Abstract
This study aims to evaluate the impact of percutaneous pedicle screw fixation (PPSF) and open pedicle screw fixation (OPSF) on the postoperative paraspinal muscle fat infiltration (FI) rate in patients with thoracolumbar fractures through magnetic resonance imaging (MRI), and explore the association between paraspinal muscle FI rate and regional kyphosis angle. We retrospectively analyzed clinical data from 35 patients who underwent either PPSF or OPSF for thoracolumbar fractures, examining data at preoperative, 1-month postoperative, and 9-months postoperative time points, which included Visual Analog Scale (VAS), Oswestry Disability Index (ODI), and regional kyphosis angle. We obtained preoperative and 9-month postoperative paraspinal muscle FI rates using T2-weighted MRI images and ImageJ software. We analyzed the correlation of FI rates with VAS, ODI, as well as the correction loss percentage of regional kyphosis angle. The analysis revealed a positive correlation between postoperative FI rate increase and correction loss percentage of regional kyphosis angle (r = 0.696, p < 0.001). The increase in paraspinal muscle FI rate was positively correlated with 9-month postoperative ODI (r = 0.763, p < 0.001). These findings indicate that an increase in postoperative paraspinal muscle FI rate may result in more significant correction loss of regional kyphosis angle and can lead to increased functional impairment in patients.
Similar content being viewed by others
Introduction
Compared to open pedicle screw fixation (OPSF) surgery, minimally invasive percutaneous pedicle screw fixation (PPSF) surgery for treating thoracolumbar fractures has been extensively proven to offer benefits1,2. One of the most widely acknowledged benefits is the reduction in damage to the paraspinal muscles. Evidence in literature indicates that when compared to open methods, minimally invasive surgery causes less harm to the paraspinal muscles, which play a crucial role in maintaining spinal stability and functionality3.
Spinal kyphotic deformity is a common complication of thoracolumbar fractures and can significantly impact patients' daily lives4. The recognition of the role of paraspinal muscles in spinal stability and degenerative changes is growing, such as Zhang's5 discovery that paraspinal muscle degeneration predicts Proximal Junctional Kyphosis and Katzman's6 observations about the potential impact of changes in paraspinal muscle composition on kyphosis. Patients with thoracolumbar fractures exhibited more prominent preoperative sagittal plane deformities, they were consequently more inclined to experience postoperative sagittal plane changes7. Literature indicates that the percutaneous approach is associated with less Cobb angle correction loss compared to the open approach8. Research also indicates that low back pain (LBP) and disability are associated with atrophy of multifidus muscles in the lumbar spine and increased FI9.
However, despite the numerous studies conducted on the role of paraspinal muscles, there is a lack of research focused on examining the relationship between changes in the FI rate at the level of the fractured vertebra and the regional kyphosis angle in postoperative populations with thoracolumbar fractures. We compared paraspinal muscle FI rate, VAS, ODI, and regional kyphosis angle between OPSF and PPSF groups at various postoperative time points to assess the impact of different surgical approaches. Subsequently, correlation analysis was employed to determine the relationship between FI rate and patients' VAS, ODI, and regional kyphosis angle. Our focus is on understanding the role of paraspinal muscle FI rate in postoperative regional kyphosis angle maintenance and its impact on pain and functional impairment in thoracolumbar spine patients. Through these insights, we can provide more precise guidance for clinical surgical decisions and formulate more tailored postoperative rehabilitation plans for patients. This will ultimately result in a decrease in functional disabilities among patients and a significant improvement in their quality of life.
Materials and methods
Patients
Approval from the ethics committee of our local ethics committee (Ethics Committee of Wuxi Hospital of Traditional Chinese Medicine.) (Approval number: 2023LUN-002-01) was obtained for this study design. The present study was performed in accordance with the contemporary amendments of the Declaration of Helsinki and within an appropriate ethical framework. The need for written informed consent from patients was waived by the Ethics Committee of Wuxi Hospital of Traditional Chinese Medicine because this was a retrospective study. In this study, we conducted observations on 35 thoracolumbar fracture patients who underwent OPSF or PPSF surgery at our institution from October 2018 to June 2022. According to AO Spine classification the inclusion criteria for our study were patients with T11-L3 single-segment type A fractures who had undergone either OPSF surgery or PPSF surgery, were between the ages of 18 and 65, and had complete follow-up and imaging data at preoperative, postoperative 1 month, and postoperative 9 months. Patients with concomitant nerve injury, scoliosis, non-traumatic fractures, a previous history of thoracic or lumbar spine surgery, or muscle diseases were excluded from the study. Both OPSF and PPSF can be applicable to type A thoracolumbar fractures. The medical team in the ward held a meeting to discuss and determine the surgical approach for the patient.
Surgical technique
OPSF: After successful anesthesia, the patient is placed in the prone position. We performed standard disinfection and draping. Next, we made an incision along the central midline of the spinous processes, dissecting the subcutaneous tissues. Following this, a vertical incision was made approximately 1.5 cm lateral to the spinous processes. Muscular dissection and detachment were carried out from the spinous process and lamina using bipolar electrocautery, allowing visualization of the lower lamina and the outer border of the facet joint. Subsequently, we placed pedicle screws on either side of the adjacent vertebrae and used a connecting rod to support and restore the position of the injured vertebra. Finally, we securely fastened the fixation nuts to complete the surgery.
PPSF: After successful anesthesia, the patient is placed in a prone position, and routine disinfection and draping are performed. Firstly, the C-arm machine is used to locate the pedicle root shadow of the adjacent two vertebral bodies of the injured vertebra. Then, approximately 2.5 cm incisions are made along the marked positions of the pedicle root shadows, and the skin and fascia are incised. The puncture needle is inserted into the pedicle roots of the adjacent two vertebral bodies, about 0.5–0.8 cm from the posterior edge of the vertebral body, and the guide wire is inserted. Confirmation of the correct position of the puncture needle and guide wire is achieved under fluoroscopy. After satisfactory placement of the guide wire, the pedicle root screws are threaded through the guide wire, and four screws are smoothly inserted into the pedicle roots. Then, two connecting rods are inserted from the incision from top to bottom, and the locking nuts are tightened.
Imaging assessments
We collected preoperative, 1-month postoperatively, and 9-month postoperative lateral X-ray images from our hospital's imaging system, and measured their regional kyphosis angle. The regional kyphosis angle was determined by calculating the angle between the tangent to the upper endplate of the vertebra overlying the fracture and the tangent to the lower endplate of the vertebra underneath the injured vertebra. This measurement method has shown strong reliability and is endorsed by the Spine Trauma Group Study in the United States as a dependable approach10,11. To better represent the postoperative change in regional kyphosis angle, we calculated the correction loss percentage of regional kyphosis angle from 1 to 9 months postoperatively, i.e., (9-mo post angles—1-mo post angles)/1-mo angles × 100%. This is because the larger the preoperative regional kyphosis angle, the greater the regional kyphosis angle correction, and subsequently, a higher susceptibility to postoperative regional kyphosis angle loss7 (Fig. 1). We also collected preoperative and 9-month postoperative MRI (1.5-T Philips Achieva, The Netherlands, FOV: 180 mm, slice thickness: 4 mm, slice gap: 1 mm) images of the patients. Specifically, we focused on the fat infiltration rate of the paraspinal muscles, including the multifidus (MF) and erector spinae (ES) muscles. We measured the paraspinal muscle FI rate at the level of the injured vertebral body and the adjacent vertebral levels. The FI rate was measured on both sides, resulting in a total of six locations (Fig. 2a). T2-weighted cross-sectional MRI images were imported into Image J software, where the regions of interest (ROI) for paraspinal muscles were manually delineated (Fig. 2b). In this analysis, red areas represented fat tissue, and any pixel with a grey-scale value > 120 in Image J was considered as fat tissue in the paraspinal muscles12,13,14 (Fig. 2c). The fat infiltration rate is defined as the proportion of the red area to the ROI. The increase in FI rate from preoperative to 9 months postoperative was represented using Δ FI rate. To ensure measurement accuracy, we calculated the average values for these six locations. Two examiners independently assessed the regional kyphosis angle and fat infiltration rate. To determine the reliability of their observations, interclass correlation coefficients (ICC) were employed using a two-way mixed effects model.
Regional kyphosis angle examples in two patient groups from different time periods. (a–c) represent the preoperative, 1-month postoperative, and 9-month postoperative angles for a patient in the PPSF group. The correction loss percentage of regional kyphosis angle for this patient was 0% [(7–7)/7*100%]. (d–f) depict the corresponding angles for a patient in the OPSF group. The correction loss percentage of regional kyphosis angle for this patient was 38.46% [(18–13)/13*100%].
Clinical outcome assessments
We collected the patients' VAS and ODI scores preoperatively, at 1 month postoperatively, and at 9 months postoperatively.
Statistical analysis
Data were analyzed using IBM SPSS (Version 25.0, SPSS, Inc., and Chicago, IL) statistical software. The normality of all data was assessed using the Kolmogorov–Smirnov test. Age, BMI, and VAS scores conformed to a normal distribution, while ODI and regional kyphosis angle exhibited distributions similar to normal. Descriptive statistics for these parameters were presented using mean and one standard deviation (SD). While for FI rate, median and interquartile range were utilized. We compared the OPSF and PPSF groups using the t-test for age and BMI, as well as Fisher's exact probability test for sex, fracture level, and fracture classification. We conducted a mixed ANOVA to assess differences in VAS, ODI, and the regional kyphosis angle between the OPSF and PPSF groups over time, followed by Bonferroni post-hoc tests for pairwise comparisons. Pairwise comparisons of the FI rates within each group before and after surgery were conducted using the Wilcoxon signed-rank test to assess differences. Differences in Δ FI rate and the correction loss percentage of the regional kyphosis angle between the two patient groups were evaluated using the Mann–Whitney U test. Use Spearman correlation coefficients to analyze the relationships between preoperative FI rate, 9-month post FI rate, and Δ FI rate with 9-month post VAS, ODI, and correction loss percentage of regional kyphosis angle for all patients. The Spearman correlation coefficient was understood as follows: less than 0.3 indicated no correlation; between 0.3 and 0.5 signified a weak correlation; a correlation between 0.5 and 0.7 was considered strong; 0.7 to 0.9 indicated a very strong correlation; and anything above 0.9 was deemed excellent. For all tests, p values < 0.05 were considered significant.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Wuxi Hospital of Traditional Chinese Medicine. This study has obtained informed consent from all participants.
Results
Patient characteristics
A total of 35 patients (13 male, 22 female; aged 31–65 years) were included, with 15 in the OPSF group and 20 in the PPSF group. Patient demographics are presented in Table 1.
Comparison of VAS
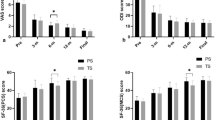
The analysis revealed a significant main effect of surgical type, F (1, 33) = 44.09, p < 0.001, partial η2 = 0.572, indicating differences in VAS scores between the two surgical procedures. Additionally, a significant main effect of assessment time was observed, with Greenhouse–Geisser adjusted F (1.43, 47.27) = 611.93, p < 0.001, partial η2 = 0.949, suggesting variations in VAS scores across different assessment times. The interaction effect between surgical type and time was not significant, as evidenced by Greenhouse–Geisser adjusted F (1.43, 47.27) = 1.70, p = 0.191, partial η2 = 0.049. Further scrutiny with Bonferroni multiple comparisons revealed that there were no significant differences in preoperative and 9-month postoperative VAS scores between the two patient groups. However, at 1 month postoperatively, the VAS scores in the PPSF group were significantly lower than those in the OPSF group, p < 0.001 (Table 2).
Comparison of ODI
The analysis revealed a significant main effect of surgical procedure type, F (1, 33) = 22.08, p < 0.001, partial η2 = 0.401, the ODI scores of patients in the PPSF group were significantly lower than those in the OPSF group. Additionally, a significant main effect of assessment time was observed, with Sphericity Assumed F (2, 66) = 12,333.19, p < 0.001, and partial η2 = 0.997, suggesting variations in ODI scores across different assessment times. The interaction effect between surgical procedure type and time was found to be significant, as evidenced by Sphericity Assumed F (2, 66) = 11.53, p < 0.001, partial η2 = 0.259. This indicates a significant interaction effect between surgical procedure type and assessment time on ODI scores. The results of the simple effects tests indicated that there were no significant differences in patients' ODI scores between the two groups preoperatively. However, at 1-month and 9-month postoperative assessments, the ODI scores of patients in the PPSF group were significantly lower than those in the OPSF group, p < 0.001 (Table 2).
Comparison of regional kyphosis angle
The main effect of group was not significant, F (1, 33) = 0.216, p = 0.645, partial η2 = 0.007, indicating no substantial differences in regional kyphosis angle between the OPSF and PPSF groups. However, a significant main effect of assessment time was observed, with Greenhouse–Geisser adjusted F (1.27, 41.89) = 44.04, p < 0.001, partial η2 = 0.572, suggesting notable variations in regional kyphosis angle across different assessment times. The interaction effect between surgical type and time was found to be significant, as evidenced by Greenhouse–Geisser adjusted F (1.27, 41.89) = 4.00, p = 0.023, partial η2 = 0.108. While the results of the simple effects tests indicated no significant differences in regional kyphosis angle between the two groups of patients at preoperative, 1-month postoperative, and 9-month postoperative assessments. Further scrutiny with Bonferroni multiple comparisons revealed that the regional kyphosis angle in both groups of patients at 1 month and 9 months after surgery was significantly smaller than the preoperative angle, p < 0.001. Additionally, in the PPSF group, there was no significant difference in the regional kyphosis angle between 9 months and 1 month after surgery. In contrast, in the OPSF group, the regional kyphosis angle at 9 months after surgery was significantly larger than at 1 month after surgery, p < 0.001 (Table 2). The OPSF group exhibits a significantly greater correction loss percentage of the regional kyphosis angle compared to the PPSF group, p < 0.001 (Table 3).
Comparison of FI rate
Both groups of patients had significantly higher FI rates at 9 months postoperatively compared to preoperative (p < 0.001) (Fig. 3). However, the Δ FI rate in the OPSF group was significantly greater than that in the PPSF group (p < 0.001) (Table 3).
Spearman correlation analysis
The results of the correlation analysis indicate that there was no significant correlation between preoperative FI rate and 9-mo post VAS. 9-mo post FI displayed a weak positive correlation with 9-mo post VAS (r = 0.367, p = 0.030), and Δ FI rate also exhibited a weak positive correlation with 9-mo post VAS (r = 0.335, p = 0.049). The correction loss percentage of regional kyphosis angle is negatively correlated with the preoperative paraspinal muscle FI rate (r = -0.349, p = 0.040), positively correlated with the Δ FI rate (r = 0.696, p < 0.001), and unrelated to the 9-mo post FI. The ODI scores at 9 months postoperatively showed no correlation with the preoperative FI rate or the FI rate at 9 months postoperatively. However, it does exhibit a positive correlation with the degree of increase in FI rate (r = 0.763, p < 0.001) (Table 4).
ICC results
Inter-observer reliability showed excellent agreement, ICC (95% confidence interval)—the regional kyphosis angle: 0.984 (0.976, 0.989) and FI rate: 0.993 (0.988, 0.995).
Discussion
A plethora of studies suggests that traditional open surgery is more likely to induce paraspinal muscle degeneration and atrophy. Tsutsumimoto et al.15 propose that in traditional open surgery, continuous traction on the paraspinal muscles raises the muscle pressure and impacts blood perfusion in paraspinal muscle capillaries. Yamamoto et al. 's16 study discovered that rats in the open surgical group, as compared to the sham surgery group, exhibited a reduced density of blood vessel distribution in the paraspinal muscles. This decrease in blood vessel density contributes to ischemic changes that exacerbate paraspinal muscle atrophy and degeneration. In the sham group, an incision was made in the skin and fascia lumbodorsalis at the midline. In the surgical group, paraspinal muscles were completely detached from the vertebrae, following the standard surgical procedure under anesthesia. Hu17 found that the splitting approach in open surgery remains a significant factor leading to multifidus muscle injury and atrophy during surgeries involving the posterior lumbar spine. Denervation and disuse may be important factors in multifidus muscle atrophy in the splitting approach, and this situation is more pronounced in conventional posterior open surgery. Compared with minimally invasive surgery, open surgery also leads to more severe paraspinal muscle edema, inflammation, and necrosis9,18. This provides a good basis for the formation of adipose tissue17. In open surgeries, the more frequent and extensive use of cautery devices may increase the likelihood of damaging the posterior branches of spinal nerves, thereby exacerbating paraspinal muscle atrophy19. Our results also showed a significantly smaller increase in the rate of paraspinal muscle FI in the PPSF group than in the OPSF group nine months after the procedure.
The relationship between paraspinal muscle FI and LBP is currently a subject of controversy. Many studies have indicated an association between an increase in the rate of paraspinal muscle FI and the occurrence of LBP20,21. Nevertheless, numerous studies have challenged the belief that an association exists between an increase in paraspinal muscle FI and LBP22,23. The correlation analysis results reveal a weak positive correlation between the postoperative 9-month paraspinal muscle FI rate and the postoperative VAS score. Additionally, Δ FI rate also exhibits a weak positive correlation with the postoperative 9-month VAS score. Despite these findings, it's crucial to recognize that, due to the inherent limitations of retrospective research, we cannot definitively conclude that an increase in paraspinal muscle fat infiltration directly leads to a higher likelihood of developing LBP. In light of this, it's essential to consider the broader context of factors influencing lower back pain. Hiyama et al.24 conducted a comparative investigation, examining short-term clinical outcomes in patients with degenerative spondylolisthesis and stenosis, focusing on the differences between extreme lateral interbody fusion and minimally invasive surgery-transforaminal interbody fusion. Their research suggests that minimally invasive surgery-transforaminal interbody fusion may lead to a more substantial improvement in lower back pain compared to extreme lateral interbody fusion. This difference in outcomes could be linked to variations in the invasiveness of the procedures, particularly their impact on posterior support elements such as facet joints, lamina, and paraspinal muscles. However, it's important to emphasize that the causes of LBP are multifaceted and diverse24. Factors such as patients' psychological backgrounds, spinal alignment, and medical histories can introduce significant biases and influence the development of LBP. Therefore, a comprehensive understanding of LBP must consider these complex interactions and individual variations.
The MF and ES muscles play essential roles in maintaining both the dynamic and static balance of the spine by providing strength and stability25. In their study on how skeletal muscle mass affects spine alignment in patients with spinal degenerative disease, Hiyama et al. found that skeletal muscle mass could be a significant factor influencing the posterior inclination of the pelvis in symptomatic spinal patients26. However, surgical procedures can cause fibrotic changes in the paraspinal muscles, leading to muscle stiffness and biomechanical alterations16. After surgery, there is a projected decrease in trunk extension strength by approximately 23%. Additionally, there is a reduction in multifidus fascicle force ranging from approximately 21% to 40% during flexion tasks and approximately 14% to 35% during upright tasks27. Biomechanical modeling further suggests that heightened muscle stiffness could lead to a more than 500% increase in compressive loads on the spine, thereby increasing the likelihood of spinal deformities28. Engaging in exercises targeting the paraspinal muscles can aid in slowing down the natural progression of spinal kyphosis29. All these pieces of evidence collectively emphasize the significance of paraspinal muscles in maintaining the sagittal balance of the spine. Furthermore, evidence indicates that it is possible to predict postoperative clinical outcomes and complications based on the preoperative morphology of the paraspinal muscles30,31,32.Our research findings similarly demonstrate a negative correlation between the preoperative FI rate and the correction loss percentage of regional kyphosis angle for the patients. We observed a positive correlation between the increase in the ΔFI rate and the correction loss percentage of regional kyphosis angle. However, no correlation was observed between the postoperative 9-month FI rate and the correction loss percentage of regional kyphosis angle. The correlation analysis between the FI rate and the postoperative 9-month ODI score yielded the same results. This is a thought-provoking issue. It seems that, in comparison to the postoperative paraspinal muscle FI rate alone, the extent of increase in postoperative FI rate may have a more significant impact on both the regional kyphosis angle and ODI score. Furthermore, these findings underscore the importance of protecting paraspinal muscles during surgery and minimizing damage to paraspinal muscles and tissues whenever possible. While this study solely observed changes in paraspinal muscle FI rate from preoperative to 9 months postoperative, existing literature indicates that the impact of surgical trauma to the paraspinal muscles persists. For instance, in a three-year patient follow-up, Lv et al.33 discovered that minimally invasive transforaminal lumbar interbody fusion provides benefits in preventing paraspinal muscle atrophy when compared to conventional transforaminal lumbar interbody fusion. It can be expected that this situation is even more pronounced in surgeries for patients with greater surgical trauma, as the removal of internal fixation devices can cause secondary harm to paraspinal muscles. Therefore, for patients undergoing surgeries with higher trauma, enhancing patient education is essential. Providing guidance on appropriate exercises targeting the paraspinal muscles, such as supine pelvic tilts to promote lumbar flexion/extension and supine bridging, can significantly contribute to the improvement of postoperative pain and functional impairments34. Therefore, when a posterior approach is not required for decompression or repositioning, we can consider an anterior approach surgery35.
The study has several noteworthy limitations. Firstly, the method used for assessing FI in this study, using T2 images, the accuracy is relatively low, with potential measurement errors arising from tissue edema or inflammation. A more precise approach, such as Chemical-shift MRI, which can generate water-only and fat-only images from dual-echo and/or multi-echo acquisitions, allowing for the quantification of both extramyocellular lipids and intramyocellular lipids36, should be considered for future investigations. Secondly, the study's relatively small sample size could potentially affect the reliability of our findings. Third, the observational period was limited to 9 months postoperatively. A longer follow-up would provide a more comprehensive understanding of the effects on sagittal spinal balance. It can be anticipated that after the removal of internal fixation, the role of paraspinal muscles in stabilizing the spine becomes more prominent. Therefore, based on this study, our research team will continue to investigate the long-term effects of postoperative paraspinal muscle degeneration on spinal stability. Furthermore, due to the retrospective design, only the regional kyphosis angle was assessed, which might have some impact on the evaluation of sagittal spinal balance. It's essential to recognize that while correlations have been identified, a definitive cause-and-effect relationship has not been firmly established. Our research team plans to design prospective studies building upon this research to further investigate the role of paravertebral muscles in maintaining spinal balance.
Conclusion
In this retrospective study, we investigated the impact of different surgical approaches on paravertebral muscle FI rate in patients. Compared to OPSF, PPSF causes less damage to the paravertebral muscles in patients, and there is a smaller increase in the paravertebral muscle FI rate after surgery in the PPSF group. In the PPSF group, the correction loss percentage of the regional kyphosis angle is smaller. The correlation results show that the more the paravertebral muscle FI rate increases after surgery, the greater the correction loss percentage of the regional kyphosis angle, and the higher the VAS and ODI scores of the patients. Although a definite causal relationship has not been elucidated, our study suggests that paraspinal muscles play a crucial role in maintaining the correction of the regional kyphosis angle.
Data availability
Raw data will be made available on reasonable request and with the permission of the institution where the data were generated. Yitao Liao was the person to be contacted if someone wanted to request the data from this study.
References
Kocis, J., Kelbl, M., Kocis, T. & Návrat, T. Percutaneous versus open pedicle screw fixation for treatment of type A thoracolumbar fractures. Eur. J. Trauma Emerg. Surg. 46, 147–152. https://doi.org/10.1007/s00068-018-0998-4 (2020).
Yang, M. et al. Comparison of clinical results between novel percutaneous pedicle screw and traditional open pedicle screw fixation for thoracolumbar fractures without neurological deficit. Int. Orthop. 43, 1749–1754. https://doi.org/10.1007/s00264-018-4012-x (2019).
Pishnamaz, M. et al. Muscular changes after minimally invasive versus open spinal stabilization of thoracolumbar fractures: A literature review. J. Musculoskelet. Neuronal. Interact. 18, 62–70 (2018).
Rajasekaran, S. et al. Osteoporotic thoracolumbar fractures-how are they different? Classification and treatment algorithm. J. Orthop. Trauma. 31(Suppl 4), S49-s56. https://doi.org/10.1097/bot.0000000000000949 (2017).
Zhang, T. T. et al. Paraspinal muscle degeneration and lower bone mineral density as predictors of proximal junctional kyphosis in elderly patients with degenerative spinal diseases: A propensity score matched case-control analysis. BMC Musculoskelet. Disord. 23, 1010. https://doi.org/10.1186/s12891-022-05960-z (2022).
Katzman, W. B., Miller-Martinez, D., Marshall, L. M., Lane, N. E. & Kado, D. M. Kyphosis and paraspinal muscle composition in older men: A cross-sectional study for the Osteoporotic Fractures in Men (MrOS) research group. BMC Musculoskelet. Disord. 15, 19. https://doi.org/10.1186/1471-2474-15-19 (2014).
Vives, M. J. The paraspinal muscles and their role in the maintenance of global spinal alignment. Another wrinkle in an already complex problem. Spine J. 16, 459–461. https://doi.org/10.1016/j.spinee.2015.11.029 (2016).
Sun, X. Y., Zhang, X. N. & Hai, Y. Percutaneous versus traditional and paraspinal posterior open approaches for treatment of thoracolumbar fractures without neurologic deficit: A meta-analysis. Eur. Spine J. 26, 1418–1431. https://doi.org/10.1007/s00586-016-4818-4 (2017).
Fan, S. et al. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Eur. Spine J. 19, 316–324. https://doi.org/10.1007/s00586-009-1191-6 (2010).
Keynan, O. et al. Radiographic measurement parameters in thoracolumbar fractures: A systematic review and consensus statement of the spine trauma study group. Spine 31, E156-165. https://doi.org/10.1097/01.brs.0000201261.94907.0d (2006).
Ruiz Santiago, F. et al. Classifying thoracolumbar fractures: Role of quantitative imaging. Quant. Imaging Med. Surg. 6, 772–784. https://doi.org/10.21037/qims.2016.12.04 (2016).
Liu, Y. et al. Fat Infiltration in the multifidus muscle as a predictor of prognosis after decompression and fusion in patients with single-segment degenerative lumbar spinal stenosis: An ambispective cohort study based on propensity score matching. World Neurosurg.. 128, e989–e1001. https://doi.org/10.1016/j.wneu.2019.05.055 (2019).
Mandelli, F. et al. Assessing fatty infiltration of paraspinal muscles in patients with lumbar spinal stenosis: Goutallier classification and quantitative MRI measurements. Front. Neurol. 12, 656487. https://doi.org/10.3389/fneur.2021.656487 (2021).
Miura, K. et al. The fatty degeneration of the lumbar erector spinae muscles affects dynamic spinal compensation ability during gait in adult spinal deformity. Sci. Rep. 11, 18088. https://doi.org/10.1038/s41598-021-97358-5 (2021).
Tsutsumimoto, T., Shimogata, M., Ohta, H. & Misawa, H. Mini-open versus conventional open posterior lumbar interbody fusion for the treatment of lumbar degenerative spondylolisthesis: Comparison of paraspinal muscle damage and slip reduction. Spine 34, 1923–1928. https://doi.org/10.1097/BRS.0b013e3181a9d28e (2009).
Yamamoto, S. et al. The effect of posterior lumbar spinal surgery on biomechanical properties of rat paraspinal muscles 13 weeks after surgery. Spine 46, E1125-e1135. https://doi.org/10.1097/brs.0000000000004036 (2021).
Hu, Z. J. et al. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J. Bone Joint Surg. Am. 95, e192. https://doi.org/10.2106/jbjs.L.01607 (2013).
James, G. et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur. Spine J. 27, 1744–1756. https://doi.org/10.1007/s00586-018-5652-7 (2018).
Sangala, J. R., Nichols, T. & Freeman, T. B. Technique to minimize paraspinal muscle atrophy after posterior cervical fusion. Clin. Neurol. Neurosurg. 113, 48–51. https://doi.org/10.1016/j.clineuro.2010.09.001 (2011).
Fryer, G., Morris, T. & Gibbons, P. Paraspinal muscles and intervertebral dysfunction: Part two. J. Manip. Physiol. Ther. 27, 348–357. https://doi.org/10.1016/j.jmpt.2004.04.008 (2004).
Wesselink, E. O. et al. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur. Spine J. 32, 787–796. https://doi.org/10.1007/s00586-022-07471-w (2023).
Ranger, T. A. et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 17, 1729–1748. https://doi.org/10.1016/j.spinee.2017.07.002 (2017).
Crawford, R. J. et al. Geography of lumbar paravertebral muscle fatty infiltration: The influence of demographics, low back pain, and disability. Spine 44, 1294–1302. https://doi.org/10.1097/brs.0000000000003060 (2019).
Hiyama, A. et al. Short-term comparison of preoperative and postoperative pain after indirect decompression surgery and direct decompression surgery in patients with degenerative spondylolisthesis. Sci. Rep. 10, 18887. https://doi.org/10.1038/s41598-020-76028-y (2020).
Le Huec, J. C., Thompson, W., Mohsinaly, Y., Barrey, C. & Faundez, A. Sagittal balance of the spine. Eur. Spine J. 28, 1889–1905. https://doi.org/10.1007/s00586-019-06083-1 (2019).
Hiyama, A. et al. Correlation analysis of sagittal alignment and skeletal muscle mass in patients with spinal degenerative disease. Sci. Rep. 8, 15492. https://doi.org/10.1038/s41598-018-33867-0 (2018).
Jamshidnejad, S. & Arjmand, N. Variations in trunk muscle activities and spinal loads following posterior lumbar surgery: A combined in vivo and modeling investigation. Clin. Biomech. (Bristol, Avon). 30, 1036–1042. https://doi.org/10.1016/j.clinbiomech.2015.09.010 (2015).
Malakoutian, M. et al. Dysfunctional paraspinal muscles in adult spinal deformity patients lead to increased spinal loading. Eur. Spine J. 31, 2383–2398. https://doi.org/10.1007/s00586-022-07292-x (2022).
Ball, J. M., Cagle, P., Johnson, B. E., Lucasey, C. & Lukert, B. P. Spinal extension exercises prevent natural progression of kyphosis. Osteoporos. Int. 20, 481–489. https://doi.org/10.1007/s00198-008-0690-3 (2009).
Jermy, J. E., Copley, P. C., Poon, M. T. C. & Demetriades, A. K. Does pre-operative multifidus morphology on MRI predict clinical outcomes in adults following surgical treatment for degenerative lumbar spine disease? A systematic review. Eur. Spine J. 29, 1318–1327. https://doi.org/10.1007/s00586-020-06423-6 (2020).
Gengyu, H. et al. The predictive value of preoperative paraspinal muscle morphometry on complications after lumbar surgery: A systematic review. Eur. Spine J. 31, 364–379. https://doi.org/10.1007/s00586-021-07052-3 (2022).
Zotti, M. G. T. et al. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis?. Eur. Spine J. 26, 2589–2597. https://doi.org/10.1007/s00586-017-4986-x (2017).
Lv, Y. et al. Three-year postoperative outcomes between MIS and conventional TLIF in1-segment lumbar disc herniation. Minim. Invasive Ther. Allied Technol. 26, 168–176. https://doi.org/10.1080/13645706.2016.1273837 (2017).
Hebert, J. J., Fritz, J. M., Thackeray, A., Koppenhaver, S. L. & Teyhen, D. Early multimodal rehabilitation following lumbar disc surgery: A randomised clinical trial comparing the effects of two exercise programmes on clinical outcome and lumbar multifidus muscle function. Br. J. Sports Med. 49, 100–106. https://doi.org/10.1136/bjsports-2013-092402 (2015).
Strube, P. et al. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J. Spinal Disord. Tech. 25, 362–369. https://doi.org/10.1097/BSD.0b013e3182263d91 (2012).
Han, G., Jiang, Y., Zhang, B., Gong, C. & Li, W. Imaging evaluation of fat infiltration in paraspinal muscles on MRI: A Systematic review with a focus on methodology. Orthop. Surg. 13, 1141–1148. https://doi.org/10.1111/os.12962 (2021).
Author information
Authors and Affiliations
Contributions
X.Z. and C.L. designed the study; Y.T.L. and X.F.L. searched relevant studies; T.C.X., C.L., and Q.M.X. analyzed and interpreted the data; Y.T.L. wrote the manuscript; and X.Z. approved the final version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liao, Y., Liu, X., Xu, T. et al. Association between paraspinal muscle fat infiltration and regional kyphosis angle in thoracolumbar fracture patients: a retrospective study. Sci Rep 14, 2364 (2024). https://doi.org/10.1038/s41598-024-53017-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53017-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.