediction model compared to clinical standard care. The primary outcomes of these RCTs evaluating prediction tools were not patient outcomes which were often difficult to change but other outcomes such as decision-making33,34, behavior change35, cost-effectiveness36 etc. Some of these studies showed that prediction models did not show good clinical benefit in clinical application level37. Previous studies have reviewed RCTs evaluating AI interventions in digital health and medical decision support systems, suggesting that the evidence of the effectiveness of AI interventions is limited and contradictory, and their quality is variable11,38,39. However, the included studies in these reviews were of small number and in specific fields, and little quantitative analysis was made.
With the increasing number of RCTs evaluating the clinical effectiveness of AI tools recently, concerns about study design and reporting have been raised as well. The Consolidated Standards of Reporting Trials (CONSORT) statement is a 37-item checklist for reporting randomized trials and is widely used in medical research40. With the growing recognition of rigorous evaluation for reporting AI trials, the CONSORT statement was planned to adapt to account for specific considerations for AI interventional studies41,42. The CONSORT-AI extension has been published recently, which is a 37-item checklist for reporting randomized trials evaluating AI interventions but included 14 new items that are specific for AI interventional trials42. Therefore, understanding the clinical effectiveness and quality of these RCTs can provide a reference for more such studies in the future, and vice versa, it may inform future research in model development and application in the early phases of model construction as well.
In this review, we aimed to conduct research of published literature of RCTs involving interventions of traditional statistical or artificial intelligence (TS/AI) prediction tools. First, the quality of these RCTs was evaluated through the Cochrane risk-of-bias tool43. Second, the clinical effectiveness of these prediction tools was evaluated according to the main findings of the trial and compared with its previous observational studies of model development and validation.
Methods
Study design
This study was a cross-sectional survey on RCTs involving traditional statistical or artificial intelligence (TS/AI) tool interventions in peer-reviewed clinical research journals. The inclusion criteria of RCTs were that (1) the study should be conducted with patients or health professionals, or both, in a clinical setting (population), (2) TS/AI prediction tools were used as a clinical intervention in RCTs (intervention), (3) any types of control group were selected (comparison), (4) quantitative outcomes of the study were presented (outcome), and (5) the article was written in English. The exclusion criteria included (1) studies that were not relevant to interventions using TS/AI tools, (2) reviews and/or meta-analysis, (3) studies of model development and/or validation, (4) observational studies, (5) study protocols or pilot studies, (6) editorial/letters/comments/case report, and (7) studies not in the field of interest. In the current review, we categorized trials into three groups according to the types of intervention tools in clinical practice. They were trials involving interventions evaluating TS, ML, and DL tools, respectively. AI tools included ML and DL algorithms. Though deep learning is a subset of machine learning, the category of ML in the current study did not include DL algorithms. TS models mainly used regression modeling methods, ML included machine learning algorithms, computer-aided diagnosis, Bayesian analysis, and DL used deep convolutional neural networks. No human subjects were involved because the study was mainly a survey of public data and no written informed consent was needed. Studies for prediction tool development and validation of each randomized trial were investigated.
Search strategy and data sources
We searched PubMed (to Oct 2020) for published papers within the title, abstract, and keywords of the articles. We divided search terms in PubMed into four groups: DL-related terms, ML-related terms, prediction tool-related terms, and terms relating to RCTs. Terms within groups and DL, ML, and prediction tool-related terms were combined with RCTs using the Boolean operator AND, respectively, and the resultant three subgroups were combined using the Boolean operator OR. We referred and modified filters from previous studies11,44 to identify AI studies, prediction tools, and RCTs and provided search strategies in Supplementary Note 1.
A search was also conducted in the clinical trial registry website (clinicaltrials.gov, to Oct 2020) using the terms ‘artificial intelligence’, ‘machine learning’, ‘deep learning’ and ‘prediction model/tool’ to identify finished clinical trials for TS/AI interventions. Furthermore, reference lists of each relevant impact analysis study were included to identify possible additional studies. Y.h.C. and Q.Z. independently screened the identified articles following the literature search to minimize selection bias. Any disagreements were resolved by discussion till all investigators reached a consensus.
We screened the abstracts of the candidate articles for inclusion and subsequently read the full text of the articles deemed eligible according to the inclusion criteria. Subsequently, we excluded those ineligible articles and articles not providing sufficient information about the application of TS/AI tools. The studies for prediction tool development and/or validation of each randomized trial were searched according to the descriptions and the citation of the references of the paper. The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines45. Supplementary Table 1 shows a completed PRISMA checklist.
Data collection and definition
Data extraction was performed by Y.h.C. and Q.Z. using an Excel spreadsheet (Excel for Windows 2013; Microsoft, Redmond, WA, USA) with the following items for each relevant article: (1) first author; (2) year of publication; (3) type of TS/AI tools (TS, ML, or DL); (4) target of TS/AI tools (assistive diagnosis, risk stratification, assistive treatment decision, or others); (5) algorithms of TS/AI tools; (6) input and output; (7) controls; (8) clinical domain or condition; (9) application setting: inpatient, outpatient, home; (10) performance of the algorithm in model development and/or validation measured by the area under the receiver operating characteristic (AUC) and accuracy, and their 95% confidence intervals; (11) primary outcome of interest: whether it was significantly positive or not, and how the outcome was being used; (12) number of enrolled participants; (13) planned sample size (sufficient or not, defined as the number of enrolled participants larger or equal to the planned number); (14) duration of studies; (15) referenced CONSORT (yes or no); (16) study design and relevant features: masking (open-label, single-blinded or double-blinded), intent-to-treat (ITT) analysis (yes or no), and subgroup analysis (yes or no). For observational studies of model development and/or validation, we exacted data including year of publication, study type (prospective or retrospective), sample size for model development, whether the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis [TRIPOD]46 was referenced (yes or no), and performance of the algorithm in model development and/or validation measured by AUC and/or accuracy.
Because of the heterogeneity of these trials, the actual effect size for each trial was not able to be synthesized. According to the statistical significance of the primary outcome of interest, we classified a trial as positive if the proposed primary outcome of interest was reached, which means the null hypothesis was rejected, if the 95% confidence intervals excluded the null hypothesis or if the pre-specified target was met. If the primary objective was not stated, a trial was considered positive if the TS/AI tool was superior to the specified control or standard treatment. In describing the TS/AI interventions, the number of predictors, the outcome the algorithm was predicting, and how the outcome was being used to make a decision in the trial were documented.
Methodological and reporting quality assessment
The quality of each article was independently performed by two reviewers (Q.Z. and Y.h.C.). RCTs were assessed according to the Cochrane Collaboration’s tool for risk of bias43. Checked risk of bias and data for published trials were presented. The quality of reporting was assessed according to whether the CONSORT statement was referenced or not40. We did not use the CONSORT-AI extension as a reference because the included articles were published before the statement extension was released.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range, IQR), as appropriate, and categorical variables as numbers and percentages. Comparisons between two groups were made using t test or Mann−Whitney U test for continuous variables and Fisher’s exact test for categorical variables because of small sample size. Subgroup analysis was performed according to the risk of bias of trials. P value < 0.05 was considered statistically significant. All statistical analyses and plots were performed using the R version 3.6.0 software (Bell Laboratories, Murray Hill, NJ; https://cran.r-project.org/bin/windows/base/old/3.6.0/).
Results
General characteristics
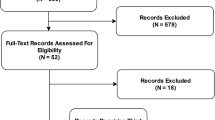
We screened 26,082 records through PubMed and the registry website from Jan 2010 to Oct 2020, and included 65 trials from 63 articles in the final review and analysis (Fig. 1). There were two articles including two trials conducted in a different population or clinical settings. As we mainly focused on the quality and effectiveness of the studies, we included all the trials separately in each article. A list of included RCTs is shown in Supplementary Table 2.
Published trials were searched on PubMed. Clinical trial registry and references in the full-text articles for eligibility were also checked to include potentially relevant trials. Clinical trial registry was the clinicaltrial.gov registry website. The observational studies for tool development and/or validation were searched according to the descriptions and the references of the clinical trial paper.
Of the 65 RCTs, 67.7% were published in 2016 or later. RCTs evaluating DL interventions emerged in 2019. The number of published trials increased over time, with 11 (16.9%), 17 (26.2%), and 37 (56.9%) trials involving DL, ML, and TS prediction tools, respectively (Fig. 2a). Most RCTs did an open-label (75.4%) randomized controlled superiority design (73.8%) with 1:1 allocation ratio (84.6%), and recruited at a single center (50.8%) over a median duration of 12 months for a median sample size of 435 (IQR: 192, 999). The function of these tools in clinical practice included assistive treatment decision (53.8%), assistive diagnosis (24.6%), risk stratification (18.5%), and others (3.1%). The top three covered conditions were acute disease (29.2%), chronic disease not including cancer (27.7%), and cancer (16.9%) (Table 1).
a The trend of published randomized controlled trials involving traditional statistical and artificial intelligence prediction tool interventions with years; b the trend of the number of trials with positive and negative results; c number of trials with positive results by three types of prediction tools; d percentage of trials with positive results by three types of prediction tools.
Quality of reporting and risk of bias assessment for trials involving interventions evaluating traditional statistical and artificial intelligence prediction models
The distributions of risk of bias by each domain and the overall risk of bias of all trials and by types of tools are depicted in Fig. 3a, b.
Blinding of participants and personnel, other bias, blinding of outcome assessment showed a more frequent high risk of bias than other domains. Seventeen trials (26.2%) were considered to have an overall low risk of bias, 38.5% some concerns, and 35.4% overall high risk. When stratified by types of tools, more (46%) trials evaluating DL tools showed a low risk of bias and less (18%) showed a high risk of bias, and nearly half of the trials (43%) involving TS as interventions showed an overall high risk of bias, but the difference was not statistically significant among the three groups (P = 0.395). Nearly three quarters (72.3%) did not reference the CONSORT statement in reporting of the trials. Nineteen RCTs (32.3%) did not perform sample size pre-estimation and seven trials (10.8%) recruited subjects less than expected. Most (60.3%) of the trials did not use or mention intent-to-treat analysis. All trials were registered in advance, but study protocols were not available in most of the trials (75.4%) (Table 1).
AI tool intervention and its performance in both observational model development and/or validation study and randomized controlled trial
All studies reported the P value from a comparison of the primary outcome of intervention and control groups. Compared with the control group, two-fifths (38.5%) of the trials showed no benefit as no statistically significant difference for the primary outcome while 61.5% showed positive results (Table 1). The number of trials with positive results for primary outcome increased with time, but the number of trials with negative results did not change much (Fig. 2b).
We found that 58 trials (89%) had model development studies. Nine of them were published in the same paper with the RCT, and 49 were published independently before the trial. Model development studies were not found in seven trials (10.8%), five from TS and two from ML. Most (64.6%; 42/65) of the trials had internal validation using methods of cross-validation, bootstrapping, random split, and split-sample by time point, and 61.5% (40/65) had external validation which was defined as making validation in independent datasets before the corresponding RCT was conducted. These observational studies had a median sample size of 1392 (IQR: 192, 10,356). Most (63.1%) of them were retrospective studies and some (16.9%) were prospective. Only two studies were reported according to the TRIPOD guidance which has been widely used for reporting clinical prediction models. In terms of model performance, 21 of them reported a median AUC of 0.81 (IQR: 0.75, 0.90) in model development. The median AUCs were 0.78 (IQR: 0.73, 0.88; n = 18), 0.83 (IQR: 0.79, 0.97; n = 11) in internal and external validation, respectively. Data of AUC were not available mainly because of different methods for assessing model performance or not being reported in the final paper. More information on observational studies is shown in Supplementary Table 3.
Table 2 and Supplementary Table 4 show the brief descriptions of 28 RCTs involving deep learning and machine learning interventions in terms of conditions, sample size, tools for intervention and control group, algorithms, the input and output of the tool, how the output is being used in clinical settings, trial outcomes, the gold standard of the outcome, trial findings. Most DL tools were developed for the diagnosis of gastroenterological oncology, but they showed slight differences in the tool outputs. The control group in these trials was routine clinical examination, such as colonoscopies, esophagogastroduodenoscopy. In order to avoid potential operational bias, one DL trial used a sham AI system as control, so that a double-masked design could be performed. Most ML tools exhibited assistive function of patient management and treatment decision for chronic disease.
Comparisons among trials involving interventions of traditional statistical, machine learning, and deep learning tools
The intervention tools were classified into three categories according to their types of algorithms (Table 3). Trials involving ML and DL tool interventions took less time duration than TS interventions (7 vs 6 vs 18 months, P = 0.005). The median sample size of trials evaluating the TS, ML, and DL tools was 435 [IQR: 194, 999], 258 [IQR: 90, 537], and 700 [IQR: 548, 994], respectively, but no statistical significance (P = 0.122). These models were implemented in different clinical settings (P = 0.015). A majority of DL interventions were for inpatients and used non-quantitative clinical data such as computed tomography (CT), magnetic resonance imaging (MRI), slit-lamp photography, colonoscopy, or esophagogastroduodenoscopy. The proportions of disease categories and the function of prediction models were not consistent among the three types of interventions (both P < 0.001). Trials evaluating DL interventions were more likely conducted in cancer research, such as colorectal cancer, upper gastrointestinal cancer, and all of them were for the purpose of disease assistive diagnosis. ML interventions were more frequently used in chronic diseases, not including cancer such as obesity, work disability, anemia, and so on, and a majority of them were used for assistive treatment decisions and some for assistive diagnosis. While TS tool interventions were more in acute diseases such as mechanical acute small bowel obstruction, acute heart failure, treatment decisions in intensive care units, and their purposes were diverse including assistive treatment decision, risk stratification, and assistive diagnosis.
The positive rates of primary analysis were different among trials involving interventions evaluating TS, ML, and DL tools (51.4%, 70.6%, 81.8%, respectively; P for Fisher exact test = 0.136, P for trend = 0.044) (Fig. 2c, d). However, when we stratified by the risk of bias (low, some concerns, high), the distribution of the positive rate of results was changed (Fig. 4). In trials with low risk of bias, the positive rate of trials involving TS tools increased to 63%, ML tools decreased to 25%, and DL tools remained (80%), but no statistically significant difference was found (P for Fisher exact test = 0.374, P for trend = 0.660; Fig. 4a, b). Only in the subgroup of high risk of bias, the positive rates were significantly different (TS, ML, DL: 44%, 100%, 100%, P for Fisher exact test = 0.035, P for trend = 0.019; Fig. 4e, f).
a The number of trials of each type of tool in trials with low risk of bias; b the percentage of positive results of each type of tool in trials with low risk of bias; c the number of trials of each type of tool in trials with some concerns; d the percentage of positive results of each type of tool in trials with some concerns; e the number of trials of each type of tool in trials with a high risk of bias; f the percentage of positive results of each type of tool in trials with a high risk of bias.
Discussion
In the current study, we found that the number of RCTs evaluating TS/AI interventions increased with year in the past decade, and trials involving AI tools multiplied in recent 2 years. However, we should be cautious about the clinical application of TS/AI prediction tools before the effectiveness has been proved in rigorous clinical research. This review showed that only a quarter of trials were assessed to be low risk of bias. Consistent with other studies reviewing the quality of RCTs in both general medical fields and in AI11,38,39,47,48, the quality of the trials in the current study tended to be suboptimal in the aspects of referenced CONSORT statement, sample size pre-estimation, randomization, masking, and intent-to-treat analysis. In addition, in this cross-sectional survey through published literature of 65 RCTs, two-fifths of TS/AI prediction tools that achieved good performance in observational model development and/or validation studies failed to show clinical benefit for patients compared to routine clinical treatment. DL and ML tools exhibited superiority to TS tools with regard to the percentage of positive results. However, in trials with a low risk of bias, this advantage in DL remained but disappeared in ML. The percentage of positive rate remained in DL trials, and increased in TS trials, but decreased a lot in ML trials.
We focused on RCTs involving interventions evaluating TS/AI prediction tools to make the assessment and evaluation of the effectiveness of the TS/AI-based interventions as well as the quality of these studies. In recent 2 years, a few studies have been published to review the current situation of AI-based interventional studies from RCTs to evaluate its effectiveness, quality, and methodology11,38,39. They were reviews including five, eight, and two AI-based RCTs, respectively. Cresswell et al.38 selected AI-based RCTs because they thought that, compared with other observational research designs, the risk of bias of RCTs was the lowest. Triantafyllidis et al.39 found that digital health intervention involving AI could be useful and effective based on eight RCTs. But five of them were pilot studies or studies of wearable devices, which were inconsistent with the inclusion criteria of the current study. In short, although a small number of previous studies have discussed the situation of AI-based interventional studies in several medical fields, these reviews were unable to make quantitative analysis and conclusions because of their small sample size and less rigorous methodology. Therefore, we made broader criteria for including RCTs to evaluate the clinical effectiveness of AI prediction tools and compared them with TS trials. In the present study, we focused on prospective RCTs not only in medical images but also in other medical fields. Although there are inherent limitations in RCTs49, we believe that as a gold standard design, the results from RCTs could help us to understand more about the progress and effectiveness of TS/AI tool interventions.
A total of 65 RCTs were included in the current study, and nearly half of them used AI tools as interventions. We found a moderate proportion of negative results in these trials. This indicated that even achieving good performance in model development and being well-validated, prediction-tool-based interventions might fail to show clinical benefit for patients when compared to routine care in RCTs. No agreement has been reached on how much evidence is needed before a prediction tool could be utilized in clinical work. Some researchers9 tried to adopt clinical trial phases for the drug development process to simulate the development process of medical image mining tools. According to their proposed process, a prospective design for validation with more than 100 sample sizes was defined as Phase III. However, the identified eight “Phase III trials” in their study were less evidence to be implemented in clinical settings50. Nagendran et al.11 reviewed 83 published clinical studies of deep learning in medical imaging diagnosis from 2010 to June 2019 and found that the superiority of AI tools over clinicians was overpromising. But there were only nine prospective deep learning studies and two randomized trials existing in medical imaging, so their conclusions were largely based on retrospective studies. Our study included more AI RCTs than previous reviews and we chose TS trials as comparisons. Through the results of these RCTs, we found that although AI tools showed more percentages of better clinical outcomes than traditional care or routine examination than that of TS tools, in the subgroup of low-risk of bias studies, the rate of a positive result in TS trials increased a lot and was not inferior to that of AI tools. Therefore, we believe that high-quality clinical trial designs, such as RCTs, are still required to assess the effectiveness of TS/AI prediction tools before they are implemented in clinical settings. CONSORT-AI group41 has been working on the reporting guideline for clinical trials evaluating AI interventions, which will help evaluate the reporting of these trials.
In addition, a high-quality and rigorous study design was required to conduct RCTs evaluating TS/AI interventions. In our study, the quality of the included trials was variable, which is consistent with several previous reviews11,38,39. Principles of trials’ design such as masking, randomization, and allocation concealment, reporting referenced CONSORT statement was not well followed in the included trials. It is worth noting that these issues are not unique to TS/AI trials. In fact, in previous RCTs in general, the quality was found to be well below an acceptable level47,48. In addition, the risk of bias assessment showed a low proportion of low risk and a high proportion of high risk of bias in the included trials. In doing the assessment, we focused on randomization and masking. Some of the trials51,52,53 gave the reason why they did not use masking for its difficulty or its nature of the intervention, and some would be not influenced by non-masking54,55,56. Masking was also related to study design (i.e. stepped-wedge cluster RCTs)57. Therefore, we did not consider such open-label trials as high risk in the blinding domain of risk of bias assessment after a comprehensive evaluation. Encouragingly, four trials used double-blinded design58,59,60,61. One of them is a DL trial61 to assess the effectiveness of an AI system compared with a sham system so that participants could be blinded to study groups. A sham system was adopted as a control, which was developed from polyp-like non-polyp structure with high sensitivity and zero specificity to detect polyps. This allowed avoiding potential operational bias. Although cluster randomization was recommended to a preferred design by researchers more than 10 years ago27, there was a fifth of trials using clustered randomization. In order to compare with human performance or routine clinical treatment, most trial hypotheses were that the prediction tool would show superiority to clinicians. Non-inferiority design (2/65) would be a choice to prove that the performance of the prediction tool is not inferior to expertise62.
This study showed that DL tools tended to obtain more positive results compared to ML and TS models. However, RCTs involving DL tools nowadays were conducted in a narrower field of diseases and had simpler targets than that of ML and TS trials. For example, DL prediction tools were mostly for the diagnosis and detection of colorectal cancer. Of note, there were seven trials concerning AI’s application in colonoscopy. This is relevant to the increasing number of published studies on the AI model construction of colonoscopy in recent years8. Consistent with other reviews in deep convolutional neural network‐based AI on colonoscopy63, AI assistive colonoscopy was promising but still need more application in different population. ML and TS RCTs were conducted in more application scenarios for different purposes, showing great flexibility and uncertainty in results. For example, Bailey et al.64 in 2013 used a logistic regression prediction model to make real-time automated alerts for patients every day and send alerts to nurses to signify the risk of transfer to intensive care. Geersing et al.54 in 2020 used a Cox regression prediction model to estimate patients’ recurrence risk and then make model-assisted treatment recommendations for patients. These RCTs tried to solve important clinical problems but unfortunately failed. Of note, the percentage of a positive rate of TS trials increased from 51% in all the trials to 63% in the low-risk trials, while ML interventions decreased from 71% in all the trials to 25%. This change after stratifying by the risk of bias was also observed in observational studies for prediction model development which showed that no performance benefit of ML over logistic regression for clinical prediction models22. The current applications of AI are not yet fully spread performed in medicine, and in the future, it will integrate more clinical problems like ML and TS tools. This gives us a hint that if AI tools will be used in a wider range of scenarios in medicine in the future, the process may be more complex and results may face more uncertainty.
Limitations
There were some limitations in the study. Firstly, although comprehensive, our search might have missed some studies that could have been included. In order to validate our search strategy, we specifically paid close attention to trials in high-quality journals and also searched for specific studies or study designs, such as trials for computer decision support, TS/AI trials using a cluster randomized controlled design, and reports of relevant trials on websites. Second, given the heterogeneity of these published trials, no meta-analysis was performed. The current study was a systematic review of trials involving interventions evaluating TS/AI prediction tools, and we analyzed the quality of methodology and risk of bias of the included trials. Third, the trials included in this review were published before the publication of CONSORT-AI extension42, so we did not evaluate the reporting of these trials according to the new guidance. We extracted information on whether these trials referenced CONSORT40 or not.
Future work
Based on our study, we made some recommendations for future research.
Rigorous trial design such as randomized controlled trial to study evaluating TS/AI tools
This could make the evidence of the performance of a TS/AI tool more reliable before it is used in clinical practice and accelerate clinical translation.
Application of CONSORT-AI for reporting
The articles reporting RCTs evaluating TS/AI tools should comply with CONSORT-AI42 before publication. This could improve the quality of reporting of RCTs evaluating TS/AI tools.
Development of specific tools of evaluating the risk of bias for RCTs evaluating TS/AI tools
Currently, there is no specific standard for the assessment of the risk of bias for RCTs evaluating TS/AI tools. With the rapid development of AI tools, it is urgent to develop a specific tool for evaluating the risk of bias of these studies, which can make the results and conclusions of this kind of trials more convincing.
Conclusion
Although negative results have been consistently reported in RCTs involving TS/AI prediction tools, an increasing proportion of studies with positive results in DL prediction tool interventions showed promising perspectives. Whereas the current applications of DL tools are not yet fully widely performed in medicine, and in the future, it will integrate more clinical problems like ML and TS tools. However, ML tools in RCTs showed variable results because in trials at low risk of bias, ML tools got a very low rate of positive results compared to the other two kinds of tools, while in trials with a high risk of bias, it performed much better. Therefore, we believe that rigorous trial is necessary to obtain evidence of DL prediction tool interventions. The experience of RCTs involving ML and TS tools indicates that we should be cautious about the effectiveness of DL when applied to more complex clinical problems and long-term interventions. In addition, high-quality RCTs with transparent reporting are needed to evaluate the efficacy of intelligence prediction models in clinical settings. Prediction tools with DL algorithms for clinical decision-making are the future trend and will be used in the treatment needs of millions of people. Using high-quality research to carefully validate the most clinically valuable tools for clinical practice will help reduce the burden on physicians and protect subjects.
Data availability
The data that support the findings of this study are available on reasonable request from the authors. A full list of records identified through database searching are included in the supplementary information.
Code availability
The codes of the paper are available on reasonable request from the authors.
References
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 350, g7594 (2015).
Pencina, M. J., Goldstein, B. A. & D'Agostino, R. B. Prediction models-development, evaluation, and clinical application. N. Engl. J. Med 382, 1583–1586 (2020).
Steyerberg, E. W. & Harrell, F. E. Jr. Prediction models need appropriate internal, internal-external, and external validation. J. Clin. Epidemiol. 69, 245–247 (2016).
Eagle, K. A. et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. Jama 291, 2727–2733 (2004).
Wilson, P. W. et al. Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847 (1998).
Shah, P. et al. Artificial intelligence and machine learning in clinical development: a translational perspective. NPJ Digit. Med. 2, 69 (2019).
van der Sommen, F. et al. Machine learning in GI endoscopy: practical guidance in how to interpret a novel field. Gut 69, 2035–2045 (2020).
Le Berre, C. et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology 158, 76–94.e72 (2019).
Sollini, M., Antunovic, L., Chiti, A. & Kirienko, M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 46, 2656–2672 (2019).
West, E., Mutasa, S., Zhu, Z. & Ha, R. Global trend in artificial intelligence-based publications in radiology from 2000 to 2018. AJR Am. J. Roentgenol. 213, 1204–1206 (2019).
Nagendran, M. et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ 368, m689 (2020).
Vollmer, S. et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ 368, l6927 (2020).
Stafford, I. S. et al. A systematic review of the applications of artificial intelligence and machine learning in autoimmune diseases. NPJ Digit. Med. 3, 30 (2020).
Benedetto, U. et al. Machine learning improves mortality risk prediction after cardiac surgery: systematic review and meta-analysis. J. Thoracic Cardiovasc. Surg. https://doi.org/10.1016/j.jtcvs.2020.07.105 (2020).
Shin, S. et al. Machine learning vs. conventional statistical models for predicting heart failure readmission and mortality. ESC Heart Fail. 8, 106–115 (2021).
Shung, D. L. et al. Validation of a machine learning model that outperforms clinical risk scoring systems for upper gastrointestinal bleeding. Gastroenterology 158, 160–167 (2020).
Xu, X. et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J. Hepatol. 70, 1133–1144 (2019).
Abrol, A. et al. Deep learning encodes robust discriminative neuroimaging representations to outperform standard machine learning. Nat. Commun. 12, 353 (2021).
Schulz, M. A. et al. Different scaling of linear models and deep learning in UKBiobank brain images versus machine-learning datasets. Nat. Commun. 11, 4238 (2020).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Christodoulou, E. et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 110, 12–22 (2019).
Cowling, T. E., Cromwell, D. A., Bellot, A., Sharples, L. D. & van der Meulen, J. Logistic regression and machine learning predicted patient mortality from large sets of diagnosis codes comparably. J. Clin. Epidemiol. 133, 43–52 (2021).
Gosselt, H. R. et al. Complex machine-learning algorithms and multivariable logistic regression on par in the prediction of insufficient clinical response to methotrexate in rheumatoid arthritis. J. Pers. Med. https://doi.org/10.3390/jpm11010044 (2021).
Smith, A. M. et al. Standard machine learning approaches outperform deep representation learning on phenotype prediction from transcriptomics data. BMC Bioinform. 21, 119 (2020).
Moons, K. G. et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 98, 691–698 (2012).
Kappen, T. H. et al. Evaluating the impact of prediction models: lessons learned, challenges, and recommendations. Diagnostic Prognostic Res. 2, 11 (2018).
Moons, K. G., Altman, D. G., Vergouwe, Y. & Royston, P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 338, b606 (2009).
Garg, A. X. et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. Jama 293, 1223–1238 (2005).
Toll, D. B., Janssen, K. J., Vergouwe, Y. & Moons, K. G. Validation, updating and impact of clinical prediction rules: a review. J. Clin. Epidemiol. 61, 1085–1094 (2008).
Wallace, E. et al. Framework for the impact analysis and implementation of Clinical Prediction Rules (CPRs). BMC Med. Inform. Decis. Mak. 11, 62 (2011).
Bothwell, L. E., Greene, J. A., Podolsky, S. H. & Jones, D. S. Assessing the gold standard—lessons from the history of RCTs. N. Engl. J. Med. 374, 2175–2181 (2016).
Caballero-Ruiz, E. et al. A web-based clinical decision support system for gestational diabetes: automatic diet prescription and detection of insulin needs. Int. J. Med. Inform. 102, 35–49 (2017).
Group, I. C. Computerised interpretation of fetal heart rate during labour (INFANT): a randomised controlled trial. Lancet 389, 1719–1729 (2017).
Sadasivam, R. S., Borglund, E. M., Adams, R., Marlin, B. M. & Houston, T. K. Impact of a collective intelligence tailored messaging system on smoking cessation: the perspect randomized experiment. J. Med. Internet Res. 18, e285 (2016).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Kent, P., Cancelliere, C., Boyle, E., Cassidy, J. D. & Kongsted, A. A conceptual framework for prognostic research. BMC Med. Res. Methodol. 20, 172 (2020).
Cresswell, K. et al. Investigating the use of data-driven artificial intelligence in computerised decision support systems for health and social care: a systematic review. Health Inform. J. 26, 2138–2147 (2020).
Triantafyllidis, A. K. & Tsanas, A. Applications of machine learning in real-life digital health interventions: review of the literature. J. Med. Internet Res. 21, e12286 (2019).
Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 63, 834–840 (2010).
Consort, A. I. & Group, S.-A. S. Reporting guidelines for clinical trials evaluating artificial intelligence interventions are needed. Nat. Med. 25, 1467–1468 (2019).
Liu, X., Cruz Rivera, S., Moher, D., Calvert, M. J. & Denniston, A. K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26, 1364–1374 (2020).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Geersing, G. J. et al. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS ONE 7, e32844 (2012).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. J. Clin. Epidemiol. 68, 134–143 (2015).
Chan, A. W. & Altman, D. G. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 365, 1159–1162 (2005).
Hopewell, S., Dutton, S., Yu, L. M., Chan, A. W. & Altman, D. G. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. Bmj 340, c723 (2010).
Sherman, R. E. et al. Real-world evidence—what is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016).
Zhou, Q., Cao, Y. H. & Chen, Z. H. Lack of evidence and criteria to evaluate artificial intelligence and radiomics tools to be implemented in clinical settings. Eur. J. Nucl. Med. Mol. Imaging 46, 2812–2813 (2019).
Clemons, M. et al. Risk model-guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2, 225–231 (2016).
Guenancia, C. et al. Clinical effectiveness of the systematic use of the GRACE scoring system (in addition to clinical assessment) for ischaemic outcomes and bleeding complications in the management of NSTEMI compared with clinical assessment alone: a prospective study. Heart Vessels 31, 897–906 (2016).
Hill, J. C. et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 378, 1560–1571 (2011).
Geersing, G. J. et al. Effect of tailoring anticoagulant treatment duration by applying a recurrence risk prediction model in patients with venous thromboembolism compared to usual care: a randomized controlled trial. PLoS Med. 17, e1003142 (2020).
Manz, C. R. et al. Effect of integrating machine learning mortality estimates with behavioral nudges to clinicians on serious illness conversations among patients with cancer: a stepped-wedge cluster randomized clinical trial. JAMA Oncol. 6, e204759 (2020).
Steinhart, B. D. et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients—results of the GASP4Ar Study. J. Card. Fail. 23, 145–152 (2016).
Hussey, M. A. & Hughes, J. P. Design and analysis of stepped wedge cluster randomized trials. Contemp. Clin. Trials 28, 182–191 (2007).
Blomberg, S. N. et al. Effect of machine learning on dispatcher recognition of out-of-hospital cardiac arrest during calls to emergency medical services: a randomized clinical trial. JAMA Netw. Open 4, e2032320 (2021).
Brier, M. E., Gaweda, A. E., Dailey, A., Aronoff, G. R. & Jacobs, A. A. Randomized trial of model predictive control for improved anemia management. Clin. J. Am. Soc. Nephrol. 5, 814–820 (2010).
Finkelstein, S. M. et al. A randomized controlled trial comparing health and quality of life of lung transplant recipients following nurse and computer-based triage utilizing home spirometry monitoring. Telemed. J. e-Health. 19, 897–903 (2013).
Wang, P. et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol. Hepatol. 5, 343–351 (2020).
Repici, A. et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology 159, 512–520.e517 (2020).
Aziz, M., Fatima, R., Dong, C., Lee‐Smith, W. & Nawras, A. The impact of deep convolutional neural network‐based artificial intelligence on colonoscopy outcomes: a systematic review with meta‐analysis. J. Gastroenterol. Hepatol. 35, 1676–1683 (2020).
Bailey, T. C. et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J. Hosp. Med. 8, 236–242 (2013).
Acknowledgements
This study was funded by the Medical Scientific Research Foundation of Guangdong Province of China (grant number A2019489).
Author information
Authors and Affiliations
Contributions
Q.Z.: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; roles/writing—original draft. Z.-h.C.: data curation; methodology; writing—review and editing. Y.-h.C.: data curation; formal analysis; methodology; software; writing—review and editing. S.P.: writing—review and editing. Q.Z. and Z.h.C. were co-first authors to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Q., Chen, Zh., Cao, Yh. et al. Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review. npj Digit. Med. 4, 154 (2021). https://doi.org/10.1038/s41746-021-00524-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-021-00524-2
This article is cited by
-
Bioinformatics in urology — molecular characterization of pathophysiology and response to treatment
Nature Reviews Urology (2024)
-
To warrant clinical adoption AI models require a multi-faceted implementation evaluation
npj Digital Medicine (2024)
-
Integrating artificial intelligence into healthcare systems: more than just the algorithm
npj Digital Medicine (2024)
-
The algorithm journey map: a tangible approach to implementing AI solutions in healthcare
npj Digital Medicine (2024)
-
Roadmap on the use of artificial intelligence for imaging of vulnerable atherosclerotic plaque in coronary arteries
Nature Reviews Cardiology (2024)