Abstract
The fusion of mononuclear trophoblasts into multinucleate syncytiotrophoblasts is the critical event in the process of syncytialization, and its dysregulation can lead to pregnancy complications, notably hypertensive disorders of pregnancy (HDP). Oxidative stress may disrupt trophoblast syncytialization in HDP. Specifically, placentas with HDP exhibit impaired mitochondria, giving rise to the generation of reactive oxygen species (ROS) and subsequent oxidative stress. Quercetin, a bioflavonoid known for its antioxidant and anti-aging properties, has the potential to mitigate oxidative stress during trophoblast syncytialization. However, the precise mechanism underlying the action of quercetin in these processes remains to be elucidated. To explore the impact of quercetin on syncytialization, mitochondrial function, and ROS generation, cyclic AMP-stimulated BeWo cells were treated with quercetin. The expression of markers associated with cell fusion, mitochondrial function, and oxidative stress was determined using qPCR and western blotting. Additionally, morphological syncytialization and mitophagy (mitochondrial degradation) were assessed by immunofluorescence analysis. Our results revealed that quercetin increased the expression of syncytialization markers and promoted cell fusion. Furthermore, this compound also upregulated markers associated with mitophagy and mitochondrial fusion, which are corroborated by visual evidence of mitophagy through the fluorescence microscope. Cell fusion naturally stimulated ROS generation, which was attenuated by quercetin. Quercetin downregulated the expression of NRF2 and HO-1 during syncytialization, while increasing the expression of sirtuin1/3/6, which are known to play essential roles in antioxidant responses. In conclusion, quercetin effectively regulates mitochondrial function through its antioxidant properties and the suppression of ROS generation, ultimately promoting trophoblast fusion, suggesting that the flavonoid has the potential to ameliorate pregnancy-related disorder stemming from placental dysplasia.
Similar content being viewed by others
Introduction
The placenta plays an essential role in maintaining pregnancy and fetal growth. The placenta is composed mainly of trophoblast cells which fuse to form multinucleated syncytiotrophoblast or differentiate into invasive extravillous trophoblasts (EVTs)1. The multinucleate syncytiotrophoblast contributes to the exchange of gases and nutrition, produces various bioactive factors, and protects the fetus from the maternal immune system1,2. Dysfunction of trophoblast syncytialization leads to the pathogenesis of pregnancy complications such as hypertensive disorders of pregnancy (HDP) and fetal growth restriction (FGR)3.
It has been recently reported that the pregnancy complications may be associated with the increase in oxidative stress and reduction of anti-oxidative capacity, accompanying the inhibition of trophoblast syncytialization4,5. Furthermore, the placenta with HDP exhibits the dysfunction of placental mitochondria such as a decrease in mitochondrial DNA expression and mitochondrial complex IV activity6. Mitochondria produces adenosine triphosphate (ATP) through oxidative phosphorylation, whereas induces various disorders by abnormal calcium transfer pathways and reactive oxygen species (ROS) produced during energy production, such as ATP7,8. Thus, mitochondrial disorders, mainly ROS generation, induce oxidative stress.
The Kelch-like ECH-associated protein 1 (Keap1)-nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway plays an important defense system against ROS-induced oxidative stress. Nrf2 is a transcription factor that induces the expression of various defense genes and usually forms a complex with Keap1 dimers, and the transcriptional activity of Nrf2 is repressed by Keap19. Heme oxygenase 1 (HO-1), also known as heat shock protein-32, is an inducible enzyme and is involved in the regulation of iron homeostasis and antioxidant activity10. These factors are important in understanding oxidative stress and have been reported to be involved in placentation. In response to ROS overproduction and oxidative stress, mitochondria continue to undergo morphological changes through repeated division and fusion, and hypofunction of mitochondria is degraded by mitophagy which is selective degradation through autophagy, which leads to various disorders accompanying the accumulation of abnormal mitochondria11,12,13. During the process of mitophagy, sequestosome1 (SQSTM1; known as p62) activates PTEN induced kinase 1(PINK1) / parkin RBR E3 ubiquitin protein ligase (PRKN) pathway, thus the decrease in SQSTM1 exhibits abnormal mitophagy13. In addition, PRKN localizes in mitochondria that lose their membrane potential, thereby promoting mitochondrial degradation14.
Quercetin (Que) is a bioflavonoid found in fruits and vegetables that has beneficial antioxidant, anti-inflammation, and anti-aging effects. Quercetin prevents cells from mitochondrial injury by regulating mitochondrial biogenesis, mitochondrial membrane potential, and ATP anabolism15. This compound may protect against aging-related diseases, through activating sirtuin1 (SIRT1), a member of an NAD+-dependent protein deacetylase16. Previous studies demonstrated that quercetin has removed senescent cells and accelerated differentiation of human endometrial stromal cells17. Furthermore, quercetin could have improved the dysfunction of trophoblast invasion by oxidative stress in EVTs18. However, the effect of quercetin such as the removal of oxidative stress and maintenance of mitochondria on trophoblast syncytialization remains unknown. In this study, we examined the effect of quercetin on the syncytialization and mitochondria function, especially mitophagy and ROS generation, in trophoblast BeWo cells.
Results
Quercetin potentiates the syncytialization of trophoblast
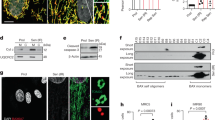
To investigate the effect of quercetin on the syncytialization of trophoblasts, the trophoblast BeWo cells were treated with cyclic AMP analog (cAMP) and quercetin. The increase in intercellular cAMP produces chorionic gonadotropin subunit beta (CGB) and progesterone19,20, and upregulates fusogenic syncytin (ERVFRD-1) through the transcription factors, glial cell missing 1 (GCM1), and ovol like transcriptional repressor 1 (OVOL1) in trophoblast cells. Quercetin increased the expression of the syncytialization markers ERVFRD-1, CGB, GCM1, OVOL1 and the number of fusogenic cells (Fig. 1A–C). Quercetin did not alter the intracellular cAMP levels (Fig. 1D).
Quercetin (Que) potentiates the syncytialization of trophoblasts. BeWo cells treated with dibutyryl cyclic AMP (cAMP, 500 µM) and Que (5 µM) for 48 h. (A) Expression of syncytialization markers ERVFRD-1, CGB, GCM1, and OVOL1 mRNA level was determined by qPCR. GAPDH was used as the reference gene. Values are represented as mean ± SEM of three independent experiments. *P < 0.05, ***P < 0.001. (B) Cells were immunostained with anti-E-cadherin antibody (green) and DAPI (blue) to visualize syncytialization. A representative picture from three independent experiments is shown and the syncytialized cells are marked with a stippled line. Scale bar = 100 µm. (C) Quantification of the number of syncytialized cells. The cell number in five areas selected randomly was counted in each experiment. The data are presented as ratios of the control and shown as mean ± SEM from three independent experiment, *P < 0.05. (D) Cyclic AMP level in the culture medium was determined by ELISA. Values are represented as mean ± SEM of three independent experiments.
Quercetin promotes mitophagy and mitochondrial fusion markers in syncytiotrophoblasts
We investigated the molecular mechanism by which quercetin promoted syncytializaton in BeWo cells. Quercetin increased the expression of mitochondrial fusion markers MFN1 and MFN2 which is involved in the maintenance of mitochondrial morphology, but did not alter the expression of dynamin 1 Like (DNM1L), a mitochondrial fission marker21, and Optic Atrophy 1 (OPA1), a GTPase which is localized in the mitochondrial inner membrane22. Quercetin upregulated the expression of the mitophagy markers SQSTM1 and PINK1, while did not alter other mitochondrial mitophagy markers FUNDC1 and BNIP3, and mitochondrial biosynthesis proteins TFAM (Fig. 2A). Quercetin also increased SQSTM1 protein expression (Fig. 2B). To further evaluate mitophagy flux, bafilomycin A1 was used to block the mitophagic flux, resulting in the mitochondrial proteins, LC3-II and SQSTM1 being rescued by bafilomycin A1 (Fig. 2C). Effect of quercetin on the mitophagy of trophoblast was further examined using mitophagy (red) and lysosomal dyes (green). Mitophagy (as merged with red and green), the selective degradation of mitochondria by fusion with lysosomes, was observed in BeWo cells treated with quercetin (Fig. 2D). In addition, the co-treatment of quercetin and cAMP significantly increased mitochondrial membrane potential (Fig. 2E).
Quercetin (Que) promotes mitophagy and mitochondrial fusion and fission in trophoblasts. BeWo cells treated with dibutyryl cyclic AMP (cAMP, 500 µM) and Que (5 µM) for 48 h. (A) Expression of MFN1/2, DNM1L, OPA1, SQSTM1, PINK1, FUNDC1, BNIP3, and TFAM was determined by qPCR. GAPDH was used as the reference gene. Values are represented as mean ± SEM of three independent experiments. **P < 0.01, ***P < 0.001. (B) Immunoblotting showing the protein levels of SQSTM1 in lysates from cAMP- and Que-treated BeWo cells. GAPDH served as the loading control. Representative data from three independent experiments are shown. The graph shows SQSTM1 levels normalized to GAPDH levels from three independent experiments. *P < 0.05. Values represent mean ± SEM. (C) Proteins extracted from BeWo cells treated with cAMP, Que, and bafilomycin A1 (100 nM) for 48 h were subjected to immunoblotting. GAPDH served as the loading control. Representative data from three independent experiments are shown. The graph shows the ratios of LC3-II/LC3-I and SQSTM1/GAPDH from three independent experiments. *P < 0.05. Values represent mean ± SEM. (D) Assessment of mitophagy. Co-localization of the mitophagy dye with the lysosome dye was evaluated. A representative picture from three independent experiments is shown. Scale bar = 100 µm. The graph shows levels of mitophagy staining intensity from three independent experiments. Values represent mean ± SEM. (E) Assessment of mitochondrial membrane potential. A representative picture from three independent experiments is shown. Scale bar = 100 µm. The graph shows levels of membrane potential staining intensity from three independent experiments. Values represent mean ± SEM. *P < 0.05.
Quercetin reduces mitochondrial ROS production and the expression of oxidative stress markers
To characterize the effect of quercetin on mitochondrial function, the production of ROS, the number of mitochondria, and oxidative stress were evaluated. Cellular fluorescence study showed that ROS was increased by cell fusion stimulation but decreased by quercetin treatment (Fig. 3A). Similar to Fig. 3A, data using flow cytometry (Fig. 3B) and microplate reader (Fig. 3C) displayed that cAMP-induced ROS level was inhibited by quercetin. In turn, the number of mitochondria was not changed regardless of cAMP and/or quercetin (Fig. 3D). Moreover, quercetin lowered the cAMP-stimulated NRF2 and HO-1 expression, whereas elevated KEAP1 (Fig. 3E). To further investigate whether quercetin affects these factors in the condition of increased ROS and/or mitochondrial damage, H2O2 as an oxidative stress inducer was treated. H2O2 decreased CGB and increased NRF2 expression, however quercetin counteracted expression of CGB and NRF2 (Fig. 3F).
Quercetin (Que) reduces mitochondrial ROS production and the expression of oxidative stress markers in trophoblasts. BeWo cells treated with cAMP inducer forskolin (cAMP, 2.5 µM) and Que (5 µM) for 48 h. (A) Fluorescence micrographs of ROS. Cells were immunostained with anti-E-cadherin antibody (red), DAPI (blue) and DCFH-DA (green) to visualize ROS. A representative picture from three independent experiments is shown. Scale bar = 20 µm (B) Flowcytometry analysis of ROS. Representative data from three independent experiments are shown. (C) Intercellular level of ROS measured by DHFH-DA dye. *P < 0.05 vs. cAMP. Values are represented as mean ± SEM of three independent experiments. (D) Flowcytometry analysis showing the number of mitochondria. Representative data from three independent experiments are shown. (E) Immunoblotting of NRF2, HO-1, and KEAP1. GAPDH served as the loading control. Representative data from three independent experiments are shown. The graph shows NRF2, HO-1, and KEAP1 levels normalized to GAPDH levels from three independent experiments. *P < 0.05. Values represent mean ± SEM. (F) BeWo cells treated with dibutyryl cyclic AMP (cAMP, 500 µM) and Que (5 µM) for 48 h, and then H2O2 (400 µM) was added for 24 h. Expression of CGB and NRF2 mRNA level was determined by qPCR. GAPDH was used as the reference gene. Values are represented as mean ± SEM of three independent experiments. *P < 0.05, ***P < 0.001.
Quercetin upregulates SIRT1 expression in syncytiotrophoblasts
SIRT1 is expressed in human trophoblasts, exerts anti-oxidative stress and anti-inflammatory effects, but detail roles of SIRT1 in trophoblast remains unknown. We examined whether quercetin regulated the expression of SIRT1, SIRT3, or SIRT6 in trophoblast cells which are localized in the cytoplasm, mitochondria, or nucleus, respectively. The expression of SIRT1, SIRT3, and SIRT6 was not altered by cAMP alone, but was increased by co-treatment with quercetin (Fig. 4A). Similar to mRNA expression, SIRT1 protein expression was increased by quercetin (Fig. 4B). We further evaluated the effect of quercetin through SIRT1 on syncytialization using an SIRT1 inhibitor. SIRT1 inhibitor treatment significantly suppressed cell fusion by quercetin (Fig. 4C,D).
Quercetin regulates SIRT1 expression in trophoblasts. BeWo cells treated with dibutyryl cyclic AMP (cAMP, 500 µM) and Que (5 µM) for 48 h. (A) The expression of the SIRT1, SIRT3, and SIRT6 was determined using qPCR analysis. GAPDH was used as the reference gene. Values are represented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01. (B) Immunoblotting of SIRT1 in lysates from cAMP- and/or Que-treated cells. GAPDH served as the loading control. Representative data from three independent experiments are shown. The graph shows SIRT1 levels normalized to GAPDH levels from three independent experiments. Values represent mean ± SEM. *P < 0.05, **P < 0.01 vs. Ctrl. (C, D) BeWo cells treated with dibutyryl cyclic AMP (cAMP, 500 µM), Que (5 µM), and SIRT1 inhibitor (1 µM) for 48 h. Cells were immunostained with anti-E-cadherin antibody (green) and DAPI (blue) to visualize syncytialization and the syncytialized cells are marked with a stippled line (C). Scale bar = 100 µm. Quantification of the number of syncytialized cells (D). The cell number in five areas selected randomly was counted in each experiment. The data are presented as ratios of the control and shown as mean ± SEM from three independent experiment, **P < 0.01.
Discussion
This study showed that quercetin promotes syncytialization induced by cAMP. Furthermore, quercetin increased some mitochondrial function markers, mitophagy, and mitochondrial membrane potential, while decreased ROS production and oxidative stress markers during cell fusion. In addition, quercetin increased the expression of SIRT1, 3, and 6, which have been reported to inhibit ROS production and oxidative stress. Thus, quercetin may promote cell fusion via SIRTs expression by restoring mitochondrial dysfunction and oxidative stress associated with trophoblast syncytialization (Fig. 5).
In pregnant women, material metabolism and hormone levels dynamically change for the maintenance of pregnancy and fetal development, compared to non-pregnant women. During pregnancy, oxidative stress and endoplasmic reticulum (ER) stress have induced dysplasia of placenta23. The source of ROS during pregnancy is placental mitochondria24. In normal pregnancy, oxidative stress increases due to the generation of ROS that occurs alongside inflammatory response8. The present study first found that mitochondrial metabolism increased during syncytialization, by which the increase in ROS production caused oxidative stress in trophoblast cells. Furthermore, quercetin decreased ROS production and oxidative stress and increased the expression of SIRT1, 3, and 6 in trophoblasts. SIRTs inhibit ROS production and oxidative stress. Furthermore, SIRT3 has mitochondrial biosynthesis and antioxidant effects25, and SIRT6 is responsible for protecting and repairing damage from oxidative stress26,27. In addition, SIRT1 has been reported to be a protective molecule in trophoblasts with preeclampsia28.
On the other hand, ER stress may regulate the invasion of extravillous trophoblast cells and relate to the pathogenesis of HDP29. Previous study showed that the ER stress inducers tunicamycin and thapsigargin inhibited trophoblast fusion. However, treatment of quercetin under induction of ER stress did not change the expression of cell fusion markers (data not shown). These findings suggest that quercetin could remove oxidative stress which is harmful to pregnancy via partially SIRTs independent of ER stress.
In this study, the expression of the mitochondrial fusion markers MFN1 and MFN2 were increased by quercetin. However, some mitochondrial fission markers and the mitophagy marker BNIP3 were not changed. This indicates that quercetin acts on SQSTM1-induced PINK1 specific for mitophagy pathway and MFN1/MFN2 fusion markers to regulate morpho-functional maintenance of mitochondria. There are several mitophagy pathways besides the SQSTM1-induced PINK1 mitophagy pathway, of which the pathways via BNIP3L and FUNDC1 were not altered by quercetin, meaning that quercetin regulated mitochondrial functions mediated by the SQSTM1/PINK1 mitophagy pathway. It has been reported that the expression PINK1 is decreased in preterm FGR placentae and that the expression of MFN2 is decreased in the placentas of HDP patients30,31. These findings suggest that quercetin could have a therapeutic effect on a disorder of placentation. Further investigation of physiological function and molecular mechanisms of how quercetin affects mitochondrial morphology, degradation, and fusion on trophoblast syncytialization is required.
Administration of antioxidants during pregnancy may increase the risk for maternal health, and hence the development of safe active ingredients for pregnant women is required. HDP occurs in about 10% of pregnant women, and there is no fundamental cure, only delivery. Oxidative stress increases the risk of pregnancy complications and spontaneous abortion and increases the expression of oxidative stress markers in the tissue of HDP placentas32. It has been reported that impaired autophagy and increased mitochondrial damage were observed in preeclampsia placenta when compared with normal placenta33. Furthermore, our RNA-seq data of HDP placenta indicates that mitochondrial damage-related transcripts are changed (data not shown). In this study, quercetin decreased the expression of cell fusion- or H2O2-induced oxidative stress markers and induced mitophagy in trophoblast cells. Quercetin is contained in foods, and experiments with mice have confirmed that there is no teratogenicity in the fetus34. Therefore, quercetin may be an effective alternative in the prevention and treatment of pregnancy complications, such as HDP35.
In conclusion, quercetin effectively regulates mitochondrial function by exerting antioxidant effects, suppressing ROS generation via SIRTs during the cell fusion process, and resulting in the promotion trophoblast cell fusion. As a result, quercetin could improve normal placentation and potentially alleviate pregnancy complications associated with placental dysplasia.
Materials and Methods
Cell culture
The human choriocarcinoma BeWo cell line, purchased from the JCRB Cell Bank (Osaka, Japan), were grown in 1:1 Ham’s F12/Dulbecco’s modified Eagle’s medium (Fujifilm Wako Pure Chemical Corp., Osaka, Japan) supplemented with 10% FBS and 1% PSA (Fujifilm Wako Pure Chemical Corp.) at 37 °C in humidified air containing 5% CO236. the cells were treated with dibutyryl cAMP (500 μM, Tokyo Chemical Industry, Tokyo, Japan) or forskolin (2.5 µM, Cayman chemical, Ann Arbor, MI USA) an adenylate cyclase activator, for 48 h to induce syncytialization. These cells were also treated for 48 h with Que (5 µM, Tokyo Chemical Industry).
RNA extraction and quantitative RT-PCR
RNA was extracted using the RNeasy Mini Kit (Qiagen, Tokyo, Japan), according to manufacturer’s instructions. Reverse transcription of mRNA was performed using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan), and the cDNA produced was subjected to qPCR amplification in a PowerUP SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). The primers used are listed in Table S1. Calibration curves were used to determine the amplification of each target gene with respect to the expression of a reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The mean crossing threshold (Ct) values for each target were calculated using Sequence Detection System software v2.3 (Thermo Fisher Scientific)37.
Cell fusion assay
BeWo cells were fixed with methanol and incubated with anti-E-cadherin antibody (1:200, #3195, CST) and AlexaFluor 594-conjugated goat anti-mouse antibody (Thermo Fisher Scientific) to distinguish cell surfaces. The nuclei were counterstained with 4′,6-diamino-2-phenylindole 2HCl (DAPI). The number of nuclei in syncytiotrophoblasts and total number of nuclei were counted in five randomly selected microscopic areas per sample, and the fusion index was calculated [(number of nuclei in syncytia/total number of nuclei) × 100] in three independent experiments29.
ELISA for intracellular cAMP
BeWo cells (1.6 × 104) were seeded in 96-well culture plates, and treated with dibutyryl cAMP (500 µM) and quercetin (5 µM) for 48 h. The concentration of cAMP in the culture medium was determined using the Cyclic AMP ELISA Kit (Abcam, Tokyo, Japan) according to the manufacturer’s instruction.
Western blotting
Harvested cells were lysed in RIPA buffer (Thermo Fisher Scientific), and then equal amounts of lysate proteins were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA) using a Trans-Blot Turbo (Bio-Rad). After blocking with Bullet Blocking One (Nacalai Tesque, Kyoto, Japan), the membranes were incubated with primary antibodies against SQSTM1 (1:5000, BC017222; ProteinTech, Chicago, IL, USA), NRF2 (1:2000, SAB1303359; Sigma-Aldrich, Tokyo, Japan), HO-1 (1:2000, ab13248; Abcam), KEAP (1:5000, BC002930; ProteinTech), SIRT1(1:5000, BC012499; ProteinTech) or GAPDH (1:5000, 5A12; Fujifilm Wako Pure Chemical Corp.). Immunoreactive bands were detected using enhanced chemiluminescence (Merck Millipore, Burlington, MA, USA) after incubation with horseradish peroxidase-labeled goat anti-rabbit or anti-mouse IgG (1:5000; Vector Laboratories, Burlingame, CA, USA). Signals were detected using a C-DiGit Blot Scanner (LI-COR), and the relative band density was quantified using Image Studio DiGit software (version 5.2)38.
Mitophagy assay
BeWo cells (1.6 × 104) treated with forskolin (2.5 µM), and quercetin (5 µM) were seeded into ibidi µ-slide 8well (Ibidi, Martinsried, Germany) coated with Matrigel (Corning, Corning, NY, USA) and cultured at 37 °C for 48 h. Mitophagy was detected using the Mitophagy detection kit (Dojindo Molecular Technologies, Kumamoto, Japan). The cells were treated with 100 nM Mtphagy Dye working solution at 37 °C for 30 min. After washing with the medium, cells were incubated with 1 µM Lyso Dye working solution for 30 min, and the levels of mitophagy were detected using fluorescence microscope. The fluorescence of colocalization was measured using the microcell count system (Keyence). Relative staining intensity was calculated [(fluorescence intensity of mitochondrial membrane potential/total staining area) × 100]. The data are presented as ratios of the control and shown as mean ± SEM from three independent experiments.
Mitochondrial membrane potential assay
BeWo cells (4 × 104) treated with forskolin (2.5 µM), and quercetin (5 µM) were seeded into 24-well coated with Matrigel (Corning) and cultured at 37 °C for 48 h. The mitochondrial membrane potential was evaluated using the MT-1 MitoMP Detection Kit (Dojin Molecular Technologies) following the manufacturer's protocol. Cells were incubated with MT-1 working solution for 30 min at 37 °C, and mitophagy were detected using BZX800 fluorescence microscope (Keyence). The fluorescence was measured using microcell count system (Keyence). Relative staining intensity was calculated (fluorescence intensity of mitochondrial membrane potential/total staining area). The data are presented as ratios of the control and shown as mean ± SEM from three independent experiments.
The measurement of ROS level
BeWo cells (1.6 × 104) treated with forskolin (2.5 µM), and quercetin (5 µM) were seeded into 96-well plates coated with Matrigel (Corning) and cultured at 37 °C for 48 h. The supernatant was removed, and a highly sensitive DCFH-DA dye working solution (Dojindo) was added and then incubated at 37 °C for 30 min. Changes in the levels of ROS were detected using fluorescence microscope, flow cytometry, and plate leader at Ex/Em: 490 nm/540 nm.
Determination of the number of mitochondria
The number of mitochondria in BeWo cells using the MitoBright IM Red for immunostaining (Dojindo). BeWo cells (16 × 104) treated with forskolin, and quercetin were seeded into 6-well plates cultured at 37 °C for 48 h. The alteration of the number of mitochondria was detected using flow cytometry.
Statistical analysis
Data are expressed as mean ± SEM, and were compared using the Dunnett’s test. A P-value < 0.05 was considered to be statistically significant. Statistical testing was performed using the R software (ver.4.0.5; www.r-project.org).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bischof, P. & Irminger-Finger, I. The human cytotrophoblastic cell, a mononuclear chameleon. Int. J. Biochem. Cell Biol. 37, 1–16. https://doi.org/10.1016/j.biocel.2004.05.014 (2005).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6, 584–594. https://doi.org/10.1038/nri1897 (2006).
Gauster, M., Moser, G., Orendi, K. & Huppertz, B. Factors involved in regulating trophoblast fusion: Potential role in the development of preeclampsia. Placenta 30(Suppl A), S49-54. https://doi.org/10.1016/j.placenta.2008.10.011 (2009).
Chiarello, D. I. et al. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165354. https://doi.org/10.1016/j.bbadis.2018.12.005 (2020).
Hubel, C. A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222, 222–235. https://doi.org/10.1177/153537029922200305 (1999).
Vangrieken, P. et al. Placental mitochondrial abnormalities in preeclampsia. Reprod. Sci. 28, 2186–2199. https://doi.org/10.1007/s43032-021-00464-y (2021).
Sherratt, H. S. Mitochondria: Structure and function. Rev. Neurol. (Paris) 147, 417–430 (1991).
Hussain, T. et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 9962860. https://doi.org/10.1155/2021/9962860 (2021).
Baird, L. & Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol https://doi.org/10.1128/mcb.00099-20 (2020).
Bindu, S. et al. Translocation of heme oxygenase-1 to mitochondria is a novel cytoprotective mechanism against non-steroidal anti-inflammatory drug-induced mitochondrial oxidative stress, apoptosis, and gastric mucosal injury. J. Biol. Chem. 286, 39387–39402. https://doi.org/10.1074/jbc.M111.279893 (2011).
Liesa, M., Palacín, M. & Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89, 799–845. https://doi.org/10.1152/physrev.00030.2008 (2009).
Ausman, J. et al. Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis. 9, 298. https://doi.org/10.1038/s41419-018-0360-0 (2018).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131. https://doi.org/10.1038/ncb2012 (2010).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803. https://doi.org/10.1083/jcb.200809125 (2008).
de Oliveira, M. R. et al. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 34, 532–549. https://doi.org/10.1016/j.biotechadv.2015.12.014 (2016).
Cui, Z. et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 13, 943321. https://doi.org/10.3389/fimmu.2022.943321 (2022).
Kusama, K. et al. Senolytic treatment modulates decidualization in human endometrial stromal cells. Biochem. Biophys. Res. Commun. 571, 174–180. https://doi.org/10.1016/j.bbrc.2021.07.075 (2021).
Ebegboni, V. J., Balahmar, R. M., Dickenson, J. M. & Sivasubramaniam, S. D. The effects of flavonoids on human first trimester trophoblast spheroidal stem cell self-renewal, invasion and JNK/p38 MAPK activation: Understanding the cytoprotective effects of these phytonutrients against oxidative stress. Biochem. Pharmacol. 164, 289–298. https://doi.org/10.1016/j.bcp.2019.04.023 (2019).
Sullivan, M. H. Endocrine cell lines from the placenta. Mol. Cell Endocrinol. 228, 103–119. https://doi.org/10.1016/j.mce.2003.03.001 (2004).
Tuckey, R. C. Progesterone synthesis by the human placenta. Placenta 26, 273–281. https://doi.org/10.1016/j.placenta.2004.06.012 (2005).
Kleele, T. et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439. https://doi.org/10.1038/s41586-021-03510-6 (2021).
Alexander, C. et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26, 211–215. https://doi.org/10.1038/79944 (2000).
Capatina, N., Hemberger, M., Burton, G. J., Watson, E. D. & Yung, H. W. Excessive endoplasmic reticulum stress drives aberrant mouse trophoblast differentiation and placental development leading to pregnancy loss. J. Physiol. 599, 4153–4181. https://doi.org/10.1113/jp281994 (2021).
Burton, G. J. & Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 287–299. https://doi.org/10.1016/j.bpobgyn.2010.10.016 (2011).
Xin, T. & Lu, C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY) 12, 16224–16237. https://doi.org/10.18632/aging.103644 (2020).
Zhang, C., Yu, Y., Huang, Q. & Tang, K. SIRT6 regulates the proliferation and apoptosis of hepatocellular carcinoma via the ERK1/2 signaling pathway. Mol. Med. Rep. 20, 1575–1582. https://doi.org/10.3892/mmr.2019.10398 (2019).
Pan, H. et al. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 26, 190–205. https://doi.org/10.1038/cr.2016.4 (2016).
Liu, Z., Wang, C., Pei, J., Li, M. & Gu, W. SIRT1: A novel protective molecule in pre-eclampsia. Int. J. Med. Sci. 19, 993–1002. https://doi.org/10.7150/ijms.73012 (2022).
Yoshida, K., Yano, A., Kusama, K., Ishikawa, G. & Tamura, K. Alpha 1 antitrypsin regulates trophoblast syncytialization and inflammatory factor expression. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23041955 (2022).
Yu, J., Guo, X., Chen, R. & Feng, L. Downregulation of mitofusin 2 in placenta is related to preeclampsia. Biomed. Res. Int. 2016, 6323086. https://doi.org/10.1155/2016/6323086 (2016).
Bartho, L. A., McKeating, D. R., Hannan, N. J., Kaitu’u-Lino, T. J. & Perkins, A. V. Transcriptional profiles of genes related to mitochondrial aging in placental pathologies. Mol. Hum. Reprod. https://doi.org/10.1093/molehr/gaac026 (2022).
Qiu, D. et al. Impaction of factors associated with oxidative stress on the pathogenesis of gestational hypertension and preeclampsia: A Chinese patients based study. Medicine 100, e23666. https://doi.org/10.1097/md.0000000000023666 (2021).
Zhou, X. et al. Impaired placental mitophagy and oxidative stress are associated with dysregulated BNIP3 in preeclampsia. Sci. Rep. 11, 20469. https://doi.org/10.1038/s41598-021-99837-1 (2021).
Prater, M. R., Laudermilch, C. L., Liang, C. & Holladay, S. D. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta 29, 802–808. https://doi.org/10.1016/j.placenta.2008.06.010 (2008).
Ożarowski, M. et al. Pharmacological effect of quercetin in hypertension and its potential application in pregnancy-induced hypertension: review of in vitro, in vivo, and clinical studies. Evid. Based Complement Alternat. Med. 2018, 7421489. https://doi.org/10.1155/2018/7421489 (2018).
Teoh, S. S., Zhao, M., Wang, Y., Chen, Q. & Nie, G. Serum HtrA1 is differentially regulated between early-onset and late-onset preeclampsia. Placenta 36, 990–995. https://doi.org/10.1016/j.placenta.2015.07.001 (2015).
Kusama, K. et al. Cordyceps militaris fruit body extract decreases testosterone catabolism and testosterone-stimulated prostate hypertrophy. Nutrients 13, 50. https://doi.org/10.3390/nu13010050 (2020).
Kusama, K., Bai, R. & Imakawa, K. Regulation of human trophoblast cell syncytialization by transcription factors STAT5B and NR4A3. J. Cell Biochem. 119, 4918–4927. https://doi.org/10.1002/jcb.26721 (2018).
Acknowledgements
This research was supported in part by JSPS KAKENHI Grants numbers JP20H03133 (K.K.), and Mishima Kaiun Memorial Foundation (K.Y.).
Funding
Mishima Kaiun Memorial Foundation, Japan Society for the Promotion of Science, 20H03133.
Author information
Authors and Affiliations
Contributions
K.Y., G.S. and S.S. performed experiments and analyses. K.Y., K.K. G.S. and K.T. wrote the main manuscript text and K.Y., K.K., and G.S. prepared all figures and tables. K.K., M.Y., and K.T. were involved in the planning of the entire experimentation. K.Y., K.K., G.S., S.S., M.Y. and K.T. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshida, K., Kusama, K., Shinohara, G. et al. Quercetin stimulates trophoblast fusion via the mitochondrial function. Sci Rep 14, 287 (2024). https://doi.org/10.1038/s41598-023-50712-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50712-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.