Abstract
Oxygen isotope anomaly of nitrate aerosol (∆17O-NO3−) contributes to understanding the atmospheric nitrogen chemistry in the polar oceans. Here, ∆17O-NO3− of the aerosol samples was analyzed based on a cruise from East Asia to the Arctic Ocean to explore the nitrate formation mechanisms. ∆17O-NO3− decreased with the increase of latitude, especially when after entering the Arctic Circle. ∆17O-NO3− (e.g., 11.5‰–21.2‰) was extremely low while crossing the sea ice-covered Arctic Ocean. This is most likely influenced by the combined enhancement of hydroxyl (OH) and peroxy (HO2 + RO2) radicals derived by sea ice under permanent sunlight period. In addition, the obvious increase in the ∆17O-NO3− of return trip with shortened daytime indicated the advantage of nocturnal pathways (NO3 related) with the higher ∆17O endmembers. The mutation of ∆17O-NO3− can reflect the change of NOx conversion pathways to nitrate, and it can be more sensitive to the change of radical chemistry related to atmospheric oxidation.
Similar content being viewed by others
Introduction
Atmospheric nitrate aerosols in polar regions can record changes in atmospheric oxidation potential and greatly influence regional atmospheric chemistry1,2, because the cycle of NOx and changes in the formation pathway of nitrate aerosols affect the production rate of O3 and the life of NOx3. Nitrates account for 75–100% of nitrogen emissions deposited from the atmosphere in the Arctic and Antarctic2. Numerous studies have well-studied the nitrate formation pathways in terrestrial and coastal areas4,5,6,7,8. However, due to observational restrictions and other limitations, the formation mechanism of nitrate in the oceans, especially in the Arctic Circle, is still unclear and needs to be further studied. Nitrate impacts the oxidation capacity of the oceanic atmosphere9. In addition, many free radical groups (e.g., HO2, RO2, OH, etc.) are involved in the formation process of nitrate10. Nitrate is widely recognized as an important factor for understanding the marine geochemical cycle, especially to understand the atmospheric oxidation related to reactive nitrogen11,12,13.
During the rapid cycle of NOx during the day, NO2 undergoes photolysis and decomposes into NO and atomic oxygen, and then O3 is generated after the atomic molecular oxygen combines with molecular oxygen14. Tropospheric NO is mainly oxidized by O3 and HO2/RO2:
And oxidized halogen XO (X stands for Cl, Br, I) can also be the oxides in certain conditions such as in the air above the open sea:
The conversion of NOx to HNO3 goes through a series of complex pathways, generally including:
Among them, dimethyl sulfide (DMS) are usually produced in the ocean by phytoplankton15. Besides, although halogens can be produced by coal combustion on land16,17, halogen chemistry plays an important role in nitrate formation mainly in the oceans and polar regions18,19:
The reaction relating to hydroxyl radical (OH) (Eq. (4)) takes place during the day because the formation of OH requires photolysis. Whereas, due to the photosensitivity of NO3 radicals, the reaction with NO3 and N2O5 (Eqs. (6) and (8)) proceeds at night. Halogen chemistry may be the main role of tropospheric nitrate formation, especially in the oceanic atmosphere20.
Oxygen isotope anomaly (expressed as ∆17O = δ17O-0.52×δ18O) is a powerful tool to reflect the mechanism involved in the formation of nitrate, which contributes to a better understanding of the atmospheric nitrogen cycle6,9,18,21,22. Different oxidants (O3, OH, HO2/RO2, XO) containing different ∆17O are involved in the different reaction pathways, leading to different ∆17O of HNO323 (Supplementary Note 1). O3 and XO (ozone oxidation product) have high ∆17O values, while the ∆17O of other oxidants (HO2/RO2 and OH) are low and can basically be ignored24. This means that nitrate produced through NO3 + HC (Eq. (6)) and XONO2 hydrolysis (Eq. (10)) pathways has the highest ∆17O, followed by N2O5 hydrolysis (Eq. (8)), and nitrate produced through NO2 + OH (Eq. (4)) pathway has the lowest ∆17O value (quantification are shown in Methods). Because ∆17O is mass-independent, it doesn’t undergo isotopic fractionation during the conversion, therefore ∆17O can identify the conversion pathway of NOx from different pathways to nitrate more accurately.
Li et al.25 recently reported the changes in the source and formation mechanism of atmospheric nitrate based on isotopes during the two cruises from Shanghai, China to the Arctic Ocean in 2017 and 2018. This provides strong support for enhancing our understanding of the N cycle of the oceanic atmospheric boundary in the Arctic region. Before their study, almost all the reported ∆17O-NO3− was above 20‰ in atmospheric particulate matter in the oceans and polar regions (Supplementary Table 1). In these two cruises, ∆17O of atmospheric nitrate was significantly lower in the high-latitude ocean (70 ~ 85°N, average ∆17O-NO3− = 20.3‰, with a minimum value of 14.8‰) than in other lower oceans (30 ~ 50°N: average 27.7‰, 50 ~ 70°N: average 30.5‰). Such a low ∆17O-NO3− (mean: 20.3‰) in the high-latitude ocean was attributed to the high contribution of the NO2 + OH (P1) pathway. However, some extremely low values (e.g., 14.8‰) remained unexplored as these values have been already below the lowest limit of the ∆17O-NO3− produced by the NO2 + OH pathway (19.8‰) in the study of Li et al., 25 which requires further investigation. Such anomalous ∆17O-NO3− of atmospheric particulate matter has been observed in an altitude station over Taiwan, China (2.7–31.4‰)26, which is due to that peroxy radical dominated the NO oxidation reaction to NO2. Low ∆17O-NO3− values were also observed in precipitation samples over the Tibetan Plateau (6.6–24.1‰)27, which may be due to the low end member of ∆17O-O3, the decrease of O3 concentration and the increase of hydroxyl and peroxy radicals. In addition, Alexander et al.28 used the GEOS-Chem model to simulate a global surface ∆17O-NO3− range of 4‰ to 33‰, with the lowest values in Amazon areas, which may be caused by the hydrolysis of alkyl nitrate. However, the cause of those low values (< 20‰) for the high Arctic regions remains a puzzle.
We also found a lot of low values in the Arctic Ocean at high latitudes during the expedition of the Chinese R/V Xuelong from East Asia to the Arctic Ocean in 2017. It shows that the lower ∆17O-NO3− is not a random event, then it suggests that there are special mechanisms affecting nitrate formation in the high Arctic Ocean and those areas mentioned above. Therefore, in this study, we tried to solve the unexplored mechanism for the lower ∆17O-NO3− in the high-latitude Arctic Ocean, using the cruise data with higher temporal and spatial resolution. TSP samples with a resolution of 48 h were collected during the expedition of the Chinese R/V Xuelong from East Asia to the Arctic.
Results and discussion
Spatial variation of atmospheric NO3 − and ∆17O-NO3 −
During the entire R/V Xuelong cruise, the concentration of atmospheric nitrate ranged from 0.03 μg m−3 to 1.07 μg m−3 (Fig. 1), with apparent spatial characteristics. The NO3− concentration decreased with increasing latitude, with the highest level in eastern Asia and the lowest level in the open ocean of the Arctic Ocean (Fig. 2), which is consistent with the results of other Chinese Arctic expeditions29. This may show the effect of atmospheric nitrogen input over East Asia on marine nitrate aerosols. A much higher concentration of NO3− was found near the continent (Fig. 2), just like the observations from East Asia to the Antarctic22. The concentration of atmospheric NO3− inside the Arctic Circle (0.08 ± 0.05 μg m−3) was significantly lower than that outside the Arctic Circle (0.45 ± 0.37 μg m−3). The NO3− concentration inside the Arctic Circle is in line with the NO3− concentration (0.04 ± 0.02 μg m−3) reported by Morin et al.30 at the Arctic from June to October and such concentration is thought to indicate less exposure to anthropogenic NOx31. Vicars et al.32 reported the average NO3− concentration in the atmosphere (0.52 ± 0.32 μg m−3) on the central coast of the Pacific, and this concentration is regarded as the critical value of the ocean boundary layer in the coastal area of the Pacific free from being subjected to anthropogenic emissions. During the whole cruise, the concentrations of atmospheric NO3− of all seas, except for those near East Asia, were consistently below the specified range, while the air mass back trajectories (Supplementary Fig. 1) for the sea areas near East Asia passed over the eastern Asian continent. This suggests the coastal seas of the East Asian continent are more vulnerable to anthropogenic emissions whereas the remote open ocean is less polluted far out than near continental sea. The distribution of other components of the WSI components (Supplementary Fig. 2 and Supplementary Table 2) of TSP had similar spatial distribution characteristics with nitrate, showing higher concentrations outside the Arctic Circle than that inside the Arctic Circle. The composition of WSI in the Arctic Ocean was significantly different from that of the coastal oceans of East Asia. Cl− and Na+ were the dominant component of WSI in the Arctic Ocean, which also was shown in the Arctic Ocean aerosol WSI of Yu et al.29. The results indicate that the contribution of sea salt aerosol to the aerosol composition in the Arctic Ocean is higher than that in the lower latitudes. However, the WSI of aerosol dominated by SO42− in the East Asian ocean may show the influence of anthropogenic emissions. The concentration of non-sea-salt sulfate (nss-SO42−), mainly from the oxidation of dimethyl sulfide (DMS)33 emitted by ocean phytoplankton and the discharge of anthropogenic sources especially fossil fuel combustion34 in the northern hemisphere, was calculated (Supplementary Note 2). The concentrations of nss-SO42− (Supplementary Table 2) in the Arctic Circle (0.12 ± 0.11 μg m−3) was significantly lower than out the Arctic Circle (1.86 ± 3.27 μg m−3). Methane sulfonic acid (MSA, mainly comes from oxidation products of DMS in summer35) can help to separate the nss-SO42− from other sources. The ratio of MSA to nss-SO42− was higher inside the Arctic Circle (0.23 ± 0.66) than outside the Arctic Circle (0.06 ± 0.09), indicating that the nss-SO42− from the contribution of anthropogenic source was obviously higher than that of low latitude area36.
The variation series of (a) oxygen isotope anomaly of nitrate (Δ17O-NO3−), (b) mass concentrations of nitrate (NO3−), (c) daytime length, and (d) temperature with latitude during the R/V Xuelong cruise from East Asia to the Arctic Ocean. The dotted line represents the location of the Arctic Circle. The red dots indicate the outward voyage to the Arctic, and blue dots indicate the return leg.
During the cruise period, ∆17O-NO3− ranged from 11.5‰ to 36.7‰ (Fig. 1). The ∆17O-NO3− values were relatively higher when the cruise approached the continent and were lower when approaching open sea (Fig. 2). This suggests that nitrate formation mechanisms vary with the changing of the land-based emissions, which is consistent with the research in the tropical Pacific Ocean by Carter et al.11. They also found high ∆17O-NO3− values near continents, and they expected that ∆17O was much lower for NO3− from marine sources compared to the continent. The change of O3 concentration also followed this spatial distribution characteristics, with higher concentrations near the continent and lower concentrations in the open ocean of the Arctic Ocean (Fig. 2). The difference of atmospheric nitrate ∆17O-NO3− in different sea areas may be caused by the different dominant species of main oxidant in the atmosphere, and O3 dominant area will lead to the formation of nitrate with high ∆17O-NO3−. Besides, compared to the open ocean, the concentration of volatile organic compounds (VOCs) including HC in the polluted continents may be higher, due to the presence of more land plants and human-caused emission sources. This will cause high ∆17O (NO3 + HC/DMS, Supplementary Table 3) in the near-continental oceans.
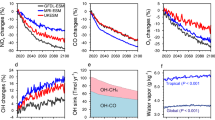
The ∆17O-NO3− is significantly correlated with latitude (r = −0.54, p < 0.01) during the entire cruise. Besides, the ∆17O of return stages from the Arctic Ocean (29.3 ± 4.0‰) was significantly higher than the ∆17O from the Western Pacific to the Arctic Ocean (20.2 ± 5.8‰) (p < 0.01). Further, the whole cruise is divided into several stages, and the relationship of ∆17O-NO3− with latitude is shown in Fig. 3. Stages I and II represent the cruise from East Asia to the Arctic Circle in July-August, and III and IV represent the return cruise in September. Overall, the ∆17O of return stages III and IV were significantly higher than stages I and II (Fig. 3a). However, no significant increase in O3 concentration during the return trip indicates that the changing ∆17O may be not caused by O3 related processes alone (Fig. 3b). This suggests that factors other than dominant oxidants and continental emissions may play a role in the formation of oceanic atmospheric nitrates.
Series of (a) oxygen isotope anomaly of nitrate (∆17O-NO3−) and (b) volume concentrations of ozone (O3) at different stages including stage I (beyond the Arctic Circle along the outward voyage, red dots), II (inside the Arctic Circle along the outward voyage, yellow), III (inside the Arctic Circle along the return leg, green), and IV (beyond the Arctic Circle along the return leg, blue) varying with latitude, respectively.
Surprisingly, during the high-latitude ocean while crossing the Arctic Ocean, ∆17O decreased sharply with increasing latitude (Fig. 3, stage II). Possibly due to the high temporal resolution of the observations in this study, more abnormally low ∆17O-NO3− values were observed in this study than in the report by Li et al.25 at high-latitude Arctic Ocean. In the ocean at 80°N to the north, the average value of ∆17O was 14.8 ± 4.1‰, with a minimum of 11.5‰. This further indicates that the appearance of low ∆17O-NO3− in the high-latitude Arctic Ocean is not accidental. These low ∆17O-NO3− values in stage II corresponded to permanent sunlight period (Supplementary Fig. 3), which suggests that there must be an unknown mechanism of nitrate formation at this stage.
NO3 − formation mechanisms during the permanent sunlight
As mentioned above, phase II (Fig. 3) in the Arctic Circle corresponded to the Arctic permanent sunlight period, and it had the lowest ∆17O-NO3− values (11.5‰–24.3‰, with an average of 16.7 ± 4.7‰) currently observed in the ocean. This was unexpected because previous studies on nitrate formation of the Arctic have mentioned the role of halogens, which would result in high ∆17O-NO3− values. Morin et al.19,37 reported for the first time the ∆17O-NO3− in the Arctic, including the permanent sunlight period in the Arctic, and found that the range of ∆17O in spring was 29–35‰. This high ∆17O-NO3− value is due to ozone depletion events (ODEs) caused by oxidizing halogens. ODEs are frequently observed in the Arctic spring38, and are associated with the release of large amounts of bromine radicals39. In addition, Morin et al.30 also monitored the ∆17O-NO3− of the Arctic for the whole year, which is probably between 25‰ and 32‰. Even though it is in summer permanent sunlight time, the ∆17O-NO3− value is also beyond 25‰, which cannot explain the lowest value (10–20‰) of the Arctic in this study. Firstly, as conventionally thought, the low ∆17O-NO3− value at stage II is due to the dominance of the NO2 + OH (P1) pathway, after all, there should be no nocturnal pathway (NO3–related pathway) in the daytime with permanent sunlight exposure. Moreover, ∆17O-NO3− with lower values is often associated with the NO2 + OH pathway. During permanent sunlight with strong UVB exposure, polar OH can come from the photolysis of O3, CH2O, HONO, and H2O generated by the photolysis of ice and snow. But the increase in OH alone is not sufficient to explain the lowest ∆17O in this study. After calculating according to the end members of ∆17O-NO3− produced by each pathway in this study (Methods, Supplementary Table 3 and Supplementary Note 1), the ∆17O-NO3− of NO2 + OH (P1), NO3 + HC/XONO2 hydrolysis (P2) and N2O5 heterogeneous (P3) was 19.3‰–21.6‰, 31.8‰–34.1‰, and 25.5‰–27.8‰, respectively. According to the end members of ∆17O-NO3− produced by NO2 + OH (P1) (19.3‰–21.6‰), even though we hypothesized that the NO2 + OH pathway dominated the formation of nitrate in the permanent sunlight period, however, the observed ∆17O-NO3− value of 16.7 ± 4.7‰ was lower than the lowest limit ∆17O-NO3− of the NO2 + OH pathway. Therefore, it is speculated that exists a special process that makes our conventional calculation of the ∆17O value of the nitrate formation mechanism higher than the actual situation. And this particular process should have a clear variation with latitude, which caused the sharp decrease of ∆17O-NO3− with increasing latitude in phase II. Interestingly, we found that phase II fit perfectly with passing through the sea ice cover area. As shown by the sea ice concentration modeled by Nimbus-7 SMMR and DMSP SSM/I-SSMIS Passive Microwave Data V002 (https://search.earthdata.nasa.gov/) in Fig. 4, the permanent sunlight period of phase II corresponded to passing through the sea ice, which is consistent with the sea ice prediction results of Mu et al.40 for the same batch of R/V Xuelong cruise. Moreover, the sea ice concentration has the characteristics of being higher near the pole. Therefore, the lowest Δ17O-NO3− during phase II and the sharp decline with increasing latitude (Fig. 3) may be attributed to the sea ice derived changing of atmospheric oxidation.
Sea ice may affect the formation mechanisms of atmospheric nitrate in two ways. One of them is that the NOx produced by the photolysis of nitrate in ice and snow re-participates in the formation of atmospheric nitrate, and the other is that atmospheric chemical processes derived from sea ice may release free radicals associated with nitrate formation processes, altering local atmospheric oxidation. Nitrate is known to be one of the most important ions in polar ice and snow41. The photolysis of nitrate has to be considered when it comes to ice and snow. Contaminant/nutrient sources from mid-to-high latitudes can be deposited on sea ice as wet or dry deposition and incorporated into the ice matrix. Therefore, the release of reactive nitrogen from melting snow and ice may be important during the melt season. Aerosol Δ17O-NO3− may be reduced if affected by the photolysis of nitrates in ice and snow42. It is assumed that the Δ17O value of NOx produced by photolysis of ice and snow will be low, and NO2 is the main product of nitrate photolysis43, so NO2 with low Δ17O generates nitrate through various pathways, which may lead to lower Δ17O-NO3−. Morin et al.30 reported one-year δ15N-NO3− observed at the Arctic Alert site (82.5°N), and summer δ15N-NO3− was highest (−1‰ on average) due to the least effect of nitrate emissions from snow, while δ15N-NO3− in spring had very low values matching the Antarctic summers, which has been attributed to snow photolysis. Berhanu et al.41,44 estimated that δ15N isotope fractionation associated with snow NO3− photolysis in Antarctica ranged from −47.9‰ to −55.8‰. This isotope fractionation will result in very low δ15N of NOx from photolysis, which in turn results in very low δ15N of aerosol NO3−. However, in this study, the δ15N value corresponding to the permanent sunlight period was −3.4 ± 1.6‰, which is much higher than the value caused by snow photolysis. Furthermore, Clark et al.45 reported that δ15N-NO3− did not show very lower values in the Arctic open ocean from previous Antarctic ice and snow sources in the summer study, and surface snow samples did not behave any significant direct evidence of NO3− photolytic loss. This indicates that the recovered snow-derived NOx is not a significant source of atmospheric nitrate in the summer Arctic ocean. So the lowest Δ17O-NO3− while crossing sea ice-covered sea during the permanent sunlight phase could not be explained by the effect of NO3− photolysis in snow and ice.
Burger et al.31 observed very low δ18O-NO3− (δ18O-NO3− of half sample is less than 31‰) in the Weddle Sea in the Southern Hemisphere, and this is due to the effect of sea ice emissions, which can enhance the production of RO2. Brough et al.46 observed an increase in HO2 + RO2 concentration when air masses arrived from sea ice cover in the Antarctic coastal region in spring because sea ice provides a source of halogen radicals such as Cl and Br. Cl can generate R through the reaction of RH+Cl→R+HCl, and then R + O2 → RO2. This reaction is important in the Arctic47. Besides, the presence of Br can increase the concentration of OH and RO248 through the reaction with O3 to form BrO, then BrO reacts with H2O2 to generate HOBr, which in turn can be photolysis to generate OH. Brough et al.46 showed that air masses passing through sea ice regions had higher OH concentrations in the study on the Antarctic coast and that the co-existence of sea ice and sunlight may lead to increased OH concentrations. The increase in OH concentration during the permanent sunlight period can also enhance the generation of peroxy radicals (CO + OH → CO2 + HO2, RH + OH → H2O + RO2)49. Therefore, the low Δ17O-NO3− value during permanent daylight can be attributed to the combined contribution of the HO2/RO2 and OH radicals. Among them, HO2/RO2 is the most important, because that Geng et al.42 also suggested that ∆17O-NO3− value is mainly sensitive to the relative abundance of O3 and HO2 + RO2, while only changing the OH + NO2 pathway has relatively little effect on ∆17O-NO3−. The increase of HO2/RO2 may result in a very large proportion of NO2 generated by HO2/RO2 oxidation relative to O3, which also implies an overestimation of the value of estimated A (Supplementary Note 1). This would then result in an overestimation of the end members of ∆17O-NO3− for each pathway. Our estimated HO2 + RO2 in the permanent sunlight period was around 10pptv, which is similar to the results previously observed in Greenland (8.4pptv) in the Arctic50. Although Morin et al.51 think that the value of A depends on the mixing ratio of O3, if sea ice causes a large increase in HO2 + RO2 in the permanent sunlight period, the value of A is likely to decrease greatly. The value of A in stage II was estimated to be 0.78 ± 0.01 (Supplementary Fig. 4), and the lowest value of the NO2 + OH (P1) pathway was calculated to be 19.3‰. Similarly, Li et al.25 attributed the observed low ∆17O-NO3− values in the high latitude Arctic ocean to the NO2 + OH pathway, but based on their calculation of the A value (0.76) and their assumption for O3*(26 × 1.5), the lower limit of ∆17O-NO3− (19.8‰) for the NO2 + OH pathway is also much higher than the observed lowest value of 14.8‰. Therefore, to match these low ∆17O-NO3− values, the A value of phase II must be higher than currently estimated. When the concentration of HO2 and RO2 is doubled (20pptv), the A value can be reduced by about 0.2, which would be similar to the simulation of the A value (around 0.5) in glacial-interglacial period by Geng et al.42. Then, the theoretical end members of ∆17O-NO3− value of the P1 pathway at stage II will change from 19.3‰ to 12.5‰. When the A value decreased by 0.2 and 0.4, the mean value of the P1 pathway decreased by 25.7% and 51.3%, respectively. If A = 0.5 was used in stage II, the SIAR quantitative analysis of nitrate formation pathways showed that 88.8 ± 9.4% nitrate was produced by NO2 + OH (P1) and 11.2 ± 9.4% by BrONO2 hydrolysis of P2 (Supplementary Figure 5b). Therefore, a complex reaction of peroxy radical generation is possible for this period of permanent sunlight and in the sea ice-covered sea, but it is not possible to discuss it in depth in this study due to the lack of measurement of peroxy radical concentration. Evidence for an increase in peroxy radicals in the atmosphere covered by Arctic sea ice remains limited and further research is needed.
Different nitrate formation mechanisms in the return cruise
The increasing trend of ∆17O on the return journey is in line with the shortening of the length of the daytime. There is a very significant negative correlation (r = −0.85, p < 0.01) relationship between daytime length and ∆17O values (Supplementary Fig. 6). The cruise was mostly carried out in July to September, corresponding to the Northern hemisphere summer, therefore, the higher the latitude, the longer the daytime. The day length of stages I, II, III and IV was 15.4 ± 1.4 h, 24 h, 14.0 ± 1.9 h and 11.5 ± 1.3 h, respectively. The increased nighttime on the return trip (III and IV) may make pathways for high ∆17O-NO3− values associated with NO3 and N2O5 (NO3 + HC/DMS of P2 and P3) more important. When longer daytimes occurred in stages I and II, the decrease in ∆17O (Fig. 3a) also indicated an increase in the proportion of diurnal pathways NO2 + OH (P1). For stages I and IV in the ocean with almost the same latitude outside the Arctic Circle, the mean ∆17O was 25.7‰ and 32.5‰, respectively. Combined with the SIAR results of quantitative estimates of nitrate pathways (Supplementary Fig. 5a, d), nitrate in stages I and IV should be mainly generated by NO2 + OH pathway (38.9 ± 11.8 %) (P1) and NO3 + HC/DMS or XONO2 hydrolysis (47.4 ± 17.0 %) (P2), respectively. In other words, the increase of ∆17O-NO3− in return stage IV is mainly caused by the increase of P2 pathway and the decrease of P1 pathway. The nitrate formation mechanism in the non-permanent sunlight period (stage III) inside the sea area of the Arctic Circle during the return journey (Supplementary Fig. 5c) also changed significantly compared with the permanent sunlight period (stage II) during the journey from Asia to the Arctic (Supplementary Fig. 5b). That is, the length of daytime significantly affects the mechanism of nitrate formation in oceanic atmosphere. Surprisingly, N2O5 heterogeneous pathway (P3) did not change significantly in stage I and stage IV (Supplementary Fig. 5a, d), indicating that the NO3 + HC/DMS pathway may be more important than the N2O5 heterogeneous hydrolysis pathway in the marine atmosphere. Study has shown that the NOx sink of N2O5 in a relatively clean environment is significantly reduced3, which may be due to insufficient NOx reacting with NO3 to form N2O5, and the concentration of aerosols may be lower than in polluted areas, so there would be not enough surface for heterogeneous reactions with N2O5. Although the NO3 + HC and BrONO2 hydrolysis pathways of P2 could not be distinguished, we attribute the high contribution of P2 in return stage to the NO3 + HC/DMS pathway, as the nitrate production via nocturnal NO3–related pathways may increase as nights get longer. This does not mean the unimportant of halogen BrONO2 hydrolysis pathway, but there is no evidence that this pathway has a good trend with latitude or day length. The previous studies in the Arctic reported a very high contribution of the BrONO2 hydrolysis pathway to nitrate formation in the context of ozone depletion events (ODEs)19,30. Brominated compounds, mainly from sea salt, catalyzed the destruction of boundary layer ozone. In our study, there was no sudden decrease in O3 concentration (Figs. 2, 3b) in Arctic sea areas, which could be because O3 depletion events mostly occurred in the arctic spring52. Savarino et al.3 showed that the NO3 + DMS and BrONO2 hydrolysis pathways (P2) in the tropical ocean boundary layer can account for about 40% and 20%, respectively. Shi et al.22 showed that the contribution of the BrONO2 hydrolysis pathway in the ocean increases toward the Antarctic in the cruise from East Asia to Zhongshan Station in Antarctica. BrONO2 hydrolysis pathway has been shown to contribute significantly to the formation of nitrate in the Pacific and Atlantic oceanic boundary layers18. It can be seen that the role of halogens can not be ignored in the study of the formation mechanism of marine atmospheric nitrate, but considering the contribution of the halogen nitrate hydrolysis pathway during the permanent sunlight day (~10 %), the contribution of this pathway to marine atmospheric nitrate in this study may not play a leading role. In summary, the analytical results of the formation mechanism of nitrate on the trip from East Asia to the North Pole in the process from outside the Arctic Circle to the inside of the Arctic Circle, and then on the return journey from the North Pole in the process from inside the Arctic Circle to outside the Arctic Circle (Supplementary Fig. 5a–d) show that the contribution of the NO2 + OH pathway increases with increasing daytime length and then decreases with decreasing daytime length, further suggesting that the large latitudinal variation of ∆17O-NO3− observed may be due to the time gap between daytime and nighttime.
Based on the Δ17O-NO3− observed from East Asia to the Arctic Ocean, this study provides valuable insights into the special mechanisms of nitrate formation in the marine atmosphere of the Arctic Ocean, which is crucial for understanding the impact of sea ice on the Arctic environment. Our findings confirm that the recently observed abnormally low ∆17O-NO3− values in the Arctic Ocean were not accidental and provide evidence for the effect of sea ice on atmospheric oxidizability. ∆17O-NO3− values showed a clear negative trend with both latitude and day length. During the permanent sunlight period, the ∆17O-NO3− values were extremely low and decreased significantly with increasing latitude, which aligns with the simulated sea ice cover area. The effect of sea ice has been shown to enhance the production of OH and peroxy radicals, and this could make the ratio of peroxy radical oxidation to form NO2 likely higher than expected, resulting in lower Δ17O-NO2 values than expected. This then acted in conjunction with the NO2 + OH pathway to cause the observed low ∆17O-NO3−. The increase in Δ17O-NO3− of the same latitude oceanic range on the return trip could be attributed to the contribution of the high-Δ17O (NO3 related) pathways at night due to the longer nighttime at the return trip.
Our study highlights the need for further research to better understand the formation mechanisms of nitrate in the Arctic marine atmosphere. Specifically, to better match the lower ∆17O-NO3− of the Arctic Ocean in this study, we recommend investigating the chemical enhancement of peroxy radicals in the upper atmosphere of the sea ice-covered ocean during the permanent sunlight phase. These findings could help to improve our understanding of the impact of sea ice of Arctic environment. Furthermore, this work shows that Δ17O-NO3− values can reflect changes in oxidants within the NOx cycle and Δ17O-NO3− may be used to improve global atmospheric chemical models. In the future, more attention should be paid to the observation and simulation research on the Δ17O of NO2, and the A value (the proportion of NO2 produced by ozone oxidation) should be corrected more accurately.
Methods
Cruise description of R/V Xuelong
In 2017, the Chinese R/V Xuelong cruise successfully passed the Trans-Arctic Channel during the eighth China Arctic Expedition, which was the first successful trial of a Chinese ship on the Northwest Passage of the Arctic. On July 20, 2017, the R/V Xuelong set sail from the Shanghai Wharf of the Chinese Polar Research Institute (121.69°E, 31.32°N), crossed the Sea of Japan, and crossed the Bering Strait to the Arctic Circle on July 31. The R/V Xuelong passed through the highest latitude sea area at around 86°N on or around August 13. By August 19, the ship had begun a clockwise circumnavigation of Greenland, viewed from the North Pole, before returning and passing through the Arctic Bay and the waters of western Canada. It wasn’t until September 24 that the R/V Xuelong completely exited the Arctic Circle, and it returned until October 10.
Collection for aerosol samples
The cruise trajectories of R/V Xuelong and the locations of sampling points are shown in Supplementary Fig. 7. The total suspended particulate matter (TSP) was collected by the large-flow sampler ASM-1 (Mingye, Guangzhou, China) at 700 L/min on R/V Xuelong. A total of 34 48 hour-TSP samples and 1 blank sample were collected. The sampler set was installed on the deck of the ship about 10 m from the sea level. The machine was closed when the ship is just sailing and other times when it is not suitable for sampling to avoid contamination by the ship’s exhaust gas. The onboard monitoring system recorded real-time information such as latitude and longitude, temperature, humidity, and wind speed. Information about the location of the sampling point and the meteorological conditions is shown in Supplementary Table 4.
All samples were collected on quartz filters (size 8 × 10 in, Pallflex). Before sampling, the filter was wrapped in aluminum foil and baked in a muffle furnace at 450 °C for 6 h to remove impurities. After sampling, the filter was placed in clean aluminum foil and stored in a refrigerator at −26 °C until chemical and isotope analysis was performed.
Chemistry and isotope analysis
The 3.14 cm2 filter cut from the quartz filter was immersed in a 15 mL vial containing 10 mL ultrapure water (18.2 MΩ). After 30 min of ultrasonic vibration and filtration, inorganic ions mainly including Na+, K+, Mg2+, Ca2+, NH4+, Cl−, NO3−, SO42− etc. were measured by ion chromatograph (ICS 5000 + , Thermo Scientific, USA). Each batch of samples contains blank samples and water blanks. The detection limit of NO3− is 0.005 μg/ml. The correlation coefficient of the calibration curve of each batch of samples reached 0.999.
Oxygen isotopes of nitrate were determined using the denitrifying bacteria method, which can be found elsewhere53. Nitrate is converted into N2O gas by denitrifying bacteria (Pseudomonas aureus, ATCC13985), and N2 and O2 are generated by pyrolysis of a gold tube at 800 °C. The ∆17O (obtained by measuring δ17O and δ18O of O2, ∆17O = δ17O-0.52 × δ18O) was measured by using an isotope ratio mass spectrometer (MAT253, Thermo Scientific, USA). The detection limit of this method is 0.4 μgN. The verification of international standard samples (USGS34, USGS35, and IAEA-NO-3) showed that the analytical accuracy of Δ17O can reach 0.19‰ and the uncertainties values were 0.3 ‰. Meanwhile, every 10 samples were accompanied by a replicate, and the standard deviation of ∆17O between the replicates was 0.5‰.
Backward trajectory analysis
The backward trajectory is the analysis of the source and transport path of the air mass based on the path of the atmospheric air mass moving in a certain period of time. The 5-day air mass backward trajectory during the whole sampling was calculated using TrajStat54 to study the long-distance transmission of pollutants, with the set of 1000 m above sea level. The global data assimilation system GDAS data provided by the US Air Resources Laboratory (NOAA ARL) was used. The result is shown in Supplementary Fig. 2. The stations near the coast were greatly affected by continental air mass, while the distant sea was less affected.
Quantification of Nitrate Formation Pathways
The simulation of global nitrate formation pathways of Alexander et al.28 showed that nitrate formation is mainly through OH (Eq. (4) ~ 41%) and N2O5 (Eq. (8) ~ 41%) pathways. In addition, the oxidative halogen XO pathway (Eq. (9) ~ 6%) and NO3 + HC (Eq. (6) ~ 4%) contributed the most. However, many studies do not consider the XO pathway. Halogen bromine chemistry (X=Br) is considered in this study because the main sources of active halogen in the troposphere are sea salt aerosols and seawater55. Here we assume that the ∆17O of OH, HO2 and RO2 is about 0‰, which is consistent with other studies10,21 (Supplementary Note 1). Therefore, it can be inferred that the nitrate formed by the reaction of O3 and XO has a higher ∆17O while formed related HO2, RO2, OH, and H2O have lower values. The ∆17O of nitrate is determined by the ∆17O of NO2 and the conversion process of NO2 to nitrate. The ∆17O value of NO2 should be expressed as A×∆17O-O3*, where the value of A represents the relative importance of the oxidation pathway in which NO is oxidized to NO2 (where the transferred oxygen atoms come from ozone (NO + O3 and NO + XO)) and ∆17O-O3* represents the ∆17O of the oxygen atom transferred by O3. Theoretical ∆17O-NO3− values calculated for each formation pathway were shown in Supplementary Table 3 based on Supplementary Note 1, in summary, the nitrate formation reactions are divided according to ∆17O into three main pathways P1, P2, P3:
Therefore, ∆17O-NO3− = P1×f1 + P2×f2 + P3×f3, where f represents the relative contribution of each pathway, and f1 + f2 + f3 = 1.
The contribution of different formation pathways was quantitatively analyzed using the SIAR (Stable Isotope Analysis in R) model (Supplementary Note 3). The average and the standard deviation value of ∆17O-NO3− of each pathway were input into SIAR. Finally, the estimated values of f1, f2, and f3 can be obtained.
Data availability
All experimental data reported in this study and Supplementary Information are available from the Open Science Framework (https://osf.io/56vsr/).
Code availability
The codes are available upon request from the corresponding author.
References
Wolff E. W. Nitrate in Polar Ice. In: Ice Core Studies of Global Biogeochemical Cycles. NATO ASI Series, Vol 30 (Springer Berlin, Heidelberg, 1995).
Blaszczak-Boxe, C. S. & Saiz-Lopez, A. Nitrate photolysis in ice and snow: A critical review of its multiphase chemistry. Atmos. Environ. 193, 224–241 (2018).
Savarino, J. et al. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl. Acad. Sci. 110, 17668–17673 (2013).
Fan, M. Y. et al. Important Role of NO3 Radical to Nitrate Formation Aloft in Urban Beijing: Insights from Triple Oxygen Isotopes Measured at the Tower. Environ. Sci. Technol. 56, 6870–6879 (2021).
He, P. et al. Atmospheric Δ17O(NO3−) reveals nocturnal chemistry dominates nitrate production in Beijing haze. Atmos. Chem. Phys. 18, 14465–14476 (2018).
He, P. et al. The observation of isotopic compositions of atmospheric nitrate in Shanghai China and its implication for reactive nitrogen chemistry. Sci. Total Environ. 714, 136727 (2020).
Lin, Y. C. et al. Formation Mechanisms and Source Apportionments of Airborne Nitrate Aerosols at a Himalayan-Tibetan Plateau Site: Insights from Nitrogen and Oxygen Isotopic Compositions. Environ. Sci. Technol. 55, 12261–12271 (2021).
Song, W. et al. Nitrogen isotope differences between atmospheric nitrate and corresponding nitrogen oxides: a new constraint using oxygen isotopes. Sci. Total Environ. 701, 134515 (2020).
Sofen, E. et al. WAIS Divide ice core suggests sustained changes in the atmospheric formation pathways of sulfate and nitrate since the 19th century in the extratropical Southern Hemisphere. Atmos. Chem. Phys. 14, 5749–5769 (2014).
Alexander, B. et al. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 9, 5043–5056 (2009).
Carter, T. S., Joyce, E. E. & Hastings, M. G. Quantifying Nitrate Formation Pathways in the Equatorial Pacific Atmosphere from the GEOTRACES Peru-Tahiti Transect. ACS Earth Space Chem. 5, 2638–2651 (2021).
Kamezaki, K. et al. Nitrogen and Triple Oxygen Isotopic Analyses of Atmospheric Particulate Nitrate over the Pacific Ocean. (EGU General Assembly Conference Abstracts, 2017).
Gobel, A. R., Altieri, K. E., Peters, A. J., Hastings, M. G. & Sigman, D. M. Insights into anthropogenic nitrogen deposition to the North Atlantic investigated using the isotopic composition of aerosol and rainwater nitrate. Geophys. Res. Lett. 40, 5977–5982 (2013).
Crutzen, P. J. The influence of nitrogen oxides on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 96, 320–325 (1970).
Scarratt, M. et al. Influence of phytoplankton taxonomic profile on the distribution of dimethylsulfide and dimethylsulfoniopropionate in the northwest Atlantic. Mar. Ecol. Prog. Ser. 244, 49–61 (2002).
Peng, X. et al. An unexpected large continental source of reactive bromine and chlorine with significant impact on wintertime air quality. Natl Sci. Rev. 8, nwaa304 (2021).
Li, Q. et al. Halogens enhance haze pollution in China. Environ. Sci. Technol. 55, 13625–13637 (2021).
Kamezaki, K. et al. Tracing the sources and formation pathways of atmospheric particulate nitrate over the Pacific Ocean using stable isotopes. Atmos. Environ. 209, 152–166 (2019).
Morin, S., Savarino, J., Bekki, S., Gong, S. & Bottenheim, J. Signature of Arctic surface ozone depletion events in the isotope anomaly (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 7, 1451–1469 (2007).
Patris, N., Cliff, S., Quinn, P., Kasem, M. & Thiemens, M. Isotopic analysis of aerosol sulfate and nitrate during ITCT‐2k2: Determination of different formation pathways as a function of particle size. J. Geophys. Res. Atmos. 112, D23301 (2007).
Michalski, G., Scott, Z., Kabiling, M. & Thiemens, M. H. First measurements and modeling of Δ17O in atmospheric nitrate. Geophys. Res. Lett. 30, 1870 (2003).
Shi, G. et al. Using stable isotopes to distinguish atmospheric nitrate production and its contribution to the surface ocean across hemispheres. Earth Planet. Sci. Lett. 564, 116914 (2021).
Savarino, J., Bhattacharya, S., Morin, S., Baroni, M. & Doussin, J. F. The NO+O3 reaction: A triple oxygen isotope perspective on the reaction dynamics and atmospheric implications for the transfer of the ozone isotope anomaly. J. Chem. Phys. 128, 194303 (2008).
Savarino, J. & Thiemens, M. H. Mass-independent oxygen isotope (16O, 17O, 18O) fractionation found in Hx, Ox reactions. J. Chem. Soc. A 103, 9221–9229 (1999).
Li, Y. et al. Significant latitudinal gradient of nitrate production in the marine atmospheric boundary layer of the Northern Hemisphere. Geophys. Res. Lett. 49, e2022GL100503 (2022).
Guha, T. et al. Isotopic ratios of nitrate in aerosol samples from Mt. Lulin, a high-altitude station in Central Taiwan. Atmos. Environ. 154, 53–69 (2017).
Xia, X. et al. Triple oxygen isotopic evidence for atmospheric nitrate and its application in source identification for river systems in the Qinghai-Tibetan Plateau. Sci. Total Environ. 688, 270–280 (2019).
Alexander, B. et al. Global inorganic nitrate production mechanisms: comparison of a global model with nitrate isotope observations. Atmos. Chem. Phys. 20, 3859–3877 (2020).
Yu, C. et al. Characteristics of aerosol WSI with high‐time‐resolution observation over Arctic Ocean. Earth Space Sci. 7, e2020EA001227 (2020).
Morin, S. et al. Tracing the origin and fate of NOx in the Arctic atmosphere using stable isotopes in nitrate. Science 322, 730–732 (2008).
Burger, J. M. et al. The importance of alkyl nitrates and sea ice emissions to atmospheric NOx sources and cycling in the summertime Southern Ocean marine boundary layer. Atmos. Chem. Phys. 22, 1081–1096 (2022).
Vicars, W. et al. Spatial and diurnal variability in reactive nitrogen oxide chemistry as reflected in the isotopic composition of atmospheric nitrate: Results from the CalNex 2010 field study. J. Geophys. Res.: Atmos. 118, 10567–10588 (2013).
Kettle, A. et al. A global database of sea surface dimethylsulfide (DMS) measurements and a procedure to predict sea surface DMS as a function of latitude, longitude, and month. Glob. Biogeochem. Cycles 13, 399–444 (1999).
Manktelow, P., Mann, G., Carslaw, K., Spracklen, D. & Chipperfield, M. Regional and global trends in sulfate aerosol since the 1980s. Geophys. Res. Lett. 34, (2007).
Jiang, B. et al. Effects of atmospheric oxidation processes on the latitudinal distribution differences in MSA and nss-SO42- in the Northwest Pacific. Atmos. Environ. 298, 119618 (2023).
Prospero, J. M., Savoie, D. L. & Arimoto, R. Long‐term record of nss‐sulfate and nitrate in aerosols on Midway Island, 1981–2000: Evidence of increased (now decreasing?) anthropogenic emissions from Asia. J. Geophys. Res. Atmos. 108, AAC 10-11-AAC 10–11 (2003).
Morin, S. et al. Major influence of BrO on the NOx and nitrate budgets in the Arctic spring, inferred from Δ17O (NO3–) measurements during ozone depletion events. Environ. Chem. 4, 238–241 (2007).
Halfacre, J. et al. Temporal and spatial characteristics of ozone depletion events from measurements in the Arctic. Atmos. Chem. Phys. 14, 4875–4894 (2014).
Abbatt, J. et al. Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmos. Chem. Phys. 12, 6237–6271 (2012).
Mu, L., Liang, X., Yang, Q., Liu, J. & Zheng, F. Arctic Ice Ocean Prediction System: evaluating sea-ice forecasts during Xuelong’s first trans-Arctic Passage in summer 2017. J. Glaciol. 65, 813–821 (2019).
Berhanu, T. et al. Isotopic effects of nitrate photochemistry in snow: a field study at Dome C, Antarctica. Atmos. Chem. Phys. 15, 11243–11256 (2015).
Geng, L. et al. Isotopic evidence of multiple controls on atmospheric oxidants over climate transitions. Nature 546, 133–136 (2017).
Boxe, C. et al. Kinetics of NO and NO2 evolution from illuminated frozen nitrate solutions. J. Phys. Chem. A 110, 3578–3583 (2006).
Berhanu, T. A. et al. Laboratory study of nitrate photolysis in Antarctic snow. II. Isotopic effects and wavelength dependence. J. Phys. Chem. 140, 244306 (2014).
Clark, S., Granger, J., Mastorakis, A., Aguilar‐Islas, A. & Hastings, M. An investigation into the origin of nitrate in Arctic Sea ice. Glob. Biogeochem. Cycles 34, e2019GB006279 (2020).
Brough, N., Jones, A. E. & Griffiths, P. T. Influence of sea ice‐derived halogens on atmospheric HOx as observed in springtime coastal Antarctica. Geophys. Res. Lett. 46, 10168–10176 (2019).
Ahmed, S. et al. The Role of Snow in Controlling Halogen Chemistry and Boundary Layer Oxidation During Arctic Spring: A 1D Modeling Case Study. J. Geophys. Res.: Atmos. 127, e2021JD036140 (2022).
Oldridge TNW. Halogen activation from sea ice: Nitrate photolysis and heterogeneous reaction with ozone. (University of Toronto, 2009).
Geng, L. et al. On the origin of the occasional spring nitrate peak in Greenland snow. Atmos. Chem. Phys. 14, 13361–13376 (2014).
Liao, J. et al. Observations of hydroxyl and peroxy radicals and the impact of BrO at Summit, Greenland in 2007 and 2008. Atmos. Chem. Phys. 11, 8577–8591 (2011).
Morin, S. et al. Comprehensive isotopic composition of atmospheric nitrate in the Atlantic Ocean boundary layer from 65 S to 79 N. J. Geophys. Res.: Atmos. 114, D05303 (2009).
Lehrer, E., Hönninger, G. & Platt, U. A one dimensional model study of the mechanism of halogen liberation and vertical transport in the polar troposphere. Atmos. Chem. Phys. 4, 2427–2440 (2004).
Yu, H. R., Cao, F., Zhang, W. Q., Zhao, Z. Y. & Zhang, Y. L. Determination of 17O Anomaly in Atmospheric Aerosol Nitrate. Chin. J. Anal. Chem. 49, 253–262 (2021).
Wang, Y., Zhang, X. & Draxler, R. R. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ. Modell. Softw. 24, 938–939 (2009).
Wang, X. et al. The role of chlorine in global tropospheric chemistry. Atmos. Chem. Phys. 19, 3981–4003 (2019).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 42192512 and No. 41977305), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_1160) and the National Natural Science Foundation of China (No. 42207136). The authors would like to thank all the staff of the R/V Xuelong and the eighth Chinese Arctic scientific expedition team for providing the opportunity for this observation.
Author information
Authors and Affiliations
Contributions
Y.Z. designed the study. Z.Z., Y.Z., and F.C. conceived and organized this paper. Z.Z. conducted the measurement of oxygen isotopes, with contributions from H.Y., H.L., and M.F. to laboratory experiments. Z.Z. and Y.H. conducted the back trajectory simulation. Z.Z. and Y.Z. prepared the manuscript with contributions from W.S., Y.L., and M.G.; All authors reviewed and commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Zhao, Z., Cao, F. et al. Changes in atmospheric oxidants over Arctic Ocean atmosphere: evidence of oxygen isotope anomaly in nitrate aerosols. npj Clim Atmos Sci 6, 124 (2023). https://doi.org/10.1038/s41612-023-00447-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41612-023-00447-7