Abstract
Microvascular invasion (MVI) is a critical risk factor for postoperative recurrence of hepatocellular carcinoma (HCC). This study aimed to firstly develop and validate nomograms based on MVI grade for predicting recurrence, especially early recurrence, and overall survival in patients with early-stage HCC after curative resection. We retrospectively reviewed the data of patients with early-stage HCC who underwent curative hepatectomy in the First Affiliated Hospital of Fujian Medical University (FHFU) and Mengchao Hepatobiliary Hospital of Fujian Medical University (MHH). Kaplan–Meier curves and Cox proportional hazards regression models were used to analyse disease-free survival (DFS) and overall survival (OS). Nomogram models were constructed on the datasets from the 70% samples of and FHFU, which were validated using bootstrap resampling with 30% samples as internal validation and data of patients from MHH as external validation. A total of 703 patients with early-stage HCC were included to create a nomogram for predicting recurrence or metastasis (DFS nomogram) and a nomogram for predicting survival (OS nomogram). The concordance indexes and calibration curves in the training and validation cohorts showed optimal agreement between the predicted and observed DFS and OS rates. The predictive accuracy was significantly better than that of the classic HCC staging systems.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is among the most frequent causes of cancer-related deaths worldwide1. Despite remarkable improvements in comprehensive HCC treatment, radical surgical resection and liver transplantation are considered the only curative treatments for patients with HCC classified as early-stage (stages 0 and A) according to the Barcelona Clinic Liver Cancer (BCLC) staging system. However, postoperative recurrence and metastasis rates of patients with early HCC vary from 50 to 70%2, resulting in poor overall survival (OS). Early recurrence after liver resection for HCC is the leading cause of death during the first 2 years3. Therefore, developing a model for predicting postoperative recurrence, especially early recurrence, for patients with early-stage HCC to guide risk stratification and treatment is urgently needed.
Microvascular invasion (MVI), a mass of cancer cells in the vascular cavity with adhesion to endothelial cells, and only visible under a microscope4, has been reported by previous studies to be an indicator of early invasive manifestation of HCC. It is a crucial independent predictive factor for early recurrence and poor OS among patients with HCC who underwent hepatectomy or received liver transplantation. Most patients with BCLC early-stage HCC with early recurrence are pathologically verified as MVI positive 5,6,7. Moreover, a previous study found that more invading tumor cells and multiple-invaded microvessels might be related to poor survival and recurrence rates4. These findings suggest that the BCLC staging system should reappraise HCC based on the presence or grade of MVI to distinguish the biological behavior of early-stage HCC.
MVI is graded according to the number of cancer cells and the distance of MVI to the tumor according to the Standard for Diagnosis and Treatment of Primary Liver Cancer8. Although predictive models for postoperative early recurrence in patients with HCC have been established, a predictive model for patients with early BCLC stage HCC patients according to the MVI grade has not been reported.
Therefore, we retrospectively investigated the clinical and histopathological characteristics of patients with early HCC after curative hepatectomy from two centers to establish a prognostic nomogram based on MVI grade to predict early recurrence and OS.
Methods
Patients and study design
The database was retrospectively derived from patients with HCC who underwent hepatectomy at the First Affiliated Hospital of Fujian Medical University (FHFU) and the Mengchao Hepatobiliary Hospital of Fujian Medical University (MMH) from March 2015 to March 2020.
The inclusion criteria for patients with HCC patients in this study were: (1) early-stage HCC (BCLC stage 0 or A) diagnosis that was confirmed by postoperative pathology; (2) Child–Pugh A or B liver function before surgery; (3) R0 surgical resection of the tumor with curative intent; (4) all patients who survived for at least 30 days after surgery; (5) no preoperative anticancer treatments that could introduce any bias; and (6) clinicopathological data and follow-up information were available. Patients with the following criteria were excluded: (1) recurrent HCC, (2) combined hepatocellular cholangiocarcinoma, (3) previous history of malignancy, and (4) age < 18 years. R0 surgical resection was defined as complete tumor resection with histopathologically tumor-free resection margins.
Nomogram models were constructed on the datasets from the FHFU, which were also validated using bootstrap resampling as internal validation, and the dataset from the MMH was used for external validation. This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Fujian Medical University and the Ethics Review Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University. Written informed consent was obtained from all subjects before the operation. All procedures were performed in accordance with the Declaration of Helsinki.
Clinical variables
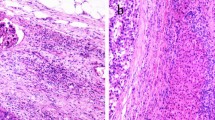
Demographic, laboratory, and HCC pathological data were collected. The laboratory tests included various tests for routine blood parameters, full sets of tests for blood clotting, full sets of tests for blood biochemistry, and hepatitis virus markers. Imaging data included, but were not limited to, the number of tumors, presence of satellite nodules, the diameter of the largest nodule, tumor capsule, and cirrhosis based on preoperative contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). The diagnosis and grade of MVI were confirmed according to the Standard for Diagnosis and Treatment of Primary Liver Cancer4 by 2 independent pathologists. In case of any doubt, the final decision was determined after MDT discussion. Briefly, the grade of MVI is defined as follows: M0: no MVI; M1: the number of MVI is < 5 and at a distance of ≤ 1 cm from the tumor; M2: the number of MVI is > 5 or at a distance of > 1 cm from the tumor (Fig. 1)4.
Follow-up
During the follow-up, serum alpha-fetoprotein (AFP) levels were measured, and ultrasonography, CT, or MRI of the chest and abdomen was done once every 2 months for the first 2 years after surgery. For patients who were free of cancer recurrence 2 years after surgery, a 6-month interval surveillance was performed. Disease-free survival (DFS) was defined as the duration from the first surgery to the first recurrence, metastasis, or death. OS was defined as the duration from the first surgery to death or the last follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Chi-squared or Fisher’s exact tests were used to assess differences in categorical variables. The Wilcoxon rank-sum test was used to compare continuous variables between groups. In this study, the cut-off values of continuous variables were established using the X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, Connecticut, United States) for widely clinical application. For DFS and OS curves during follow-up, Kaplan–Meier curves, log-rank Mantel-Cox test, and Cox proportional hazards regression analyses were used. Nomograms were generated using the rms package in R software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria)9. The predictive accuracy and discriminative ability of the nomogram were assessed using the concordance index (C-index) and calibration curves. The larger the C-index, the more accurate the prognostic prediction is. A value of P < 0.05 was considered significant. All covariates met the Cox proportional hazard assumption, as determined by the Schoenfeld residuals. Variance inflator factor was used to assess multicollinearity in all estimated models; there was no indication of multicollinearity.
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Fujian Medical University and the Ethics Review Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University. Written informed consent was obtained from all subjects before the operation. All procedures were performed in accordance with the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from every patient with HCC to perform tumor resection for analysis and publication.
Results
Characteristics of patients in study and validation cohorts
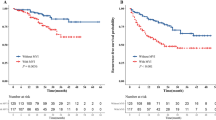
Overall, 703 patients (490 from FHFU used as training cohort, and 213 from MMH used as external validation cohort) with BCLC early-stage HCC were included. The baseline characteristics of the two cohorts are shown in Supplementary Table 1. The average age of the entire cohort was 53.7 ± 10.9 years, with a male to female ratio of 4.72:1 (580/130). The average follow-up time for all patients was 18.8 ± 10.2 months. The 8-month, 1-, 2-, and 3-year recurrence or metastasis rates were 5.8%, 8.1%, 11.8%, and 12.7%, respectively. The 8-month, 1-, 2-, and 3-year survival rates were 1.7%, 2.4%, 3.9%, and 4.2%, respectively. Among them, M1 was observed in 173 cases (24.6%), while M2 was observed in 102 cases (14.5%). Between-group differences in sex, age, operative time, follow-up time, ASA scores, laboratory, and HCC pathological data were not significant (Supplementary Table 1). The association of MVI grade relative to OS or DFS is shown in Fig. 2. The Kaplan–Meier curves of OS and DFS showed that the M2 group had significantly poorer outcomes than the M1 and M0 groups (both P < 0.001).
The univariate analysis results for DFS and OS in the study cohort are shown in Table 1. Multivariate analysis revealed that eight factors including neutrophils, alkaline phosphatase (ALP), urea, low-density lipoprotein (LDL), apolipoprotein A1 (Apo-A1), thrombin time (TT), tumor size, and MVI grade were independent prognostic factors for DFS, while six factors including TT, MVI grade, mean corpuscular haemoglobin (MCH), monocyte, prealbumin (PAB) and α-fucosidase (AFU) were prognostic factors for OS (Table 1). Therefore, these variables were included in the subsequent analysis to establish predictive models.
Establishment of nomogram model for postoperative early-relapse/OS and evaluation of its discriminability and calibration
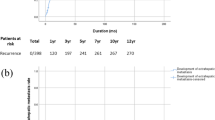
Based on the independent prognostic factors, nomograms for DFS and OS in the study cohort were established (Fig. 3). The results are shown in Supplementary Table 2. The C-index of the nomogram for DFS was 0.775 (95% confidence interval [CI] 0.720–0.830). The C-index for OS was 0.812 (95% CI 0.732–0.892). The validation showed excellent consistency between the observed and predicted 8-month, 1-, 2-and 3-year DFS, and 8-month, 1-, 2- and 3-year OS (Fig. 3); with a C-index of 0.865 (95% CI 0.806–0.924) for DFS and a C-index of 0.839 for OS (95% CI 0.675–1.00) in the internal validation cohort, and with a C-index of 0.857 (95% CI 0.763–0.951) for DFS and a C-index of 0.842 (95% CI 0.708–0.970) for OS in the external validation cohort (Supplementary Table 2). Calibration curves of internal verification and external verification with slopes closed to 1 and all p-value greater than 0.05 in the Hosmer and Lemeshow test, showed good consistency between the observed and predicted events (Fig. 4). Taken together, the nomogram models were able to accurately predict postoperative relapse and OS in patients with BCLC early-stage HCC.
Nomograms for predicting disease-free survival (DFS) and overall survival (OS) in patients with early-stage HCC after curative hepatectomy. (a) DFS; (b) OS. MCH, mean corpuscular hemoglobin; ALP, alkaline phophatase; PAB, prealbumin; AFU, α-fucosidase; Apo-A1, apolipoprotein A1; TT, thrombin time; MVI, microvascular invasion.
Calibration curves for predicting disease-free survival (DFS) and overall survival (OS) using the nomograms. (a) 8-month, 1, 2, and 3-year DFS in the training cohort; (b) 8-month, 1, 2, and 3-year DFS in the internal validation cohort; (c) 8-month, 1, 2, and 3-year DFS in the external validation cohort; (d) 8-month, 1, 2, and 3-year OS in the training cohort; (e) 8-month, 1, 2, and 3-year OS in the internal validation cohort; (f) 8-month, 1, 2, and 3-year OS in the external validation cohort.
Comparison of predictive accuracy between the nomogram models and the classical staging systems
The predictive value of the constructed model, in terms of clinical practicability, was compared with that of the 8th edition American Joint Committee on Cancer (AJCC) staging system, the BCLC staging system, the Japan Integrated Staging Score (JIS) and the Hong Kong Liver Cancer prognostic classification scheme (HKLC). The results are shown in Supplementary Table 2. In the training cohort, the C-index of the nomogram for DFS and OS was 0.775 and 0.812, respectively, which was significantly higher than the AJCC (DFS: 0.591; OS: 0.588), BCLC (DFS: 0.601; OS: 0.599), JIS (DFS: 0.589; OS: 0.592), and HKLC (DFS: 0.595; OS: 0.612) staging systems. Similarly, in the validation cohort, the C-index of the nomogram for DFS (internal cohort: 0.865; external cohort: 0.857) and OS (internal cohort: 0.839; external cohort: 0.842), was also significantly higher than the AJCC (internal cohort: 0.622, external cohort: 0.586 for DFS; and internal cohort: 0.615, external cohort: 0.578 for OS), BCLC (internal cohort: 0.602, external cohort: 0.574 for DFS; and internal cohort: 0.608, external cohort: 0.571 for OS), JIS (internal cohort: 0.606, external cohort: 0.581 for DFS; and internal cohort: 0.599, external cohort: 0.574 for OS), HKLC (internal cohort: 0.625, external cohort: 0.558 for DFS; and internal cohort: 0.619, external cohort: 0.541 for OS) staging systems. Overall, the nomogram models exhibited superior predictive accuracy to that of these authoritative staging systems for DFS and OS.
Discussion
Although patients with BCLC early-stage HCC typically have a more favorable prognosis compared to those with late-stage HCC characterized by macrovascular invasion and multiple intrahepatic metastases, a significant proportion of patients still experience recurrence and metastasis. The presence of macrovascular invasion is universally recognized as a highly influential factor in predicting poor prognosis among patients with early-stage HCC4,8,9. Moreover, recent research has demonstrated a strong correlation between the grade of macrovascular invasion and postoperative recurrence, particularly early recurrence8,10,11,12,13. Neither of the two most commonly used pathological staging systems for hepatocellular carcinoma (HCC) incorporates the presence of MVI as a criterion. Currently, there is a lack of reported predictive models utilizing the MVI grading system to identify patients with early-stage HCC who are at a high risk of recurrence or have a poor prognosis. The development of such a model would be advantageous in order to establish a system for early and continuous monitoring or prompt postoperative adjuvant therapy for HCC patients. Consequently, nomograms were developed utilizing the MVI grading system to predict recurrence and overall survival in early-stage HCC patients who underwent curative surgery. Subsequent validation demonstrated a favorable concordance between the nomogram predictions and observed outcomes in terms of predictive probability. Furthermore, our nomograms exhibited superior predictive efficacy compared to the conventional BCLC and AJCC staging systems.
The prognosis of patients with HCC is mainly affected by: (1) patient factors, such as immune function, nutritional state, liver function, and status of hepatitis virus infection; (2) tumor factors, such as tumor diameter, MVI classification, and satellite nodules; and (3) factors of treatment, in particularly adjuvant treatment after surgery. In our study, nine of the twelve risk factors associated with recurrence or OS were patient factors, including neutrophil, monocyte, ALP, PAB, MCH, Urea, LDL, Apo-A1, and TT levels, while three factors were tumor-related factors including tumor size, MVI classification, and AFU. These results indicate that the prognosis of HCC is a multifactorial and complex process.
The histopathological types and grades of MVI serve as indicators of the histopathological transformations that transpire when a cancer embolus within a vessel develops into a satellite lesion or a metastatic site. Consequently, the histopathological type of MVI can be employed as a morphological marker for assessing the biology and advancement of HCC4,14,15. The detectability rate of MVI in patients with early-stage HCC ranges from 12.4% to 33.1%, and the prognostic significance of MVI in this patient population following curative surgery is still a matter of debate16,17,18. In our study, we found that MVI was an independent risk factor associated with DFS and OS (Fig. 2, P < 0.001), with a detection rate of 39.1% (275/703). Recently, studies indicated that the tumor microenvironment in MVI-positive HCC patients was more immunosuppressive than that in MVI-negative HCC patients. This environment could promote tumor progression by activating signaling pathways that enhance tumor cell proliferation, migration, and angiogenesis including HIF-1 pathway, Wnt pathway, MAPK pathway, and Ras pathway19,20,21. Additionally, it recruits inhibitory immune cells and upregulates immune checkpoints to mediate immune escape in tumors22,23,24. Ultimately, these mechanisms jointly contribute to recurrence and metastasis in MVI-positive HCC patients after curative hepatectomy. The relationship between tumor size and patient prognosis is widely acknowledged, particularly in cases of HCC. Tumor enlargement has been consistently associated with a poor prognosis in HCC patients, leading to the establishment of cut-off values in various guidelines to predict prognosis. This is due to the non-linear nature of the relationship between tumor size and poor prognosis. For the purpose of this study, the cut-off values of 5 and 10 cm were utilized. Notably, our study revealed that tumors with a diameter exceeding 10 cm were identified as a significant risk factor for recurrence. Interestingly, despite AFP being widely recognized as a conventional clinical marker for diagnosing and prognosticating patients with HCC, our study found that it did not independently correlate with prognosis in early-stage HCC following curative hepatectomy. This observation may be attributed to the limited sensitivity of AFP in predicting the prognosis of early-stage HCC. Previous reports have indicated that AFP remains undetectable in approximately 30–35% of individuals with primary HCC, while elevated AFP levels can also be observed in individuals with normal health25. It is noteworthy that AFU emerged as a significantly independent factor associated with OS in early-stage HCC. Existing literature reports AFU as a specific marker for HCC, demonstrating superior sensitivity and specificity compared to AFP in the diagnosis of HCC. Particularly, AFU exhibits high accuracy in distinguishing AFP-negative cases and early-stage HCC. Consequently, the dynamic monitoring of AFU holds immense importance in the diagnosis and prognosis of early-stage HCC26. Besides that, ALP is also a valuable predictor of early-stage HCC patients’ DFS after curative hepatectomy in our nomogram. ALP is an important indicator of liver function and highly associated with some hepatic diseases including hepatitis, cirrhosis and HCC27. It has recently been reported that ALP levels could be used to monitor and predict recurrence and metastasis in HCC patients28. Previous studies have found that high level of ALP was related to tumor cell proliferation and epithelial-mesenchymal transition (EMT)29,30. Besides, the liver is highly susceptible to oxidative stress-induced damage, with ALP serving as a dependable and sensitive marker for assessing oxidative stress. The detrimental effects of oxidative stress on hepatocytes encompass lipid, protein, and DNA impairment, ultimately leading to liver injury31. Consequently, these processes can eventually promote the metastasis and recurrence of HCC.
Previous research has indicated a correlation between immune function and nutritional status and the prognosis of patients diagnosed with HCC32,33,34,35. Within our nomogram models, we have identified several influential immune and nutritional indices, namely neutrophil, monocyte, MCH, PAB, and urea, which can effectively predict prognosis. Notably, patients with low levels of neutrophils and urea, indicative of inadequate protein intake, exhibit a poorer prognosis. The tumor microenvironment is a crucial factor in the development of tumors. The immune and nutritional status, as components of the tumor microcirculation, undoubtedly impact the prognosis of patients with HCC. A growing body of evidence indicates a significant association between fundamental nutritional status, systemic inflammation, and the long-term prognosis of individuals diagnosed with cancer36,37,38,39. The presence of malnutrition and compromised immune function not only impacts the efficacy of treatment in individuals diagnosed with malignant tumors, but also increases the susceptibility of patients with HCC to relapse and metastasis36.
In recent times, metabolic disorders, specifically lipid metabolism disorders, have gained prominence as a crucial microenvironment contributing to the development of HCC40,41. In this study, LDL and Apo-A1, serving as indicators of hepatic lipid metabolism, emerged as significant prognostic factors for early-stage HCC. It is well-established that alterations in liver lipid metabolism are intricately linked to the onset of liver cancer, and it is plausible that non-alcoholic fatty liver disease may be recognized as a principal etiological factor for primary liver cancer in the future42. Furthermore, prior research has demonstrated that lipid metabolism disorders can facilitate the proliferation of tumor cells by impeding the apoptosis of hepatocellular carcinoma cells, consequently leading to an unfavorable prognosis43.
However, there remains scope for additional enhancements. Initially, our model predominantly relies on datasets obtained retrospectively from two Chinese institutions. Despite the satisfactory performance of the models, certain indices were not consistently gathered in certain countries, such as South Africa. The inclusion of additional cohorts from other institutions across regions may improve the predictive accuracy and universality of models. Furthermore, our models would benefit from external validation using a completely unseen and more diversity dataset, for a more accurate description of models performance. Second, though the sample size in this study is adequate, a larger sample size in conjunction with meaningful information including postoperative adjuvant treatment collected in the future may improve the accuracy of our results. Third, although, HBV infection could strongly influence on prognosis in HCC patients, the HBV-related HCC accounted for most of HCC patients in our country, which leaded it limited influence in our models. Cohort with different eitologies, multi-population and across regions will be included in the future to determine the influence of HBV infection on the prognosis in early-stage HCC patients after curative resection.
In summary, we developed and validated nomograms for predicting recurrence, especially early recurrence, and OS in patients with early-stage HCC after curative surgery. The predictive performances were superior to the common typical HCC staging systems, and they can establish patients with a high risk of recurrence or poor prognosis to provide comprehensive and accurate guidance of postoperative monitoring and adjuvant therapy after surgery, such as developing the optimal frequency of follow-up examination and individualized postoperative treatment strategies, thereby resulting in improved clinical outcomes in this group of patients.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona Clinic Liver Cancer
- OS:
-
Overall survival
- MVI:
-
Microvascular invasion
- FHFU:
-
The First Affiliated Hospital of Fujian Medical University
- MMH:
-
Mengchao Hepatobiliary Hospital of Fujian Medical University
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- AFP:
-
α-Fetoprotein
- DFS:
-
Disease-free survival
- C-index:
-
Concordance index
- ALP:
-
Alkaline phosphatase
- LDL:
-
Low-density lipoprotein
- Apo-A1:
-
Apolipoprotein A1
- TT:
-
Thrombin time
- MCH:
-
Mean corpuscular haemoglobin
- PAB:
-
Prealbumin
- AFU:
-
α-Fucosidase
- CI:
-
Confidence interval
- AJCC:
-
American Joint Committee on Cancer staging system
- JIS:
-
Japan Integrated Staging Score
- HKLC:
-
Hong Kong Liver Cancer prognostic classification scheme
- HBV:
-
Hepatitis B virus
References
Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48. https://doi.org/10.3322/caac.21763 (2023).
Dhir, M. et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann. Surg. 263, 1112–1125. https://doi.org/10.1097/sla.0000000000001556 (2016).
Poon, R. T. et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89, 500–507 (2000).
Janssen, K. J. et al. Missing covariate data in medical research: To impute is better than to ignore. J. Clin. Epidemiol. 63, 721–727. https://doi.org/10.1016/j.jclinepi.2009.12.008 (2010).
Adam, R. et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: Does size define the best oncological strategy?. Ann. Surg. 256, 883–891. https://doi.org/10.1097/SLA.0b013e318273bad0 (2012).
Harrell, F. E., Lee, K. L. & Mark, D. B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15, 361–387. https://doi.org/10.1002/(sici)1097-0258(19960229)15:4%3c361::Aid-sim168%3e3.0.Co;2-4 (1996).
Zhou, J. et al. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer 12, 405–444. https://doi.org/10.1159/000530495 (2023).
Iguchi, T. et al. New pathologic stratification of microvascular invasion in hepatocellular carcinoma: Predicting prognosis after living-donor liver transplantation. Transplantation 99, 1236–1242. https://doi.org/10.1097/tp.0000000000000489 (2015).
Tsilimigras, D. I. et al. Utilizing machine learning for pre- and postoperative assessment of patients undergoing resection for BCLC-0, A and B hepatocellular carcinoma: Implications for resection beyond the BCLC guidelines. Ann. Surg. Oncol. 27, 866–874. https://doi.org/10.1245/s10434-019-08025-z (2020).
Banerjee, S. et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 62, 792–800. https://doi.org/10.1002/hep.27877 (2015).
Chan, A. W. H. et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 69, 1284–1293. https://doi.org/10.1016/j.jhep.2018.08.027 (2018).
Erstad, D. J. & Tanabe, K. K. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann. Surg. Oncol. 26, 1474–1493. https://doi.org/10.1245/s10434-019-07227-9 (2019).
Roayaie, S. et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137, 850–855. https://doi.org/10.1053/j.gastro.2009.06.003 (2009).
Feng, L. H. et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 143, 293–303. https://doi.org/10.1007/s00432-016-2286-1 (2017).
Zhao, H. et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 8, 5474–5486. https://doi.org/10.18632/oncotarget.12547 (2017).
Huang, C. et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer 17, 58. https://doi.org/10.1186/s12885-017-3050-x (2017).
Shindoh, J. et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: Reappraisal of the staging system for solitary tumors. Ann. Surg. Oncol. 20, 1223–1229. https://doi.org/10.1245/s10434-012-2739-y (2013).
Wang, H., Wu, M. C. & Cong, W. M. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol. Res. 49, 344–354. https://doi.org/10.1111/hepr.13241 (2019).
Mavragani, C. P. et al. Type I and II interferon signatures can predict the response to anti-TNF agents in inflammatory bowel disease patients: Involvement of the microbiota. Inflamm. Bowel Dis. 26, 1543–1553. https://doi.org/10.1093/ibd/izaa216 (2020).
Cai, M.A.-O. et al. T-cell exhaustion interrelates with immune cytolytic activity to shape the inflamed tumor microenvironment. J. Pathol. 251, 147–159. https://doi.org/10.1002/path.5435 (2020).
Dang, H. et al. LINC01419 promotes cell proliferation and metastasis in hepatocellular carcinoma by enhancing NDRG1 promoter activity. Cell. Oncol. (Dordr.) 43, 931–947. https://doi.org/10.1007/s13402-020-00540-6 (2020).
Lim, C. J. et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 68, 916–927. https://doi.org/10.1136/gutjnl-2018-316510 (2019).
Kurebayashi, Y. et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology https://doi.org/10.1002/hep.29904 (2018).
Yang, J. et al. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: A systematic review and meta-analysis. Cancer Cell. Int. 21, 92. https://doi.org/10.1186/s12935-021-01790-6 (2021).
Li, J. et al. A novel prognostic index-neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio (NγLR) predicts outcome for patients with hepatocellular carcinoma. Sci. Rep. 7, 9229. https://doi.org/10.1038/s41598-017-09696-y (2017).
Wang, K. et al. Alpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: A large-scale, long-term study. Br J Cancer 110, 1811–1819. https://doi.org/10.1038/bjc.2014.102 (2014).
Wu, S. J. et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int. J. Surg. 36, 143–151. https://doi.org/10.1016/j.ijsu.2016.10.033 (2016).
Yoon, J. H. et al. Early extrahepatic recurrence as a pivotal factor for survival after hepatocellular carcinoma resection: A 15-year observational study. World J. Gastroenterol. 28, 5351–5363. https://doi.org/10.3748/wjg.v28.i36.5351 (2022).
Yamamoto, K., Awogi, T., Okuyama, K. F. & Takahashi, N. 17Nuclear localization of alkaline phosphatase in cultured human cancer cells. Med. Electron Microsc. 36, 47–51. https://doi.org/10.1007/s007950300006 (2003).
Iwadate, Y., Matsutani, T., Hirono, S., Shinozaki, N. & Saeki, N. 18Transforming growth factor-β and stem cell markers are highly expressed around necrotic areas in glioblastoma. J. Neurooncol. 129, 101–107. https://doi.org/10.1007/s11060-016-2145-6 (2016).
Ping, L.A.-O. et al. 19Systematic oxidative stress indexes associated with the prognosis in patients with T lymphoblastic lymphoma/leukemia. Oxid. Med. Cell. Longev. 2022, 2679154. https://doi.org/10.1155/2022/2679154 (2022).
Fan, W. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One 10, e0119312. https://doi.org/10.1371/journal.pone.0119312 (2015).
Goh, B. K. et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (≥10 cm) hepatocellular carcinoma. J. Surg. Oncol. 113, 621–627. https://doi.org/10.1002/jso.24197 (2016).
Liao, W. et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl. Oncol. 7, 248–255. https://doi.org/10.1016/j.tranon.2014.02.011 (2014).
Lin, Z. X. et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J. Gastroenterol. 21, 10898–10906. https://doi.org/10.3748/wjg.v21.i38.10898 (2015).
Huang, P. Y. et al. Predictive effects of inflammatory scores in patients with BCLC 0-A hepatocellular carcinoma after hepatectomy. J. Clin. Med. https://doi.org/10.3390/jcm8101676 (2019).
Galizia, G. et al. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Ann. Ital. Chir. 90, 404–416 (2019).
Kosuga, T. et al. Value of prognostic nutritional index as a predictor of lymph node metastasis in gastric cancer. Anticancer Res. 39, 6843–6849. https://doi.org/10.21873/anticanres.13901 (2019).
Bai, X. & Feng, L. Correlation between prognostic nutritional index, glasgow prognostic score, systemic inflammatory response, and TNM staging in colorectal cancer patients. Nutr. Cancer 72, 1170–1177. https://doi.org/10.1080/01635581.2019.1675725 (2020).
Chen, K. et al. Advancing the understanding of NAFLD to hepatocellular carcinoma development: From experimental models to humans. Biochim. Biophys. Acta Rev. Cancer 1871, 117–125. https://doi.org/10.1016/j.bbcan.2018.11.005 (2019).
Shi, C. et al. Acetaminophen aggravates fat accumulation in NAFLD by inhibiting autophagy via the AMPK/mTOR pathway. Eur. J. Pharmacol. 850, 15–22. https://doi.org/10.1016/j.ejphar.2019.02.005 (2019).
Dou, X. et al. Glutathione disulfide sensitizes hepatocytes to TNFα-mediated cytotoxicity via IKK-β S-glutathionylation: A potential mechanism underlying non-alcoholic fatty liver disease. Exp. Mol. Med. 50, 1–16. https://doi.org/10.1038/s12276-017-0013-x (2018).
Bai, Y. et al. ApoM is an important potential protective factor in the pathogenesis of primary liver cancer. J. Cancer 12, 4661–4671. https://doi.org/10.7150/jca.53115 (2021).
Acknowledgements
This work was supported by the Talent programs granted from The First Affiliated Hospital of Fujian Medical University (Grant number YJRC3600), Joint Funds for the innovation of Science and Technology, Fujian Province (Grant number 2020Y9127) and the National Natural Science Youth Foundation of China (Grant number 82303728) for data collection and analysis.
Author information
Authors and Affiliations
Contributions
Z.J.F., G.G.X. and C.H.K. made the conception and design. Y.H.H., and Y.L.F. developed the methodology. Y.H.H., L.F.Z., S.Y.J. and Z.A.X. collected the data. C.H.K., Y.H.H. and Y.L.F. analysed and interpreted the data. Z.J.F., G.G.X. and C.H.K. wrote, reviewed, and/or revised the manuscript. Z.J.F. and G.G.X. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, H., Ye, H., Ye, L. et al. Novel nomograms based on microvascular invasion grade for early-stage hepatocellular carcinoma after curative hepatectomy. Sci Rep 14, 3470 (2024). https://doi.org/10.1038/s41598-024-54260-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54260-0
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.