Abstract
Phytobiotic compositions are commercially used in broiler production, mostly to improve general health and the production parameters. Moreover, some of their active substances may change the expression of miRNA in different tissues. Therefore, the purpose of this study was to evaluate the effect of the phytobiotic composition (PBC) containing white mustard, calamus, turmeric, and common ivy on production parameters, oxidative stress markers and expression of selected miRNAs in pectoral muscle of broiler chickens. The experiment was performed on broiler chickens fed the control diet (without PBC), and a diet supplemented with 60 or 100 mg/kg of PBC for 35 days. After the experiment, samples (blood and muscle) were collected for analyses. The analyzed production parameters included: feed conversion ratio, feed intake and body weight. There was no effect on growth performance of broiler chickens but feeding diet supplemented with 60 mg/kg phytobiotics significantly increased the expression of miR-30a-5p, miR-181a-5p, and miR-206, and decreased that of miR-99a-5p, miR-133a-5p, miR-142-5p, and miR-222 in pectoral muscle of chickens. The addition of 100 mg/kg phytobiotics significantly increased miR-99a-5p and miR-181a-5p expression, and caused down-regulation of the expression of miR-26a-5p and miR-30a-5p. Chickens fed diet supplemented with 100 mg/kg PBC had lower level of lipid peroxidation products in blood, while in the muscle tissue it was higher in birds fed a diet with the addition of 60 mg/kg as compared to the control group. The results suggest that this unique composition of phytobiotics does not affect productive traits but can change expression of miRNAs that are crucial for muscle physiology and pathology in broiler chickens. This additive may also protect against the oxidative stress but the effect is dose dependent.
Similar content being viewed by others
Introduction
Phytobiotics are commercially used feed additives in broiler production around all over the world. Previous observation showed that dietary addition of white mustard (Sinapis alba L.), calamus (Acorus calamus L.), turmeric (Curcuma longa L.), and common ivy (Hedera helix L.) mixture may be an effective alternative for coccidiostats, particularly in a production according to antibiotic reduction programmes1. The effects of this phytobiotic composition on broilers may be broader and comparable to antibiotic growth promoters. White mustard is a source of a well-known glucosinolate, sinalbin, which is mostly recognised as an antimicrobial agent but also showed an antioxidant, proapoptotic, and antiproliferative effect (related to the modulation of the MAPK pathway)2. Also, it was shown that white mustard enhanced the activity of several antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase3. Calamus, similarly to white mustard, presented antimicrobial and antioxidant activity4,5,6. Moreover, number of studies showed that it has anti-inflammatory and wound-healing properties7,8. In poultry, several studies related to calamus were performed. Most of them were associated with coccidiosis prevention and control9, antioxidant activity and cholesterol concentration of quails’ eggs10. Turmeric has a several properties that were investigated in many studies related to different tissues and animal models. In broiler chickens the effect of turmeric addition on growth performance and meat quality was also analysed11. Moreover, it was found that dietary supplementation with turmeric alleviated the aflatoxin-induced down-regulation of sod, gst, eh gene expressions, and up-regulation of il-6, cyp1a1, and cyp2h1 in the liver of chickens12. These results demonstrated beneficial effects of turmeric on antioxidant defence, biotransformation, and immune system of the liver of chicks challenged with aflatoxin B. Feeding diet supplemented with turmeric may also improve antioxidant defence in blood13. Common ivy is another constituent of phytobiotic composition with saponins being its most important bioactive compounds. Saponins may affect growth performance of broiler chickens, meat quality, and can be used for the control of coccidiosis and other diseases such as necrotic enteritis or colibacillosis14,15,16.
Only a few studies have been performed on the effect of phytobiotic composition on miRNA expression so far. Most of them were related to mammal, human and cell culture models and only singe studies were performed on a broiler chickens17,18,19. MicroRNAs (miRNAs) are small, non-coding, interfering molecules of RNA that regulate gene expression through the sequence-specific base pairing to messenger RNA (mRNA). Previous studies showed that miRNAs are related to the key physiological and pathological processes in various tissues in animals and may also be a great diagnostic tool also in veterinary medicine or animal production20. Moreover, in mammal models, there is a group of miRNAs, called myomirs, that are highly enriched in skeletal and/or cardiac muscles and includes: miR-1, miR-133a, miR-133b, miR-206, miR-208, miR-208b, miR-486. These miRNAs are involved in skeletal muscle development. Previous studies showed that the expression of these miRNAs may be modulated by several natural nutriceutical substances21,22. In birds, there are only a few studies related to myomirs that may be associated with physiological and pathological processes in muscle tissue.
The oxidative stress is a phenomenon that may contribute to the development of skeletal muscle pathogenesis but its role is extremely complex. Transient oxidative stress may be beneficial, on the other hand, uncontrolled production and accumulation of reactive oxygen species might have pathological implications23. It is well known that oxidative stress may induce muscle aging and myopathies like white stripping or wooden breast24,25. Moreover, it has been demonstrated that dietary antioxidants could combat oxidative stress and improve broiler performance as well as meat quality26. However, it is unknown how natural antioxidants present in phytobiotic composition (PBC) affect oxidative stress markers in muscle tissue and whether they influence the expression of miRNA related to muscle development. The research hypothesis assumed that bioactive compounds present in the phytobiotic mixture will beneficially affect muscle tissue. Therefore, the aim of the study was to determine the effect of dietary level of PBC containing white mustard, calamus, turmeric, and common ivy on growth performance, miRNA expression in pectoral muscle and oxidative stress markers in broiler chickens.
Results
Production parameters
In production parameters being recorded, i.e. body weight (BW), feed intake (FI) and feed conversion ratio (FCR), no differences between groups were observed (Table 1). The highest body weight was observed in a group fed with 60 mg/kg of phytobiotics mixture. Moreover, also this group presented the lowest FCR.
MicroRNA expression
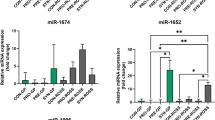
Feeding diet supplemented with 60 mg/kg phytobiotics significantly increased the expression of miR-30a-5p, miR-181a-5p, and miR-206 and decreased that of miR-99a-5p, miR-133a-5p, miR-142-5p, and miR-222 in pectoral muscle of chickens. The expression of miR-26a-5p was unaffected by the treatment (Table 2).
In pectoral muscle of chickens fed a diet with the addition of 100 mg/kg phytobiotics, there was a significant increase of miR-99a-5p and miR-18a-5p expression. Feeding this diet caused significant down-regulation of the expression of miR-26a-5p and miR-30a-5p and had no effect on miR-133a-5p, miR-142-5p, miR-206, and miR-222 expression (Table 3).
Functional analysis of identified miRNAs
Based on the Pathway Studio Mammal Plus Web Software 12.5 (Elsevier, USA) analysis and available literature a number of links between the individual components of the phytobiotic mixture, key physiological and pathological processes in muscle tissue and the miRNAs analyzed were found (Fig. 1). Selected relations in details are presented in Pathway Studio Summary List as single figures and tables (Supplementary material 1, 1a). Interestingly, this type of analysis has shown that the links of the individual components of the product to specific processes are already known, but also their relationships with miRNAs related to these processes. Among the key processes that have been described earlier in works on the impact of phytobiotics are: muscle cell differentiation and proliferation, muscle hypertrophy, muscle tissue development processes, muscle damage and repair processes, as well as apoptosis and oxidative stress. The abovementioned processes were also described in muscle tissue as those associated with the analyzed miRNA:
-
muscle tissue development, muscle cell proliferation and differentiation: miR-26a, miR-30a, miR-99a, miR-133a, miR-206, miR-222, miR-181a,
-
muscle hypertrophy, muscle growth: miR-133a, miR-142, miR-181, miR-142,
-
muscle regeneration: miR-133a, miR-142,
-
inflammation: miR-26a, miR-206, miR-142,
-
cell apoptosis: miR-181a, miR-142, miR-133, miR-222, miR-206,
-
muscle injury and myopathy: miR-99, miR-181a,
-
oxidative stress: miR-142, miR-222.
Prediction and ontological analysis of miRNA target genes (TG)
In order to identify dedicated target genes, set of eight statistically significant miRNAs was analysed using TargetScan Release 8.0 (David Bartel and Whitehead Institute Bioinformatics and Research Computing Group, USA). Analysis showed 4845 unique targets for all miRNAs (Supplementary Material; Table S2). After removing duplicated genes, 3216 unique targets were identified. Among them, only 2953 genes were previously described in Gallus gallus. Among the group of eight miRNAs, the highest regulation potential to the target genes was presented by three miRNAs: miR-222, miR-142-5p; (936 identified target genes for each of them), and miR-133 (816 identified target genes). The lowest regulation potential was noticed for miR-99a-5p, i.e. 30 identified target genes.
Ontological analysis of all identified potential target genes
Functional analysis using Pathway Studio, D.A.V.I.D. 6.7 and Panther 17.0 software showed that all TG for analysed miRNAs were associated with several processes which may be key players in several physiological (protein metabolism, muscle tissue development, apoptosis) and pathological (inflammation, oxidative stress, myopathies, myositis) conditions in muscle tissue (Supplementary Material; Table S3). Moreover, functional network analyses of target genes of differently expressed miRNAs (based on KEGG data27) showed their involvement in regulation of actin cytoskeleton, MAPK signalling, FoxO signalling pathway, Wnt signalling pathway, TGF-β signalling pathway, ubiquitin mediated proteolysis, and mTOR signalling pathway suggesting their involvement in breast muscle growth (also on protein metabolism level) in chickens (Table 4).
Oxidative stress markers
Dietary level of phytobiotic did not affect prooxidant-antioxidant balance in blood and pectoral muscle of chickens but there was an effect on thiobarbituric acid-reactive substances (TBARS) concentration (Table 5). Chickens fed diet supplemented with 100 mg/kg PBC had lower TBARS level in blood, while in the muscle tissue it was higher in birds fed diet with the addition of 60 mg/kg as compared with the control group.
Discussion
There was no effect on analyzed production parameters (BW, FI, FCR). Despite the lack of statistically significant differences between the control group and both groups where the supplement was administered, it should be emphasized that the best (lowest) FCR and the highest final BW were obtained in the group that received the supplement at a dose of 60 mg/kg. The lack of significant differences may be due to several factors. One of them may be too low doses of a phytobiotic additive, which is in line with previous observations on another phytobiotic supplement with similar composition19. The second factor that may affect the obtained results is the duration of the experiment, i.e., 35 days, which may be too short period to observe an improvement in production parameters. However, it is sufficient to improve the parameters related to oxidative stress or to change the expression of selected miRNAs involved in physiological and pathological processes in muscle tissue.
Analysis of the expression of selected miRNAs showed differences in both experimental groups as compared to the control group. Interestingly, only one miRNA (miR-181a-5p) showed consistent direction of expression for both groups. The remaining miRNAs showed different expression directions for each group, or their expression level was negligible for either group. This clearly indicates a dose-dependent phenomenon and it is necessary to carry out more detailed analyses for each of the tested doses. Moreover, the results suggest that a 60 mg/kg dose seems to positively affect miRNAs that are involved in processes of muscle growth and muscle protection. On the other hand, the results of miRNAs for a higher dosage may indicate that 100 mg/kg may have no effect on muscle growth and myoprotection.
Most of analysed miRNAs are known as myomiRs or are closely related to physiology and pathology of muscle tissue (miR-133, miR-206, miR-222, miR-142, miR-99a-5p and miR-181a-5p). The crucial roles of two muscle-specific miRNAs, i.e., miR-133 and miR-206, in the regulation of myogenesis have been well documented28,29 in human or other mammal models but only single papers were associated with birds. What is interesting, the down-regulation of miR-133a-5p, miR-222, miR-99a-5p, and miR-181a-5p and up-regulation of miR-206 and miR-30a-5p in pectoral muscle of chickens fed a diet supplemented with 60 mg/kg phytobiotic composition is in line with the direction of changes observed for the intensification of muscle cell differentiation (also in birds) and the growth of muscle tissue30,31,32. One miRNA, miR-142, seems to be also interesting in the hypertrophy aspect. Its downregulation was described during cardiac hypertrophy and is able to inhibit cytokine signalling and function in the myocardium33. Moreover, the same direction of expression was noticed previously after using β-hydroxy-β-methylbutyrate, which is known as a muscle mass builder, in equine satellite cells22. As it was mentioned above, only one miRNA, i.e., miR-181a-5p had the same direction of expression in both groups fed phytobiotic-supplemented diets. Surprisingly, in previous studies on human and other mammals up-regulation of its expression was observed in muscle tissue/cells that were during the aging process and inhibited regeneration/proliferation34. However, these studies also indicated that overexpression of miR-181a-5p facilitated the differentiation of muscle cells.
Surprisingly, in group of chickens fed diet with higher level of phytobiotics (100 mg/kg) the expression of only four miRNAs was significantly altered. These miRNAs (miR-26a-5p, miR-30a-5p, miR-99a-5p, and miR-181a-5p) were previously described as those involved in muscle cell proliferation/differentiation, muscle aging, muscle atrophy and regeneration35. Among the analysed miRNAs, miR-99a seems to be the most interesting, as the latest research indicated that it is involved in the processes of proliferation and differentiation in chicken breast muscles36. Authors noticed that knock down of this miRNA may decrease proliferation of muscle cells while its overexpression promoted the cell viability. These results suggest that different doses of phytobiotic mixture may differently affect the same miRNAs and associated processes.
While the majority of the analysed miRNAs were previously described as those associated mainly with the physiological and pathological processes of muscle tissue, and single ones with oxidative stress, apoptosis, the phenomenon of tissue regeneration, etc., the target genes show that a large group of them is also clearly related to the characteristic processes for muscle tissue. Pathway analysis for TG clearly shows that large groups of genes are associated with key metabolic pathways in muscle tissue, i.e., MAPK pathways involved in protein degradation in muscle cells37, and oxidative stress38. Moreover, MAPK pathway is a key player in a muscle energy metabolism through modulating lipid metabolism, skeletal muscle growth, and also involved with muscular myopathies and atrophy37,39. Among identified pathways also FOXO signalling is closely related to muscular dystrophies/atrophy and possibility to modulate the expression of related genes seems to be an effective tool in controlling this kind of muscle disorders40. In addition to the pathways mentioned, Wnt signalling, which plays an essential role during embryonic muscle development and is responsible for skeletal muscle homeostasis in the adult41, mTOR, which controls both the anabolic and catabolic signalling of skeletal muscle mass and finally modulate the muscle hypertrophy and muscle wastage, deserves attention42. The analysis of signalling pathways in terms of the phytobiotic mixture used is interesting because there are single studies on selected ingredients of the additive tested in the current study, confirming the effect on some of them, e.g., curcumin and mTOR signalling pathway43 or common ivy and the MAPK pathway44. It should be emphasized, however, that the presented analyses are purely theoretical and the obtained results should be verified using dedicated methods based, among others, on gene silencing in specific cell or animal models.
Due to previous reports on the potential antioxidant effect of individual components of the phytobiotic mixture, the miRNA related to this process was selected for the analysis and two oxidative stress markers, TBARS and PAB, were assessed. The method of PAB analysis was established to be used for the evaluation of age-related and metabolic diseases such as diabetes in humans and can be useful in monitoring antioxidant therapy effectiveness45. Since the method was designed to evaluate the balance in biological samples such as blood and urine45, an attempt was made to applied it in animal studies to evaluate antioxidant effect of chicory inulin in young pigs46,47. The PAB was successfully determined in blood plasma as well as in tissue homogenates. Its analysis revealed antioxidant effect of 2% and 3% dietary addition of inulin and 4% dietary level of dried chicory root in blood plasma and liver of 50-day old pigs46,47. Lower PAB values indicated the prevalence of antioxidants in a sample and improved antioxidant status. Thus, it serves as a useful marker in studies related to oxidative stress prevention. To the best of our knowledge, this is the second study in which PAB was determined in chicken blood and muscle tissue. The first one, published also by our research group, demonstrated very high levels of balance values in broiler chickens fed diets supplemented with different doses of PBC consisting of red pepper fruit, white mustard seeds, turmeric, soapwort root, and calamus rhizome48. Our analyses revealed that the balance values in broiler chicken blood were even ten times greater than those previously determined in pigs46,47. Also, PAB values measured in pectoral muscle of chickens were considerably greater than those measured in the liver and kidney of pigs47. However, this did not have to mean that there was a prevalence of oxidants in broiler chicken body. The results might rather reflect species specificity and suggested a necessity to adjust the scale of PAB values for chickens. In the current study, blood plasma was diluted ten times prior to the analysis to fit the linear range of the assay. After dilution, the raw absorbance values (0.4–0.8) were in the bottom range of the standard curve and indicated low level of oxidants and high content of antioxidants in blood (35–138 HKU). Similarly, the raw results obtained for muscle supernatants were in the range of 40–70 HKU and also indicated the prevalence of antioxidants in pectoral muscle of broiler chickens. Nonetheless, despite the antioxidative activity of constituents of the phytobiotic composition used in the current study, there was no improvement in the PAB values in blood and muscle tissue, which is in line with our previous research48. Probably, the level of dietary supplementation was too low to increase the concentrations of antioxidants circulating in the blood and their accumulation in peripheral tissues. Another reason might be the lack of phytobiotic effect on blood level of small-molecule antioxidants such as uric acid, creatinine, and bilirubin. Unfortunately, these parameters were not measured in the current study, therefore further research is required. Despite the lack of effect on PAB values, feeding diets supplemented with the phytobiotic composition reduced the level of lipid peroxidation products in the blood of chickens. On the other hand, dietary inclusion of 60 mg/kg phytobiotics increased the concentration of TBARS in pectoral muscle of broiler chickens in comparison with the other groups. This is difficult to explain, especially as the miR-142-5p expression in pectoral muscle was down-regulated in this group. As suggested previously49, inhibition of miR-142-5p in the liver cell line might upregulate the protein level of nuclear factor-erythroid 2-related factor 2 (Nrf2) and its target antioxidant genes (ho1, nqo1, mn-sod), and in consequence, reduce the production of reactive oxygen species and oxidative stress. However, the role of miR-142-5p in oxidative stress in muscle tissue of broiler chickens may differ from its role in the liver cell line. It was suggested that tissue or cell line specificity could be responsible for discrepancies in miR-142-5p expression during aging process in different tissues and pointed out the need of care in the selection of this miRNA as a marker for detection of tissue or organ aging50. It may be also truth in the case of its use as a marker of oxidative stress in muscle tissue. Nonetheless, the current study suggests that the antioxidant effect of the phytobiotic composition is dose-dependent and tissue-specific. Similar results were obtained on pigs47, in which antioxidant effect of chicory inulin was found in the blood and liver but not in the kidneys.
Materials and methods
Birds, housing, and experimental design
The trial involved 48 one-day-old female Ross 308 broiler chickens, purchased from a local commercial hatchery. Birds were divided into three groups (n = 16) fed cereal-based diets (starter, grower, and finisher) without the addition of PBC (control) or supplemented with 60 or 100 mg/kg of PBC (AdiMax® AP, AdiFeed Sp. z o.o., Warsaw, Poland, Supplementary Material S1). The doses were chosen based on the previous study and observation for similar product48. The mix consisted of white mustard, calamus, turmeric, and common ivy. According to the producer’s declaration, this product contained herbal phytoncides, including phytoanticipins and phytoalexins, and the following nutrient content: 12.7% crude protein, 18.7% crude fiber, 7.5% ether extract, 7.5% crude ash, 0.54% lysine, 0.20% methionine, and less than 0.05% sodium. Birds had free access to feed and water throughout the trial. Composition of diets (Table 6) was the same as that previously published by Chodkowska et al.48.
In the first week of life, chicks were kept in electrically heated battery brooders with wire-mesh floor in groups of eight birds each and fed the appropriate experimental diet. The housing temperature was maintained at 32 ± 1 °C and light-dark cycle was set at 18 h of light and 6 h of dark. At eighth day of life birds were placed in an individual cages with wire-mesh floors and feeding the respective experimental diets was continued until the slaughter. During this period, chickens were kept at temperature of 32 °C, which was gradually decreased according to normal management practice, and at 18/6 h light-dark cycle. Body weight and feed intake were measured weekly. At 35 day of age chickens were sacrificed by decapitation and exsanguination. Blood was collected into heparinised tubes, centrifuged (3350g, 10 min, 4 °C), and plasma was stored at − 80 °C until further analyses. Pectoral muscle samples were taken, snap-frozen in liquid nitrogen and stored at − 80 °C. Blood and muscle tissue samples were collected from 10 birds per group.
Chemical analyses of diets
Nutrient content in the experimental diets was analysed according to standard procedures51, while gross energy concentration was determined using a KL-12Mn adiabatic oxygen bomb calorimeter (Precyzja-BIT, Bydgoszcz, Poland).
Total RNA isolation
Total RNA, including miRNA, was isolated from muscle tissue using miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer`s protocol. The integrity of RNA was checked by electrophoresis on 2% agarose gel stained with ethidium bromide. The concentration and purity of RNA were measured spectrophotometrically using NanoDrop ND-1000 (Thermo-Scientific, Wilmington, DE, USA).
Real-time qPCR
The expression of selected miRNA was analysed by the real-time quantitative PCR. The miRCURY LNA RT Kit (Qiagen, Hilden, Germany) was used for polyadenylation and reverse transcription into cDNA according to the manufacturer’s protocol. Gene expression analysis was performed using the miRCURY LNA SYBR Green PCR Kit (Qiagen) and specific primers (Table 7) designed using the GeneGlobe tool (http://geneglobe.qiagen.com), and miRNA sequences retrieved from the miRbase, the microRNA database (http://www.mirbase.org52). The reaction mix consisted of: 5 μl 2× miRCURY SYBR® Green Master Mix, 1 μl PCR primer mix (Qiagen), 3 μl cDNA template (diluted 1:60), and 1 μl nuclease-free water. The PCR cycling conditions for miR-26a-5p, miR-30a-5p, miR-133a-5p, miR-142-5p, and miR-206 were as follows: initial activation at 95 °C for 2 min and 37 cycles of denaturation (95 °C, 10 s) and combined annealing/extension (56 °C, 60 s). For miR-99a-5p and miR-222 the number of cycles was reduced to 36, while for miR-181a-5p and U6 snRNA it was increased to 40 and 45, respectively. Reactions were performed on a MIC qPCR thermocycler (Bio Molecular Systems, Upper Coomera, QLD, Australia) and relative gene expression was calculated using the 2−ΔΔCt method53 and U6 snRNA as a reference gene.
Target gene prediction and ontological analyses
MicroRNA target gene prediction was performed using the TargetScan Release 8.0 database (TargetScanHuman 8.0, David Bartel and Whitehead Institute Bioinformatics and Research Computing Group, USA). The analysis was performed for all phytobiotic-affected miRNAs. For each predicted target of individual miRNA, the sum of the context++ scores were automatically calculated54. Predicted targets of each miRNA family were automatically sorted by cumulative weighted context ++ score.
Ontological analyses revealing molecular functions, biological processes, and pathways of miRNA targets were performed in D.A.V.I.D. 6.7 (DAVID Functional Annotation Bioinformatics Microarray Analysis Bioinformatic Resources; Laboratory of Human Retrovirology and Immunoinfromatics, Frederic, MD, USA) using Fisher’s exact test with P ≤ 0.05. Detailed analysis of the role of phytobiotics-modulated miRNAs and target genes in various metabolic and signal pathways was performed using Panther 17.0 Classification System (http://www.pantherdb.org). Detailed analysis of the role of phytobiotic mixture on miRNAs and potential target genes in various metabolic and signal pathways was performed using Pathway Studio Web Software 12.5 (Elsevier, USA). Relationships between all differentially expressed miRNAs were visualized with Pathway Studio’s Build Pathway functionality which is based on the wave-propagation algorithm developed for the navigation through complex networks.
Lipid peroxidation assay
The degree of lipid peroxidation in blood plasma and pectoral muscle was estimated spectrophotometrically based on the concentration of TBARS as described previously48. Before analysis, muscle samples were weighted to 0.2 g, homogenized in 0.8 ml of ice-cold 0.9% NaCl, and centrifuged (12,850g, 10 min, 4 °C), while blood plasma samples were assayed directly after 1 h-incubation of samples with 15% trichloroacetic acid and 0.37% thiobarbituric acid at 100 °C, followed by centrifugation (10,000g, 10 min). The absorbance was measured using a SpectraMax iD3 microplate reader (Molecular Devices, San Jose, CA, USA) at a wavelength of 532 nm. TBARS concentration was calculated from a standard curve for malonyldialdehyde.
Analysis of prooxidant-antioxidant balance
The balance between oxidants and antioxidants was determined according to the method of Koliakos and Hamidi Alamdari45 based on simultaneous redox and enzymatic reactions. Muscle samples were prepared as described above. Briefly, 10 μl of blood plasma or muscle supernatant were placed into a 96-well plate. Then, 200 μl of the solution containing 3,3′,5,5′-tetramethylbenzidine, its cation, and horseradish peroxidase were added and incubated for 12 min at room temperature, in the dark. After the incubation, 100 µl of 2 M HCl solution was added to each well and incubated for 45 min. at room temperature, in the dark. Then, the absorbance was measured at 450 nm and 620 nm (reference wavelength) on a SpectraMax iD3 microplate reader (Molecular Devices, San Jose, CA, USA). The balance between oxidants and antioxidants was calculated from the standard curve prepared using 1 mM H2O2 solution as representative of oxidants and 6 mM uric acid solution as representative of antioxidants, and expressed in arbitrary units of Hamidi Alamadari and Koliakos (HK) per 1 ml of blood plasma or 1 g of tissue.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Growth parameters and oxidative stress markers were analysed by one-way analysis of variance followed by Tukey’s HSD test. Data were checked for a compliance with the normal distribution by the Shapiro-Wilk test. All these analyses were performed using SPSS 11.5 software (SPSS Company, Chicago, IL, USA). The results of qPCR were analysed by a two-tailed Student’s t-test using the micPCR 2.10 programme (Bio Molecular Systems, Upper Coomera, QLD, Australia). P-values less than 0.05 were considered statistically significant.
Institutional review board statement
The protocol of the study was approved by the animal welfare body of The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences (Jabłonna, Poland), in accordance with the principles of the European Union and the Polish Law on Animal Protection. Reporting in the manuscript follows the recommendations in the ARRIVE guidelines.
Conclusions
To our knowledge, this is the first study in which the effect of phytobiotic mixture, on selected miRNAs expression associated with muscle physiology was evaluated in broiler chickens. This preliminary study showed that the feed additive consisting of white mustard, calamus, turmeric, and common ivy may modulate the expression of miRNAs related to muscle cell proliferation, differentiation, hypertrophy, vitality etc. Moreover, it was observed that it also may influence the oxidative stress markers and miRNAs that are also involved in antioxidant activity. However, some of the results are not clear, which suggests the need for further research, including analyses of antioxidant enzymes activity. The results suggest that this unique phytobiotic composition may be a potential tool used in broiler chicken nutrition as a myoprotectant. Modulation of miRNAs by dietary factors seems to be very promising not only as potential therapeutic tool in animal disease but also may be helpful in reducing some of myopathies that affect the final meat quality and have an impact on the economic aspect of poultry production.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Chodkowska, K. A. & Abramowicz-Pindor, P. Can eubiotic solutions be employed to reduce the use of chemotherapeutic agents in broiler chicken? In 45th Czech Poultry Conference within International Participation, WPSA & WVPA, Brno, 35–36, 15–16 October (2019).
Boscaro, V. et al. Antiproliferative, proapoptotic, antioxidant and antimicrobial effects of Sinapis nigra L. and Sinapis alba L. extracts. Molecules 23(11), 3004 (2018).
Yuan, H. et al. Mustard seeds (Sinapis alba Linn) attenuate azoxymethane-induced colon carcinogenesis. Redox Rep. 16(1), 38–44 (2011).
Li, K. S. & Wah, C. S. Antioxidant and antibacterial activity of Acorus calamus L. leaf and rhizome extracts. Jurnal Gizi Klinik Indonesia 13(4), 144–158 (2017).
Subathraa, K. & Poonguzhali, T. V. In vitro studies on antioxidant and free radical scavenging activities of aqueous extract of Acorus calamus L. Int. J. Curr. Sci. 13, 169–173 (2012).
Dinev, T., Tzanova, M., Velichkova, K., Dermendzhieva, D. & Beev, G. Antifungal and antioxidant potential of methanolic extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Appl. Sci. 11(11), 4745 (2021).
Kim, H., Han, T. H. & Lee, S. G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 122(1), 149–156 (2009).
Jain, D. K., Gupta, S., Jain, R. & Jain, N. Anti-inflammatory activity of 80% ethanolic extract of Acorus calamus Linn. leaves in albino rats. Res. J. Pharm. 3(3), 882–884 (2010).
Chauhan, S., Singh, V. & Thakur, V. Influence of herbal supplementation on dressed yield and percent organ weights of broilers during coccidiosis. Pharma Innov. J. 10, 249–252 (2021).
Nuningtyas, Y. F. & Widodo, E. Increasing antioxidant activity of quail (Cortunix-cortunix japonica) eggs with the addition of sweet flag (Acorus calamus) powder as a feed additive. IOP Conf. Ser. Earth Environ. Sci. 207(1), 012029 (2018).
Mondal, M. A. et al. Effect of dietary supplementation of turmeric (Curcuma longa) powder on the growth performance and carcass traits of broiler chicks. SAARC J. Agric. 13(1), 188–199 (2015).
Yarru, L. P. et al. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 88(12), 2620–2627 (2009).
Gowda, N. K., Ledoux, D. R., Rottinghaus, G. E., Bermudez, A. J. & Chen, Y. C. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 102(11), 1629–1634 (2009).
Wina, E. The role of saponin as feed additive for sustainable poultry production. WARTAZOA Indones. Bull. Anim. Vet. Sci. 27(3), 117–124 (2018).
Oelschlager, M. L., Rasheed, M. S. A., Smith, B. N., Rincker, M. J. & Dilger, R. N. Effects of Yucca schidigera-derived saponin supplementation during a mixed Eimeria challenge in broilers. Poult. Sci. 98(8), 3212–3222 (2019).
Bera, I. et al. Effect of dietary saponin rich soapnut (Sapindus mukorossi) shell powder on growth performance, immunity, serum biochemistry and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. 103(6), 1800–1809 (2019).
Hong, M., Wang, N., Tan, H. Y., Tsao, S. W. & Feng, Y. MicroRNAs and Chinese medicinal herbs: New possibilities in cancer therapy. Cancers 7(3), 1643–1657 (2015).
Dunislawska, A., Pietrzak, E., Wishna Kadawarage, R., Beldowska, A. & Siwek, M. Pre-hatching and post-hatching environmental factors related to epigenetic mechanisms in poultry. J. Anim. Sci. 100(1), skab370 (2022).
Chodkowska K. A. & Abramowicz-Pindor, P. Effect of phytobiotics mixture containing Hot Pepper Fruit, White Mustard Seed, Soapwort Root, Turmeric Rhizome and Thymol on miR-26a-5p, miR-30a-5p and miR-99a in a muscle tissue of chicken broiler. In 26th World’s Poultry Congress, Paris (2022).
Chodkowska, K. A., Sadkowski, T. & Ostaszewski, P. MicroRNA function in domestic animal physiology and diseases: A promising diagnostic tool for veterinary use. Med. Vet. 73, 156–165 (2017).
Chodkowska, K. A., Ciecierska, A., Majchrzak, K., Ostaszewski, P. & Sadkowski, T. Simultaneous miRNA and mRNA transcriptome profiling of differentiating equine satellite cells treated with gamma-oryzanol and exposed to hydrogen peroxide. Nutrients 10(12), 1871 (2018).
Chodkowska, K. A., Ciecierska, A., Majchrzak, K., Ostaszewski, P. & Sadkowski, T. Effect of β-hydroxy-β-methylbutyrate on miRNA expression in differentiating equine satellite cells exposed to hydrogen peroxide. Genes Nutr. 13, 1–20 (2018).
Scicchitano, B. M., Pelosi, L., Sica, G. & Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 170, 37–44 (2018).
Carvalho, L. M., Delgado, J., Madruga, M. S. & Estévez, M. Pinpointing oxidative stress behind the white striping myopathy: Depletion of antioxidant defenses, accretion of oxidized proteins and impaired proteostasis. J. Sci. Food Agric. 101(4), 1364–1371 (2021).
Abasht, B., Mutryn, M. F., Michalek, R. D. & Lee, W. R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One 11(4), e0153750 (2016).
Salami, S. A., Majoka, M. A., Saha, S., Garber, A. & Gabarrou, J. F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: Science and market. Avian Biol. Res. 8(2), 65–78 (2015).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Williams, A. H., Liu, N., van Rooij, E. & Olson, E. N. MicroRNA control of muscle development and disease. Curr. Opin. Cell Biol. 21(3), 461–469 (2009).
Chen, J. F., Callis, T. E. & Wang, D. Z. MicroRNAs and muscle disorders. J. Cell Sci. 122(Pt 1), 13–20 (2009).
Chen, J. F. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38(2), 228–233 (2006).
Cardinali, B. et al. Microrna-221 and microrna-222 modulate differentiation and maturation of skeletal muscle cells. PLoS One 4, e7607 (2009).
Zhang, J. & Luo, Z. The molecular expression differences of muscle growth in purebred and crossbred pigs. Acta Vet. Zootech. Sin. 47(12), 2370–2378 (2016).
Sharma, S. et al. Repression of miR-142 by p300 and MAPK is required for survival signalling via gp130 during adaptive hypertrophy. EMBO Mol. Med. 4(7), 617–632 (2012).
He, M. et al. MicroRNA-181a regulates the proliferation and differentiation of Hu sheep skeletal muscle satellite cells and targets the YAP1 gene. Genes 13(3), 520 (2022).
Wang, J. et al. Effects of microRNAs on skeletal muscle development. Gene 668, 107–113 (2018).
Cao, X. et al. MiR-99a-5p regulates the proliferation and differentiation of skeletal muscle satellite cells by targeting MTMR3 in chicken. Genes 11(4), 369 (2020).
Kramer, H. F. & Goodyear, L. J. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J. Appl. Physiol. 103(1), 388–395 (2007).
Wang, Y. et al. Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol. Environ. Saf. 206, 111329 (2020).
Brennan, C. M., Emerson, C. P. Jr., Owens, J. & Christoforou, N. p38 MAPKs—Roles in skeletal muscle physiology, disease mechanisms, and as potential therapeutic targets. JCI Insight 22(12), e149915 (2021).
Xu, M., Chen, X., Chen, D., Yu, B. & Huang, Z. FoxO1: A novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget 8(6), 10662–10674 (2017).
von Maltzahn, J., Chang, N. C., Bentzinger, C. F. & Rudnicki, M. A. Wnt signaling in myogenesis. Trends Cell Biol. 22(11), 602–609 (2012).
Yoon, M. S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 8, 788 (2017).
Rattis, B. A. et al. Modulation of the mTOR pathway by curcumin in the heart of septic mice. Pharmaceutics 14(11), 2277 (2022).
Shokry, A. A., El-Shiekh, R. A., Kamel, G., Bakr, A. F. & Ramadan, A. Bioactive phenolics fraction of Hedera helix L. (Common Ivy Leaf) standardized extract ameliorates LPS-induced acute lung injury in the mouse model through the inhibition of proinflammatory cytokines and oxidative stress. Heliyon 8(5), e09477 (2022).
Koliakos, G. & Hamidi Alamdari, D. Measurement of the oxidants-antioxidants balance in liquids. United States Patent Application Publication No. US 2009/0123956 A1 (USA) (2009).
Herosimczyk, A. et al. Proteome changes in ileal mucosa of young pigs resulting from different levels of native chicory inulin in the diet. J. Anim. Feed Sci. 27, 229–237 (2018).
Lepczyński, A. et al. Diet supplemented either with dried chicory root or chicory inulin significantly influence kidney and liver mineral content and antioxidative capacity in growing pigs. Animal 15, 100129 (2021).
Chodkowska, K. A. et al. Effect of phytobiotic composition on production parameters, oxidative stress markers and myokine levels in blood and pectoral muscle of broiler chickens. Animals 12(19), 2625 (2022).
Teimouri, M. et al. Inhibiting miR-27a and miR-142-5p attenuate nonalcoholic fatty liver disease by regulating Nrf2 signaling pathway. IUBMB Life 72, 361–372 (2020).
Houri, K. et al. MiR-142 induces accumulation of reactive oxygen species (ROS) by inhibiting pexophagy in aged bone marrow mesenchymal stem cells. Sci. Rep. 10, 3735 (2020).
AOAC. Official Methods of Analysis of AOAC International 18th edn. (AOAC, 2011).
Kozomara, A., Birgaoanu, M. & Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 47(1), 155–162 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4), 402–408 (2001).
Agarwal, V., Bell, G. W., Nam, J. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (2015).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.A.C.; methodology, K.A.C., M.B., A.T.; software; K.A.C.; formal analysis, K.A.C., M.B., A.T.; investigation, K.A.C.; writing—original draft preparation, K.A.C., M.B., A.T.; writing—review and editing, K.AC., M.B., A.T.; visualization, K.A.C.; supervision, K.A.C.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chodkowska, K.A., Barszcz, M. & Tuśnio, A. MicroRNA expression and oxidative stress markers in pectoral muscle of broiler chickens fed diets supplemented with phytobiotics composition. Sci Rep 14, 4413 (2024). https://doi.org/10.1038/s41598-024-54915-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54915-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.