Abstract
The association between Helicobacter pylori (H. pylori) infection and coronary heart disease (CHD) remains controversial, with an unclear causal link. This study employed bidirectional Mendelian randomization (MR) method, using H. pylori infection as the exposure, to investigate its causal relationship with CHD diagnosis, prognosis, and potential pathogenesis. H. pylori infection exhibited a causal association with body mass index (BMI) (β = 0.022; 95% CI 0.008–0.036; p = 0.001). Conversely, there was no discernible connection between H. pylori infection and the diagnosis of CHD (OR = 0.991; 95% CI 0.904–1.078; p = 0.842; IEU database; OR = 1.049; 95% CI 0.980–1.118; p = 0.178; FinnGen database) or CHD prognosis (OR = 0.999; 95% CI 0.997–1.001; p = 0.391; IEU database; OR = 1.022; 95% CI 0.922–1.123; p = 0.663; FinnGen database). Reverse MR analysis showed no causal effect of CHD on H. pylori infection. Our findings further support that H. pylori infection exerts a causal effect on CHD incidence, mediated by BMI. Consequently, eradicating or preventing H. pylori infection may provide an indirect clinical benefit for patients with CHD.
Similar content being viewed by others
Introduction
Coronary heart disease (CHD) is caused by atherosclerosis, which includes angina pectoris and myocardial infarction (MI) and is the leading cause of mortality in many countries1. The etiology, pathogenesis and prognosis of CHD are complex and have not been fully understood until recently. Helicobacter pylori (H. pylori) is a gram-negative bacterium that primarily inhabits the stomach and duodenum2. More than half of the world's population has been infected with H. pylori3. In addition to causing gastrointestinal diseases4, H. pylori can also induce systemic reactions, including abnormal glucose5 and lipid metabolism6, heightened blood hypercoagulability7,8, and chronic inflammatory reactions9,10,11, and is accompanied by vitamin (including vitamin B12, vitamin C, and vitamin D) deficiency12. While these reactions represent risk factors for CHD, it remains uncertain whether H. pylori influences the occurrence of CHD through these reactions.
However, the relationship between H. pylori infection and CHD is still controversial. Several studies have shown that H. pylori infection is not significantly related to the occurrence or severity of CHD13,14; however, some studies have shown that H. pylori infection is one of the main causes of CHD15,16. Studies have reported that eradication therapy for H. pylori can reduce the levels of peripheral blood inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) in patients. These inflammatory cytokines are implicated in the development of atherosclerosis and CHD, and their elevation increases the incidence of restenosis in patients after percutaneous transluminal coronary angioplasty (PTCA)17,18,19. The probability of MI in H. pylori-infected patients is twice that in uninfected individuals20. Another study used infrared radiation spectroscopy to measure the levels of triglycerides, C-reactive protein, homocysteine, low-density lipoprotein (LDL), and TNF-α in peripheral blood. The results showed that, compared with healthy individuals, CHD patients with H. pylori infection had elevated triglyceride levels and inflammation21. An Asian study also confirmed that H. pylori infection can increase the risk of CHD in the next 10 years22. At present, the evidence for a link between H. pylori infection and CHD is based on observational studies, and there may be some unknown confounding factors that affect judgment of the results. To address this controversial clinical issue, a study that removes confounding factors to accurately determine the causal relationship between H. pylori infection and CHD is urgently needed. In addition, although the infection rate of H. pylori is relatively high, H. pylori infection is not routinely screened, and many infected individuals are unaware of having this infection. Exploring the causal relationship between the two will help determine whether routine screening and treatment of H. pylori is one of the prevention and treatment strategies for CHD.
Mendelian randomization (MR) has emerged as a popular epidemiological statistical method that can remove confounding factors and accurately determine the causal relationship between two variables. The method relies on the use of the public genome-wide association study (GWAS) database to obtain instrumental variables (IVs) that are strongly related to exposure but are not related to outcomes or confounding factors. IVs are usually single nucleotide polymorphisms (SNPs), and the causal relationship between exposure and outcomes can be accurately inferred using IVs. In this study, we used H. pylori infection as the exposure and applied the bidirectional MR method to infer the relationship between H. pylori infection and the diagnosis, prognosis, and possible pathogenesis of CHD. We also used CHD as the exposure to explore the reverse causal relationship between CHD and H. pylori infection, two step MR analyses were used to explore indirect pathogenic factors of H. pylori infection, with the aim of clarifying this relationship and providing clinical suggestions for the diagnosis and treatment of CHD, providing new insights for CHD.
Methods
Study design
For the current study, we used IVs as a proxy for exposure, and then conducted an MR analysis to test the association between exposure and outcome23. MR is based on three principle assumptions: (1) correlation assumption: IVs are strongly correlated with exposure; (2) exclusivity hypothesis: IVs are not associated with outcomes; and (3) independence hypothesis: IVs are independent of other confounding factors24 (Fig. 1).
Description of the data sources
The genetic association of CHD was derived from the CARDIoGRAMplusC4D Consortium, which included 60,801 cases and 123,504 control subjects from 48 studies, and of which 77% of the participants were of European ancestry and 19% were of South and East Asian ancestry25. We also collected summary statistics for CHD, MI and angina pectoris, which were derived from the FinnGen database (https://www.fifinngen.fifi/en)26. H. pylori infection data were derived from the European Bioinformatics Institute (EBI) database (https://gwas.mrcieu.ac.uk/datasets/ieu-b-4905/) and included 1058 cases and 3625 controls. GWAS data were also collected to investigate the causal effect between H. pylori infection and the prognostic data for CHD, including major adverse cardiovascular events (MACE; Neale laboratory and FinnGen database), heart failure (Neale laboratory), heart arrhythmia, heart attack, stroke, target heart rate (HR) reached, and maximum HR data [MRC Integrated Epidemiology Unit (MRC-IEC), https://www.bristol.ac.uk/integrative-epidemiology]. In addition, GWAS data on the possible pathogenesis between H. pylori and CHD were also obtained, including fasting blood glucose data from the EBI database, body mass index (BMI) data from the MRC-IEU database, and lipid trait data from the UK Biobank database. Vitamin data were obtained from the MRC-IEU database. Inflammation data were downloaded from the public database IEU (https://gwas.mrcieu.ac.uk/). The GWAS data are detailed in Table 1 and have been approved by the author or the Consortium.
The demographic characteristics of GWAS data for H. pylori infection are as follows: pregnant women residing in Avon, UK, with expected delivery dates between April 1, 1991, and December 31, 1992, were invited to participate in the ALSPAC study. The overall sample size for analyses, incorporating data collected after the age of seven, was determined. Serum antibody levels related to H. pylori infection were measured using ELISA, ultimately providing GWAS data associated with H. pylori27. In the FinnGen database, the average age of GWAS data is 63 years, with a male proportion of 43.5% (source: https://www.nature.com/articles/s41586-022-05473-8). For the UKB database, the average age of GWAS data is 56.9 years, with a male proportion of 45.8%28. The remaining GWAS datasets may have been obtained through meta-analysis, making it challenging to acquire information on gender and age.
Selection of genetic IVs for H. pylori Infection
The genetic IVs were acquired from previous literature29,30,31. This study involved bidirectional MR analysis of H. pylori infection and non-alcoholic fatty liver disease. The SNPs rs368433 and rs10004195, located in the Toll-like receptor 10 (TLR10) gene (4p14) and the Fc gamma RIIA (FCGR2A) gene (1q23.3), respectively, have been reported to be strongly associated with H. pylori infection and are used as IVs29. Instrument strength was evaluated using the F-statistic for each allele, and if the F-statistic was greater than 10, it was considered that the potential weak instrument bias was minimized30,31. The F-statistic for each SNP was derived from the following equation:
where R2 is the proportion of variation explained by IVs, N is the sample size of the exposure dataset, and MAF indicates the minor allele frequency. In our study, all F-statistics were greater than 100 and, therefore, suitable for our analysis (Supplementary Table S1).
Selection of genetic IVs for CHD and BMI
The genetic IVs for CHD and the potential pathogenesis of H. pylori infection were obtained from the GWAS summary statistics. The following three steps were subsequently used to screen for strong correlations with CHD but not with H. pylori infection or confounding factors to ensure that the effect of each allele (containing each SNP) was the same. First, SNPs strongly related to exposure were screened (p < 5 × 10˗8). Second, independence was set to remove linkage disequilibrium (LD; r2 < 0.001, window size = 10,000 kb, p < 5 × 10˗8) and calculate the statistical strength (F-statistical > 10). Third, the exposure and outcome datasets were harmonized to ensure that the effect alleles belonged to the same allele. The SNPs screened by these strict procedures can be used as IVs for subsequent analysis (Supplementary Table S2). The genetic IVs for BMI were obtained by the same screening method (Supplementary Table S3).
Statistical analysis and data visualization
All analyses were performed using R programming software (R4.1.2, https://www.rproject.org/). The primary MR analysis was conducted using the Wald ratio and the inverse variance weighting (IVW) method, and a two-sided p-value < 0.05 was considered indicative of statistical significance. Due to the multiple comparisons, we further applied a Bonferroni corrected threshold for statistical significance (0.05/number of analyses)32 (Table 2). In reverse MR analysis and two step MR analysis, because of the large number of IVs, we applied two complementary methods (MR‒Egger and weighted-median) to increase the stability of the results. MR analyses were performed using the R-based package “TwoSampleMR” (version 0.5.6). Forest plots were generated using the “ggplot2” R package (version 3.4.0).
Results
Causal effect of H. pylori infection on the diagnosis of CHD
According to previous studies, the SNPs rs10004195 (T>A) and rs368433 (T>C) are strongly related to H. pylori infection30. Conventional IVs typically consist of two or more. Although there were only two IVs in this study, these two SNP loci were strongly correlated with H. pylori infection, with F values greater than 100, and their efficacy was more than 10 times that of conventional IVs (Supplementary Table S1). The two corresponding genes are TLR10 and FCGR2A. TLR10 is a key gene that regulates the release of inflammatory factors during H. pylori infection33, and FCGR2A is also a key gene that regulates the intestinal34 and cardiac inflammatory responses35. We therefore used these two SNPs as IVs of H. pylori infection to predict the relationship between H. pylori infection and the diagnosis of CHD, MI, or angina pectoris29. H. pylori infection was not associated with the occurrence of CHD (IEU) [odds ratio (OR), 0.991; 95% confidence interval (CI) 0.904–1.078; p-value = 0.842], CHD (Finn) (OR, 1.049; 95% CI 0.980–1.118; p-value = 0.178), angina pectoris (OR, 1.105; 95% CI 1.019–1.191; p-value = 0.023), or MI (OR, 0.993; 95% CI 0.896–1.091; p-value = 0.889) according to the IVW method. (Fig. 2, Supplementary Table S4). Although the causal analysis between H. pylori infection and angina pectoris showed a p value < 0.05, it is imperative to consider that this study included four distinct outcomes, each subjected to separate analyses. In accordance with the Bonferroni threshold correction method, the adjusted significance level dictates that the effective p value should be < 0.0125 to account for the multiple comparisons conducted (Table 2). Therefore, our analysis revealed that there is no causal relationship between H. pylori infection and CHD diagnosis.
Causal effect of H. pylori infection on the prognosis of CHD
MR analyses were further performed to examine the causal association between H. pylori infection and the prognosis of CHD, including MACE, heart arrhythmia, heart attack, stroke, heart failure, target HR achieved, and maximum HR during the fitness test. The incidence of MACE, which mainly includes heart arrhythmia, heart attack, stroke, and heart failure, is currently the main method for determining the prognosis of CVD patients. In addition, the maximum HR and target HR in cardiopulmonary exercise tests can also predict the prognosis of CHD patients and are negatively correlated with their prognosis36,37. Therefore, this study used the above factors as prognostic indicators for CHD. The analysis showed that H. pylori infection had no causal effect on MACE (OR, 0.999; 95% CI 0.997–1.001; p-value = 0.391; IEU database; OR, 1.022; 95% CI 0.922–1.123; p-value = 0.663; FinnGen database), heart arrhythmia (OR, 1.000; 95% CI 0.999–1.001; p-value = 0.823), heart attack (OR, 0.998; 95% CI 0.996–1.000; p-value = 0.124), stroke (OR, 0.999; 95% CI 0.998–1.001; p-value = 0.525), heart failure (OR, 1.000; 95% CI 0.999–1.001; p-value = 0.741), target HR achieved (OR, 0.994; 95% CI 0.983–1.005; p-value = 0.252), or maximum HR (OR, 0.972; 95% CI 0.937–1.007; p-value = 0.115) (Fig. 3, Supplementary Table S5).
Mendelian randomization results for the effect of H. pylori infection on the prognosis of CHD patients. CHD, coronary heart disease; H. pylori, Helicobacter pylori; MACEs, major adverse cardiovascular events; Maximum HR, maximum heart rate during fitness test; OR, odds ratio; Target HR achieved, reached target heart rate.
Causal effect of H. pylori infection on the pathogenesis of CHD
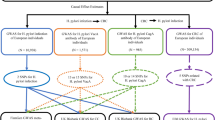
Based on previous research, we summarized the pathogenic mechanisms of H. pylori infection on CHD, and these included abnormal glucose and lipid metabolism, vitamin deficiency (including vitamin B12, vitamin C, and vitamin D), and chronic inflammatory reactions. In addition to metabolic abnormalities and chronic inflammation, vitamin deficiency has also been found to be associated with the occurrence and development of CHD. H. pylori infection can cause damage to gastric wall cells, leading to a decrease in the secretion of endogenous factors by gastric wall cells and a decrease in the absorption of vitamin B12 in the small intestine. Moreover, deficiencies in vitamin C and vitamin D, both of which are associated with the H. pylori infection progression, represent risk factors for CHD. Therefore, we used these factors as indicators of CHD pathogenesis38,39. To explore the causal relationship between H. pylori infection and CHD pathogenesis, we used H. pylori infection as the exposure and pathogenesis as the outcome for MR analysis. According to the MR analyses of abnormal glucose and lipid metabolism, H. pylori infection had no association with fasting blood glucose levels (β, 0.006; 95% CI − 0.011 to 0.023; p-value = 0.511), triglyceride (TG) levels (β, 0.005; 95% CI − 0.006 to 0.016; p-value = 0.409), high-density lipoprotein cholesterol (HDL-C) levels (β, − 0.006; 95% CI − 0.047 to 0.035; p-value = 0.788), or low-density lipoprotein cholesterol (LDL-C) levels (β, 0.013; 95% CI − 0.026 to 0.051; p-value = 0.515). In the vitamin deficiency MR analysis, we obtained negative results for water-soluble vitamins, including vitamin C (β, − 0.002; 95% CI − 0.006 to 0.002; p-value = 0.318) and vitamin B12 (β, 0.008; 95% CI − 0.029 to 0.044; p-value = 0.685). The same result was also observed for the fat-soluble vitamins of vitamin D (β, − 0.0003; 95% CI − 0.003 to 0.002; p-value = 0.775). In addition, we also analyzed whether H. pylori infection contributed to the occurrence of CHD through inflammatory mechanisms and found no causal relationships between H. pylori infection and IL-4 (β, − 0.066; 95% CI − 0.258 to 0.125; p-value = 0.497), IL-6 (β, − 0.041; 95% CI − 0.117 to 0.035; p-value = 0.294), IL-8 (β, 0.017; 95% CI − 0.055 to 0.088; p-value = 0.645), IL-10 (β, − 0.079; 95% CI − 0.276 to 0.117; p-value = 0.429), IL-18 (β, 0.022; 95% CI − 0.041 to 0.086; p-value = 0.493) or TNF-α (β, 0.020; 95% CI − 0.275 to 0.316; p-value = 0.893). However, there was a significant causal relationship between H. pylori infection and BMI (β, 0.022; 95% CI 0.008–0.036; p-value = 0.001), and there was a causal relationship between BMI and CHD incidence (Fig. 4, Supplementary Tables S6, and S7). A study showed that, compared to those in the control group, patients infected with H. pylori had increased growth hormone levels and decreased obesity, which promoted appetite increase40. Another study suggested that H. pylori can affect appetite and dietary habits through the brain-gut axis41. Therefore, we speculate that the mechanism by which H. pylori promotes an increase in BMI is through the brain-gut axis to alter appetite and promote energy intake.
Two step Mendelian randomization results for the effect of H. pylori infection on CHD incidence (the pathogenic mechanism of CHD). BMI: body mass index; CRP, C-reactive protein; FBG, fasting blood glucose; H. pylori, Helicobacter pylori; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio. TG, triglyceride; TNF-α, tumor necrosis factor-α. Dark blue dots represent significant differences as indicated by the P values.
Reverse causal effect of CHD on H. pylori infection
The IVs of CHD, MI, and angina pectoris were identified from public GWAS summary data. Three MR analysis methods, namely, IVW, weighted median, and MR‒Egger, were used for this analysis. None of the three methods had a significant causal effect on H. pylori infection (Fig. 5, Supplementary Table S8).
Discussion
In this study, we used large-scale public GWAS data to analyze the causal relationship between H. pylori infection and the risk of CHD using the MR method. The causal effect of H. pylori infection on CHD incidence was mediated by BMI.
The association between H. pylori infection and CHD is currently controversial. Several studies have reported that H. pylori infection is related to the occurrence and prognosis of CHD19,42,43. A prospective study revealed that H. pylori-infected patients had an increased occurrence of CHD44 and adverse events42. According to other studies, MI patients infected with H. pylori have a higher mortality rate45, and the probability of restenosis after PTCA is higher17. It has also been shown that MI has a reverse causal effect on H. pylori. Young people with MI have twice the probability of H. pylori infection as healthy individuals46,47. However, some studies have been unable to detect a correlation between the occurrence and development of CHD and H. pylori infection, especially among older individuals48. A prospective study with a small sample14 and meta-analyses of five large samples14,49 have provided evidence that H. pylori infection is not significantly related to the severity or prognosis of CHD. A prospective study involving 180 patients who underwent stent implantation in a native coronary artery revealed that there is no significant association between H. pylori infection and restenosis following PTCA50. However, the pathogenic link between H. pylori infection and CHD remains controversial. First, in terms of metabolism, the influence of H. pylori infection on glucose and lipid metabolism and BMI is controversial. Regarding lipids, a study showed that H. pylori infection can reduce the level of HDL and increase the levels of LDL and TG51. However, other studies have presented opposite findings52,53. Meta-analyses and prospective studies of large samples have shown that eradication of H. pylori infection has no significant effect on the levels of HDL, TG, or LDL6,54. In terms of glucose metabolism, evidence suggests that H. pylori infection may participate in the onset of diabetes and impaired glucose control in diabetes patients55,56. Infection with H. pylori can increase insulin resistance in both young people and diabetes patients57. One study revealed that, compared with that in the control group, the improvement in glucose homeostasis in diabetes patients after successful eradication of H. pylori infection was not statistically significant58. In terms of body weight, the eradication of H. pylori infection has been associated with increased weight in children59 and has variable effects on weight in adults—either increasing60 or decreasing61 it. Additionally, there is a higher observed incidence of H. pylori infection among obese individuals62. Second, H. pylori infection induces alterations in the gastrointestinal microenvironment, potentially impeding the absorption of nutrients, resulting in a deficiency of micronutrients63. Poor vitamin B12 absorption has been shown to be related to H. pylori infection64. The levels of vitamin C and vitamin D are closely related to CHD incidence65, but the relationship between vitamins and H. pylori infection remains to be confirmed. Third, H. pylori can cause an inflammatory reaction. Chronic inflammation caused by H. pylori may have dual effects. On the one hand, low-grade inflammation is a common feature of obesity, diabetes, insulin resistance, and dyslipidemia, and H. pylori may cause a chronic inflammatory reaction through abnormal metabolism66. On the other hand, H. pylori causes damage to the gastrointestinal tract67, stimulating an increase in interleukin levels10,11. H. pylori infection has been associated with elevated levels of TNF-α and IL-6 in patients with CHD68,69,70,71. Conflicting data have also been reported regarding inflammation72. The factors involved in the pathogenesis of H. pylori infection, which include glucose and lipid metabolism, vitamin deficiency, and chronic inflammatory reactions, are all causes of CHD.
The discrepancy between H. pylori infection and CHD could be attributed to multiple factors, such as differences in the race and age of the selected sample population, the small sample size, the low incidence of MACE, the detection method for H. pylori infection, and the different follow-up times. These confounding factors may lead to the poor statistical efficiency of the data and may affect the reliability of the experimental results.
This study revealed that H. pylori infection has no direct causal effect on the diagnosis or prognosis of CHD. According to our analysis of pathogenesis, H. pylori infection has a causal effect on BMI, and BMI has a causal effect on CHD incidence. Therefore, the causal effect of H. pylori infection on CHD incidence is mediated by BMI. However, H. pylori infection has no causal effect on inflammatory factors (IL-4, IL-6, IL-8, IL-10, IL-18, or TNF-α), vitamins (vitamin B12, vitamin C, or vitamin D), or glucose and lipid metabolism, and there is no reverse causal effect of CHD on H. pylori infection.
This study used the MR method to reveal a bidirectional causal relationship between H. pylori infection and CHD for the first time and could increase the recognition of pathogenic factors of CHD from the perspective of systems biology. The advantage of MR studies is that the sample size is large, and they involve a natural randomized controlled trial, which eliminates confounding factors. However, this study has several limitations. The GWAS of H. pylori infection was based on serological samples, which may not be truly representative of H. pylori infection. Furthermore, the samples were obtained from individuals of European ancestry and therefore may not be representative of all populations worldwide. Finally, the screening of IVs in this study was strict, which may have led to negative results.
Conclusions
Our findings confirm that the causal effect of H. pylori infection on CHD incidence is mediated by BMI. Therefore, the eradication or prevention of H. pylori infection may indirectly benefit patients with CHD indirectly in the clinic.
Data availability
All data generated or analysed during the study is included in this published article. The datasets for this study are shown in Table 1.
References
Nichols, M., Townsend, N., Scarborough, P. & Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 35, 2950–2959. https://doi.org/10.1093/eurheartj/ehu299 (2014).
Warren, J. R. & Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet (London, England) 1, 1273–1275 (1983).
Hooi, J. K. Y. et al. Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429. https://doi.org/10.1053/j.gastro.2017.04.022 (2017).
de Brito, B. B. et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 25, 5578–5589. https://doi.org/10.3748/wjg.v25.i37.5578 (2019).
Jeon, C. Y. et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care 35, 520–525. https://doi.org/10.2337/dc11-1043 (2012).
Watanabe, J., Hamasaki, M. & Kotani, K. The effect of helicobacter pylori eradication on lipid levels: A meta-analysis. J. Clin. Med. https://doi.org/10.3390/jcm10050904 (2021).
Torgano, G. et al. Treatment of Helicobacter pylori and Chlamydia pneumoniae infections decreases fibrinogen plasma level in patients with ischemic heart disease. Circulation 99, 1555–1559. https://doi.org/10.1161/01.cir.99.12.1555 (1999).
Yusuf, S. W. & Mishra, R. M. Effect of Helicobacter pylori infection on fibrinogen level in elderly patients with ischaemic heart disease. Acta Cardiol. 57, 317–322. https://doi.org/10.2143/ac.57.5.2005446 (2002).
Kabir, S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter 16, 1–8. https://doi.org/10.1111/j.1523-5378.2010.00812.x (2011).
Rocha, G. A. et al. Interleukin-27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori-associated gastroduodenal diseases. Helicobacter 25, e12667. https://doi.org/10.1111/hel.12667 (2020).
Rasool, K. H., Mahmood Alubadi, A. E. & Al-Bayati, I. F. I. The role of Serum Interleukin-4 and Interleukin-6 in Helicobacter pylori-infected patients. Microb. Pathogenesis 162, 105362. https://doi.org/10.1016/j.micpath.2021.105362 (2022).
Li, W. Q. et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ (Clin. Res. Ed.) 366, l5016. https://doi.org/10.1136/bmj.l5016 (2019).
Koenig, W. et al. Infection with Helicobacter pylori is not a major independent risk factor for stable coronary heart disease: Lack of a role of cytotoxin-associated protein A-positive strains and absence of a systemic inflammatory response. Circulation 100, 2326–2331. https://doi.org/10.1161/01.cir.100.23.2326 (1999).
Rogha, M. et al. Association of helicobacter pylori infection with severity of coronary heart disease. ARYA Atherosclerosis 7, 138–141 (2012).
Sun, L. et al. Helicobacter pylori infection and risk of cardiovascular disease. Helicobacter 28, e12967. https://doi.org/10.1111/hel.12967 (2023).
Gonciarz, W. et al. Antibodies towards TVLLPVIFF amino acid sequence of TNF receptor induced by Helicobacter pylori in patients with coronary heart disease. J. Clin. Med. https://doi.org/10.3390/jcm11092545 (2022).
Kowalski, M. Helicobacter pylori (H. pylori) infection in coronary artery disease: Influence of H. pylori eradication on coronary artery lumen after percutaneous transluminal coronary angioplasty. The detection of H. pylori specific DNA in human coronary atherosclerotic plaque. J. Physiol. Pharmacol. 52, 3–31 (2001).
Vizzardi, E. et al. Helicobacter pylori and ischemic heart disease. Panminerva Medica 53, 193–202 (2011).
Wang, J. W. et al. Association between Helicobacter pylori eradication and the risk of coronary heart diseases. PloS One 13, e0190219. https://doi.org/10.1371/journal.pone.0190219 (2018).
Rahmani, Y. et al. Association of Helicobacter pylori with presence of myocardial infarction in Iran: A systematic review and meta-analysis. Ethiopian J. Health Sci. 27, 433–440. https://doi.org/10.4314/ejhs.v27i4.15 (2017).
Gonciarz, W. et al. Searching for serum biomarkers linking coronary heart disease and Helicobacter pylori infection using infrared spectroscopy and artificial neural networks. Sci. Rep. 12, 18284. https://doi.org/10.1038/s41598-022-23191-z (2022).
Chung, J., Min, K. W., Son, B. K., Kim, D. H. & Kim, H. L. Association between histological severity of Helicobacter pylori infection and cardiovascular risk scores in the Korean population. Atherosclerosis 333, 124–130. https://doi.org/10.1016/j.atherosclerosis.2021.08.019 (2021).
Burgess, S., Scott, R. A., Timpson, N. J., Davey Smith, G. & Thompson, S. G. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30, 543–552. https://doi.org/10.1007/s10654-015-0011-z (2015).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. JAMA 318, 1925–1926. https://doi.org/10.1001/jama.2017.17219 (2017).
Nikpay, M. et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130. https://doi.org/10.1038/ng.3396 (2015).
Luo, J., le Cessie, S., van Heemst, D. & Noordam, R. Diet-derived circulating antioxidants and risk of coronary heart disease: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 77, 45–54. https://doi.org/10.1016/j.jacc.2020.10.048 (2021).
Chong, A. H. W. et al. Genetic analyses of common infections in the Avon longitudinal study of parents and children cohort. Front. Immunol. 12, 727457. https://doi.org/10.3389/fimmu.2021.727457 (2021).
Richardson, T. G. et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 17, e1003062. https://doi.org/10.1371/journal.pmed.1003062 (2020).
Mayerle, J. et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 309, 1912–1920. https://doi.org/10.1001/jama.2013.4350 (2013).
Liu, Y. et al. No evidence for a causal link between Helicobacter pylori infection and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. Front. Microbiol. 13, 1018322. https://doi.org/10.3389/fmicb.2022.1018322 (2022).
Burgess, S. & Thompson, S. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40, 755–764. https://doi.org/10.1093/ije/dyr036 (2011).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. https://doi.org/10.1002/gepi.21758 (2013).
Neuper, T. et al. TLR2, TLR4 and TLR10 shape the cytokine and chemokine release of H. pylori-infected human DCs. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21113897 (2020).
Asano, K. et al. Contribution of susceptibility variants at FCGR2A and 13q12 to the risk of relapse among Japanese patients with ulcerative colitis. J. Gastroenterol. 50, 1094–1102. https://doi.org/10.1007/s00535-015-1062-3 (2015).
Kuo, H. C. et al. Identification of an association between genomic hypomethylation of FCGR2A and susceptibility to Kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheumatol. (Hoboken, N.J.) 67, 828–836. https://doi.org/10.1002/art.38976 (2015).
Duarte, C. V., Myers, J. & de Araújo, C. G. Exercise heart rate gradient: A novel index to predict all-cause mortality. Eur. J. Preventive Cardiol. 22, 629–635. https://doi.org/10.1177/2047487314520784 (2015).
Kurl, S., Jae, S. Y., Voutilainen, A., Hagnäs, M. & Laukkanen, J. A. Exercise heart rate reserve and recovery as risk factors for sudden cardiac death. Progress Cardiovasc. Diseases 68, 7–11. https://doi.org/10.1016/j.pcad.2021.09.002 (2021).
Toh, J. W. T. & Wilson, R. B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21176451 (2020).
Săsăran, M. O., Mărginean, C. O., Lupu, A. & Koller, A. M. Vitamin D and its association with H. pylori prevalence and eradication: A comprehensive review. Nutrients. https://doi.org/10.3390/nu15163549 (2023).
Ulasoglu, C. et al. Effect of Helicobacter pylori eradication on serum ghrelin and obestatin levels. World J. Gastroenterol. 19, 2388–2394. https://doi.org/10.3748/wjg.v19.i15.2388 (2013).
Arzani, M. et al. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 21, 15. https://doi.org/10.1186/s10194-020-1078-9 (2020).
Shmuely, H. et al. Association of Helicobacter pylori with coronary artery disease and myocardial infarction assessed by myocardial perfusion imaging. Israel Med. Assoc. J. IMAJ 16, 341–346 (2014).
Fang, Y., Fan, C. & Xie, H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: A systematic review and meta-analysis. Medicine 98, e18348. https://doi.org/10.1097/md.0000000000018348 (2019).
Liu, J., Wang, F. & Shi, S. Helicobacter pylori infection increase the risk of myocardial infarction: A meta-analysis of 26 studies involving more than 20,000 participants. Helicobacter 20, 176–183. https://doi.org/10.1111/hel.12188 (2015).
Alkout, A. M. et al. Quantitative assessment of IgG antibodies to Helicobacter pylori and outcome of ischaemic heart disease. FEMS Immunol. Med. Microbiol. 29, 271–274. https://doi.org/10.1111/j.1574-695X.2000.tb01533.x (2000).
Danesh, J. et al. Helicobacter pylori infection and early onset myocardial infarction: Case–control and sibling pairs study. BMJ (Clin. Res. Ed.) 319, 1157–1162. https://doi.org/10.1136/bmj.319.7218.1157 (1999).
Rubinfeld, G. D., Berger, J. S. & Smilowitz, N. R. Acute myocardial infarction following hospitalization for gastrointestinal bleeding: Incidence, predictors, management, and outcomes. Am. J. Med. 135, e263–e278. https://doi.org/10.1016/j.amjmed.2022.03.030 (2022).
Pilotto, A. et al. Lack of association between Helicobacter pylori infection and extracardiac atherosclerosis in dyspeptic elderly subjects. Age Ageing 28, 367–371. https://doi.org/10.1093/ageing/28.4.367 (1999).
Danesh, J. Coronary heart disease, Helicobacter pylori, dental disease, Chlamydia pneumoniae, and cytomegalovirus: Meta-analyses of prospective studies. Am. Heart J. 138, S434-437. https://doi.org/10.1016/s0002-8703(99)70270-x (1999).
Schiele, F. et al. Cytomegalovirus, Chlamydia pneumoniae, and Helicobacter pylori IgG antibodies and restenosis after stent implantation: An angiographic and intravascular ultrasound study. Heart (Br. Cardiac Society) 85, 304–311. https://doi.org/10.1136/heart.85.3.304 (2001).
Laurila, A. et al. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis 142, 207–210. https://doi.org/10.1016/s0021-9150(98)00194-4 (1999).
Kayo, S. et al. Oxidized low-density lipoprotein levels circulating in plasma and deposited in the tissues: Comparison between Helicobacter pylori-associated gastritis and acute myocardial infarction. Am. Heart J. 148, 818–825. https://doi.org/10.1016/j.ahj.2004.05.042 (2004).
Esmaeili Dooki, M. R. et al. Helicobacter pylori infection and type 1 diabetes mellitus in children. J. Diabetes Metabolic Disord. 19, 243–247. https://doi.org/10.1007/s40200-020-00497-1 (2020).
Elizalde, J. I. et al. Influence of Helicobacter pylori infection and eradication on blood lipids and fibrinogen. Alimentary Pharmacol. Therap. 16, 577–586. https://doi.org/10.1046/j.1365-2036.2002.01202.x (2002).
Kato, M., Toda, A., Yamamoto-Honda, R., Arase, Y. & Sone, H. Association between Helicobacter pylori infection, eradication and diabetes mellitus. J. Diabetes Investigat. 10, 1341–1346. https://doi.org/10.1111/jdi.13011 (2019).
Mansori, K. et al. Helicobacter pylori infection as a risk factor for diabetes: A meta-analysis of case-control studies. BMC Gastroenterol. 20, 77. https://doi.org/10.1186/s12876-020-01223-0 (2020).
Buzás, G. M. Metabolic consequences of Helicobacter pylori infection and eradication. World J. Gastroenterol. 20, 5226–5234. https://doi.org/10.3748/wjg.v20.i18.5226 (2014).
Vafaeimanesh, J. et al. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J. Gastroenterol. 14, 55–58. https://doi.org/10.1016/j.ajg.2013.03.002 (2013).
Yang, Y. J., Sheu, B. S., Yang, H. B., Lu, C. C. & Chuang, C. C. Eradication of Helicobacter pylori increases childhood growth and serum acylated ghrelin levels. World J. Gastroenterol. 18, 2674–2681. https://doi.org/10.3748/wjg.v18.i21.2674 (2012).
Lane, J. A. et al. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Alimentary Pharmacol. Therap. 33, 922–929. https://doi.org/10.1111/j.1365-2036.2011.04610.x (2011).
Kebapcilar, L. et al. The influence of Helicobacter pylori eradication on leptin, soluble CD40 ligand, oxidative stress and body composition in patients with peptic ulcer disease. Internal Med. (Tokyo, Japan) 48, 2055–2059. https://doi.org/10.2169/internalmedicine.48.2562 (2009).
Arslan, E., Atilgan, H. & Yavaşoğlu, I. The prevalence of Helicobacter pylori in obese subjects. Eur. J. Internal Med. 20, 695–697. https://doi.org/10.1016/j.ejim.2009.07.013 (2009).
Aimasso, U. et al. Helicobacter pylori and nutrition: A bidirectional communication. Minerva Gastroenterologica e Dietologica 65, 116–129. https://doi.org/10.23736/s1121-421x.19.02568-6 (2019).
Franceschi, F. et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J. Gastroenterol. 20, 12809–12817. https://doi.org/10.3748/wjg.v20.i36.12809 (2014).
Knekt, P. et al. Antioxidant vitamins and coronary heart disease risk: A pooled analysis of 9 cohorts. Am. J. Clin. Nutr. 80, 1508–1520. https://doi.org/10.1093/ajcn/80.6.1508 (2004).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investigat. 127, 74–82. https://doi.org/10.1172/jci88883 (2017).
Walduck, A., Andersen, L. P. & Raghavan, S. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter 20(Suppl 1), 17–25. https://doi.org/10.1111/hel.12252 (2015).
Schumacher, A. et al. Positive Chlamydia pneumoniae serology is associated with elevated levels of tumor necrosis factor alpha in patients with coronary heart disease. Atherosclerosis 164, 153–160. https://doi.org/10.1016/s0021-9150(02)00043-6 (2002).
Yildirim, Z. et al. Increased exhaled 8-isoprostane and interleukin-6 in patients with Helicobacter pylori infection. Helicobacter 21, 389–394. https://doi.org/10.1111/hel.12302 (2016).
Chen, S. T., Ni, Y. H., Li, C. C. & Liu, S. H. Hepcidin correlates with interleukin-1β and interleukin-6 but not iron deficiency in children with Helicobacter pylori infection. Pediatr. Neonatol. 59, 611–617. https://doi.org/10.1016/j.pedneo.2018.02.007 (2018).
Liu, J., Zhang, F., Zhang, Z. & Zheng, C. Everolimus ameliorates Helicobacter pylori infection-induced inflammation in gastric epithelial cells. Bioengineered 13, 11361–11372. https://doi.org/10.1080/21655979.2021.2018533 (2022).
Watanabe, J. & Kotani, K. The effect of Helicobacter pylori eradication on C-reactive protein: Results from a meta-analysis. Arch. Med. Sci. AMS 18, 958–964. https://doi.org/10.5114/aoms/130288 (2022).
Acknowledgements
We thank Mengmeng Li for guiding our figure processing. We thank the editors at AJE for editing the English text of this manuscript.
Author information
Authors and Affiliations
Contributions
H.C. and Y.Z. contributed to the conceptualization, manuscript revision, and research supervision. B.L. analyzed the data and wrote the main manuscript text. Y.T.Z. collected the data and prepared figures. All authors reviewed the manuscript. Y.Z. and H.C. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, B., Zhang, Y., Zheng, Y. et al. The causal effect of Helicobacter pylori infection on coronary heart disease is mediated by the body mass index: a Mendelian randomization study. Sci Rep 14, 1688 (2024). https://doi.org/10.1038/s41598-024-51701-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51701-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.