Abstract
This paper introduces a novel approach for detecting the SARS-CoV-2 recombinant spike protein combining a label free electrochemical impedimetric immunosensor with the use of purified chicken IgY antibodies. The sensor employs three electrodes and is functionalized with an anti-S IgY antibody, ELISA and immunoblot assays confirmed the positive response of chicken immunized with SARS-CoV2 S antigen. The developed immunosensor is effective in detecting SARS-CoV-2 in nasopharyngeal clinical samples from suspected cases. The key advantage of this biosensor is its remarkable sensitivity, and its capability of detecting very low concentrations of the target analyte, with a detection limit of 5.65 pg/mL. This attribute makes it highly suitable for practical point-of-care (POC) applications, particularly in low analyte count clinical scenarios, without requiring amplification. Furthermore, the biosensor has a wide dynamic range of detection, spanning from 11.56 to 740 ng/mL, which makes it applicable for sample analysis in a typical clinical setting.
Similar content being viewed by others
Introduction
Solid-support interfaces play a crucial role in biorecognition reactions involving various analytes such as cells, microbes, DNA, proteins, enzymes, antibodies, and antigens1,2,3. These reactions cause significant changes at three key components: the immobilized macromolecule, the macromolecule-fluid interface within the fluid boundary layer, and the bulk fluid surrounding the macromolecule. These changes impact optical and electric properties, including refractive index, voltage, current, capacitance, and impedance. Understanding interfacial interactions is vital for applications like surface wetting, cell adhesion, and biosensor technologies in diverse biological systems4.
Compared to optical, colorimetric, and piezoelectric biosensors, electrochemical biosensors are gaining popularity due to their affordability, portability, simplicity, rapidity, sensitivity, and suitability for point-of-care diagnostics, particularly in resource-limited regions5,6,7,8,9,10. Electrochemical impedance spectroscopy (EIS) is widely used in corrosion monitoring, electrodeposition, batteries, biotechnology, drug discovery, disease detection, tissue engineering, cell culture, and environmental monitoring6,7,8,9,10,11. EIS biosensors can also be used in clinical applications and detection of various analyte11,12,13,14,15.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over five million global deaths16,17,18. SARS-CoV-2 is measured by diagnostic methods such as RT-PCR and immunosensors (ELISA assays and agglutination tests), in addition to other less common tools, including loop-mediated isothermal amplification (LAMP), clustered regularly interspaced short palindromic repeats (CRISPR), plasmonic metasensors, and electrochemical impedance spectroscopy (EIS)7,8,19,20,21,22.
Efforts have been made to enhance biosensors for SARS-CoV-2 detection, focusing on reproducibility, cost reduction, and achieving optimal detection limits utilized the carboxymethyl chitosan conjugation EDC/NHS method and conducted an electrochemistry spectroscopy experiment on spike protein and its antibody. The immunosensors' specificity has been observed by comparing control samples with varying concentrations of inactivated SARS-CoV-2, demonstrating low detection limits of 0.179 fg/mL and selectivity for proteins other than spike protein7,8.
In a study by Mojsoska et al.23, a graphene electrode was utilized in an EDC conjugation approach and successfully detected signals above 20 µg/mL within 45 min. Another investigation conducted by Eissa et al.24 achieved a detection limit of 0.8 pg/mL for N protein in 30 min. Similarly, in 2020, researchers employed similar methods and reported detection limits of 10 fM within 30 s for saliva and 1 fg/mL for nasopharyngeal samples25,26.
In this work, we have developed an electrochemical biosensor based on impedance spectroscopy and cyclic voltammetry to detect SARS-CoV-19 spike protein. The immunosensor, equipped with three electrodes and functionalized with anti-S IgY antibodies obtained from purified Polyclonal Chicken IgY antibodies which demonstrates high specificity and sensitivity. The process involved in immobilizing the Anti-Spike protein IgY antibody onto the electrodes included chemisorbing the thiol sites of cysteamine onto the surface of gold nanoparticles. Subsequently, glutaraldehyde was applied to establish a connection between the cysteamine and glutaraldehyde groups, ensuring the linkage of the antibody's amine groups to the aldehyde groups of glutaraldehyde. The developed immunosensor effectively detects SARS-CoV-2 in nasopharyngeal clinical samples from suspected cases. Its remarkable sensitivity allows it to detect very low concentrations of the target analyte, with a detection limit of 5.65 pg/mL. This high sensitivity makes it suitable for practical point-of-care (POC) applications, especially in low target count clinical scenarios, without the need for amplification. Additionally, the biosensor has an extensive dynamic range of detection, ranging from 11.56 to 740 ng/µL, making it applicable for sample analysis in a typical clinical setting.
Materials and methods
In this section, we will describe the methods and materials used in this work.
Chicken accommodation for experiment
Animal Experiments were approved by Yarmouk University Animal Care and Use Committee (No.AICUC/2021/11) following Jordanian and US National Institute of health guidelines. All studies involving animals. The study in this work is reported in accordance with ARRIVE guidelines.
Two rooms in the animal house at Yarmouk University were reconstructed for the accommodation of the 22 weeks old Novogen White (Longhorn) egg layer hens. After area disinfection, straw was spread on floor and clean feeder, drinks and buckets were used. Light was programmed in such a way it is on for 18 h and off for 6 h, and rooms temperature was maintained at 26 °C. Healthy hens were purchased from a local farm. Eggs laying was observed for each hen and collected daily and kept in a refrigerator until used.
Chicken immunization and production of anti-S IgY
Chicken (3 per group) were immunized intramuscularly in the chest with pure antigens of SARS-CoV-2 proteins (S) containing 400 µg mixed 1:1 with complete or incomplete Freund’s adjuvant (CFA) (Thermo Fisher, USA) following a standard immunization protocol. First injection was prepared with FCA and three shots with FIA were given 2 weeks a part. Control group was injected with normal saline mixed with matching adjuvant. Blood samples were collected from immunized and control chicken in plain vacutainer tubes (FL Medical, Italy) and left to clot. Serum was collected after centrifugation of tubes at 2000×g (MPW-251, Poland) for 10 min.
Evaluation of anti S IgY by ELISA
Antibodies binding activities were tested by ELISA. In brief, 96-well plate (Greiner Bio-One, Germany) was coated with 5 µg/mL recombinant S antigens (RayBiotech, USA) prepared in carbonate-bicarbonate buffer (0.05 M, pH 9.6) (PS Park, UK). Purified anti- antibodies with different dilutions were used to determine the antibodies titer. Finally, HRP-conjugated Rabbit anti-IgY (abcam, UK) diluted 1:1000 was added as tracer. Optical density was measured after the addition of substrate solution (TMB, Sigma-Aldrich, Germany) at 450 nm using Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher, USA).
Extraction of chicken IgY from eggs yolk
Chicken IgY was prepared using the PEG technique. Briefly, egg white-free yolk was collected in 50 mL tubes and mixed with twice volume PBS (Vivantis, Malaysia) and 3.5% poly ethylene glycol (PEG) 6000 (Bio basic, Canada) then vortexed by vortex (Velp Scientifica, Italy), followed by 30 min rolling on a rolling mixer (BIOBASE, China). Aqueous phase was transferred to a new tube and 9.5% PEG 6000 was added then vortexed and rolled for 15 min. Centrifugation step was repeated and pellet that contains precipitated IgY was collected and dissolved again in 12% PEG 6000 and treated as before. After centrifugation the pellet is dissolved in PBS and dialyzed overnight with 1 L of PBS using cellulose membrane (Sigma-Aldrich, Germany). The protein content (mg/mL) of the dialyzed samples was measured by the Bradford protein assay (Sigma-Aldrich, USA) and samples were stored at -20C until further use.
Purification of polyclonal chicken antibodies using HiTrap IgY column
HiTrap IgY purification column (Cytiva, Sweden), is a prepacked, ready to use, column for purification of IgY from egg yolk. In brief, the column was loaded with 5 mL crude egg PEG extract diluted in binding buffer after being washed with 5 × binding buffer (20 mM sodium phosphate, 0.5 M K2SO4, pH 7.5). The column was washed with 10 × binding buffer until no material appears in the effluent. Chicken IgY was eluted using elution buffer (20 mM sodium phosphate, pH 7.5). The eluent was concentrated by Vivispin concentrator with MWCO of 30 kDa (Merck Millipore, USA). Antibodies were washed twice with PBS, pH 7.4 and stored at − 20 °C for further processing.
Characterization of purified chicken IgY using SDS-PAGE
SDS-PAGE was performed for the evaluation of IgY purity. (19:1) Acrylamide/Bisacrylamide, 40% solution (Fisher Biotech, USA) was used. This was performed using Desaphor VE minigel (Cleaver scientific, UK) in the discontinuous buffer system using 0.5 mm thick 12% acrylamide–bisacrylamide gels under reducing conditions. Ten microliter of egg extract or purified IgY were mixed with an equal volume of sample buffer (pH 6.8) and loaded to gel after being boiled for 5 min. For band size determination, molecular weight prestained protein standard (Bio-helix, New Taipei City, Taiwan), was used after being processed in a similar way as extract samples. Electrophoresis was carried out using running buffer with pH 8.3 at 120 V for 60–120 min. The gel was stained with Coomassie brilliant blue R-250 (BDH pool, Dorset, UK), and destained by 20% acetic acid (LOBA Chemie, India) until clear bands were seen.
Identification of antibody-protein reactivity by western blot
The reactivity of IgY anti S antigen was determined by western blot. Recombinant SARS-CoV2 antigens were electrophoresed in 12% SDS-PAGE gel, transferred into the nitrocellulose membrane, and blocked with 3% bovine serum albumin. The membrane was incubated with purified chicken IgY and incubated for 1 h at RT. On a plate rocker. After membrane washing, monoclonal mouse anti-chicken IgY (H&L) HRP conjugated (MY BioSource, USA) diluted 1:1000 in PBS-Tween was added and incubated for 1 h at RT. Ready to use TMB substrate was poured on washed strips until color development.
Sensor fabrication
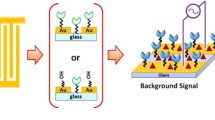
In all experiments, a three electrodes gold sensor (manufactured by BVT technologies, Czech Republic) was consistently employed. It consists of a counter electrode (CE) and a working electrode (WE) both are made of gold, and a silver/silver chloride reference electrode (RE), as illustrated in Fig. 1. The biosensor preparation involved several stages. Initially, the electrode surface was rinsed with deionized water and left to dry at room temperature (RT). After the cleaning process, the fabrication proceeded by adding 3 µL of 2 mM cysteamine (Sigma-Aldrich, Germany) exclusively to the working electrode. This step aimed to chemisorb the thiol sites of cysteamine onto the surface of gold nanoparticles. Subsequently, a coating of 30 µL of 5 mM glutaraldehyde (Alfa Aesar, Germany) was applied to the electrode to create a bond between cysteamine and glutaraldehyde groups. This was followed by the addition of 30 µL of 5 µg anti-spike protein IgY antibody, which linked the antibody’s amine groups to the aldehyde groups of glutaraldehyde. Then 30 µL of 5 mM ethanolamine (Sigma-Aldrich, Germany) was applied to the electrode to prevent unbound spaces. All these preparation steps involved a 10-min incubation at room temperature (23 °C) controlled using the room air conditioner.
Measurement procedure
To detect the spike protein, the electrodes surface was prepared to create the biosensor before each experiment. The biosensor was coated with 30 µL of SARS-CoV-2 spike (S) protein (740, 370, 185, 92.5, 46.25, 23.125, and 11.56 ng/mL), and incubated for 15 min at RT. Before doing electrochemical measurements, biosensors were cleansed with deionized water, rinsed with 1 mL of PBS, and then dried at room temperature.
In all experiments, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed using a versatile portable potentiostat (PalmSens4 BV, v5.9, Netherlands). The measurements were carried out using PBS containing 5 mM potassium ferri/ferrocyanide [Fe(CN)6]3−/[Fe(CN)6]4− (Thermo Fisher, USA). All CVs were conducted in a voltage range of − 0.2 to 0.5 V with a potential step of 0.01 V and a scan rate of 0.1 V/s. In the EIS measurements, the frequency range was 100 kHz to 0.5 Hz, and the AC and DC voltages were 0.005 V and 0.0 V, respectively.
To identify the spike protein, the electrochemical signal of the custom sensor is recorded after performing a blocking step using ethanolamine, which serves as the background signal. Subsequently, the sensor is exposed to the analyte for incubation, and the electrochemical signal is measured again. As the concentration of the analyte increases, the results obtained from cyclic voltammetry demonstrate a decrease in the peak current at the activation potential. Additionally, impedance spectroscopy reveals an increase in impedance with the rise in analyte concentration. Finally, to obtain the accurate analyte signal, the background signal is subtracted from each measured analyte signal.
The anti-S immunosensor was then further evaluated to determine the limit of detection (LOD) through dilution ranging from 180 pg/mL down to 2.83 pg/mL of SARS-CoV2 recombinant S antigen. To validate the effectiveness of the anti-spike protein biosensors, random COVID-19-positive nasopharyngeal samples were examined after being pipetted out from lysis solution included in the RAPID assay (ATLAS Biomedical, Jordan) and diluted in PBS to varying dilutions. The samples were collected by a qualified staff in a clinics assigned to do RAPID SARS-CoV-2 antigen test by Ministry of Health in Jordan. Vials containing the sample buffers were the nasopharyngeal swabs were washed were used in this study.
Results
Antibody IgY response to SARS-CoV2 recombinant proteins
Serum samples collected from chicken during the experiment time revealed the production of chicken IgY in response to antigen immunization. ELISA results showed an increase in antibody titer reaching highest concentration after the 3d booster. Chicken laid eggs were collected and IgY was extracted from egg yolk using PEG extraction protocol. The total yield of purified IgY per egg was between 17.5 and 25 mg for about 10 extraction and purification trials. Furthermore, purification of extracted IgY using affinity columns revealed the presence of pure IgY with heavy and light chains of the expected sizes (Fig. 2). The reactivity of the purified anti Covid 19 IgY were confirmed using immunoblot against recombinant antigens (Fig. 3).
Results from the electrochemical impedance spectroscopy
The electrochemical impedance spectroscopy (EIS) was employed throughout the experiments to assess the behavior of the electrode after each step of surface modification. The PStrace 5.8 software was used to display the Nyquist Plots that represent the impedance of spike protein at different concentrations ranging from 11.56 to 740 ng/mL (Fig. 4). The subsequent addition of phosphate-buffered saline (PBS) to the sensor as a control analyte resulted in a relatively minor increase in impedance compared to the ethanolamine step. However, introducing spike protein at a concentration of 11.56 ng/mL with a 15-min incubation time significantly increased the measured impedance. Consequently, the measured impedance increased with higher protein concentrations (Fig. 4A). We have included more electrochemical impedance spectroscopy results in the Supplementary file (S.4 Supplementary Figs. 1–6).
Electrochemical impedance spectroscopy for anti-s protein biosensor; (A) Nyquist plot resulted from response to various spike protein concentrations ranging from 11.56 to 740 ng/mL under the effect of ferri/ferrocyanide oxidation–reduction; (B) Capacitance curve obtained from several trials (n = 7 for each concentration, mean ± SEM).
A data fitting was done for the measured impedance spectrum and a circuit model was developed to represent a quantitative measurement for the impedance at different protein concentrations. This circuit, depicted in the inset of Fig. 4, consists of different components arranged in a specific configuration: R1, C1, R2, and W. It incorporates the solution resistance (Rsol), a double layer capacitance denoted as C1, the charge transfers resistance (Rct), and the Warburg impedance (W). The reaction is believed to occur in a single step, and the overall process is described by a combination of kinetic and diffusion processes through a layer that is considered to have infinite thickness. The circuit model, shown in the inset of Fig. 2 presents a parallel arrangement of double layer capacitance C1 and a Warburg impedance (W). Additionally, there is a resistance (Rct) to account for the accessibility of ions within porous structures and surface functionalities, as well as the polarization resistance. To consider the resistance of the electrolyte and the contact resistance of the current collector, a series resistance (Rsol) is introduced.
In analytical applications, the Warburg impedance can be disregarded in the complete equivalent circuit by selecting a frequency range where a 45° line is not visible in the Nyquist plot. This is because the Warburg impedance is directly proportional to the square root of the frequency. As a result, at higher frequencies, the contribution from the Warburg impedance becomes negligible due to its small magnitude. Upon analyzing the data and performing fitting procedures, it was observed that the capacitance values in the generated models decreases with the increase in the analyte concentration. By normalizing the capacitance data with the ethanolamine data, the resulting calibration curve exhibited an almost linear rise in capacitance values as the spike protein concentrations increased, ranging from 11.56 to 740 ng/mL (Fig. 4B).
Results from cyclic voltammetry
The results from cyclic voltammetry confirmed the results achieved from impedance spectroscopy and clearly demonstrated the reversible peaks of the ferri/ferrocyanide electron transfer from one step to another. The results in Fig. 5 show that as the concentration of the analyte increases, the current peak decreases at approximately the same activation voltage. This observation aligns with Ohm's law, which state that current is inversely proportional to impedance. We have included more cyclic voltammetry results in the Supplementary file (S.6 Supplementary Figs. 9–13).
cyclic voltammograms (CVs) of spike protein at different concentrations (ng/mL); (A) Cyclic voltammogram of spike protein from 11.56 to 740 ng/mL with activation potential from 0.220 to 0.260 V; (B) Cyclic voltammetry of spike protein for several trials (n = 8 for each concentration, mean ± SEM), the activation potential is 0.28 ± 0.35.
Additionally, the limit of detection (LOD) of the anti-S immunosensor was 5.65 pg/mL (Fig. 6), indicating that the sensor was able to detect very low concentrations of the target analyte, making it a very sensitive and reliable immunosensor. we have included more LOD results in the Supplementary file (S.5 Supplementary Figs. 7, 8).
Test validation on spike proteins using clinical samples
The anti-S biosensors were then validated using positive and negative clinical COVID-2 nasopharyngeal specimens diluted to 1:1000 for measurement of capacitance, which was then converted to ng/mL concentrations (Figs. 7 and 8, Table 1). The correlation was 84.6% for positive samples and 75% for negative samples (Table 2). However, if RAPID antigen test results were considered as reference or gold standard then the sensitivity and specificity of developed EIS would be 86% and 82%, respectively.
We have included a table for fitted date from capacitance for each electrode (n = 7), before and after normalization with ethanolamine (blocking step) in the Supplementary file (Supplementary Table 1).
Discussion
In this paper, a highly sensitive biosensor with enhanced performance was developed to rapidly diagnose a wide range of diseases, including epidemic diseases like SARS-CoV-2. The biosensor is based on an electrochemical impedimetric spectroscopy utilizing chicken IgY antibodies. The selection of the spike protein as the target analyte was due to its high immunogenicity and specific capability to detect SARS-CoV-2, as well as its lower sequence similarities to earlier SARS-CoV and MERS viruses compared to other proteins used in the literature26,27.
The use of egg-derived IgY polyclonal antibodies offers several advantages over antibodies produced from other sources, such as their specificity against highly conserved mammalian antigens and cost-effective and rapid extraction procedures in addition to being stressful free to animal28,29,30. The yield of purified IgY was comparable with other studies that showed a yield of 18.5–31.5 mg per egg31,32. However, IgY polishing using affinity purification reduced the yield but removed artifacts that may affect the biosensor fabrication and consequently its sensitivity.
Electrochemical impedance spectroscopy (EIS) was employed to measure the impedance change of the working electrode surface during the process of adding each layer of cysteamine, glutaraldehyde, and anti-spike antibody. The results were comparable to those achieved in previous studies7,8,27,33.
The biosensor exhibited remarkable sensitivity and linearity, enabling the detection of a broad spectrum of spike protein concentrations, ranging from 11.56 to 740 ng/mL, with a detection limit as low as 5.65 pg/mL. Cyclic voltammetry analysis provided additional confirmation of the sensor's sensitivity and linearity34.
The calibration curves showed that the developed electrochemical sensor outperformed previous studies in terms of sensitivity, linearity, and wider detection range7,8,26,27,33. This makes the sensor highly suitable for building models in portable devices for clinical applications.
Random clinical SARS-CoV-2 samples were tested on the immunosensor, confirming its ability to efficiently detect the spike protein in both recombinant and real samples. However, further investigations are needed to optimize the detection precision and incubation time. In comparison to other studies who could reach as low as 0.01 ag/mL detection limit35 our rapid sample analysis (15 min) is considered as good achievement that comply with POC time saving requirements.
As RT-PCR was the gold standard for any test for the calculation of sensitivity and specificity, the lack of PCR confirmed clinical samples due to pandemic cessation was a big constraint for our study. However, the reference technique in this study, the RAPID N detection system, revealed 63% to about 75% sensitivity when compared to nucleic acid based techniques36,37. Moreover, the fact that the lateral flow test is based on the measurements of N and not S antigen may affect decision about sensitivity and specificity.
In conclusion, the developed IgY based immunosensor shows great potential for the rapid diagnosis of various diseases, particularly epidemic outbreaks like SARS-CoV-2, making it a valuable tool for clinical applications.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary material.
References
Stills, H. F. Polyclonal antibody production. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents 259–274 (Elsevier, 2012).
Ma, C. et al. The critical experimental aspects for developing pathogen electrochemical biosensors: A lesson during the COVID-19 pandemic. Talanta 253, 124009 (2022).
Patolsky, F., Katz, E., Bardea, A. & Willner, I. Enzyme-linked amplified electrochemical sensing of oligonucleotide–DNA interactions by means of the precipitation of an insoluble product and using impedance spectroscopy. Langmuir 15(11), 3703–3706 (1999).
Twardowski, M. & Nuzzo, R. G. Molecular recognition at model organic interfaces: Electrochemical discrimination using self-assembled monolayers (SAMs) modified via the fusion of phospholipid vesicles. Langmuir 19(23), 9781–9791 (2003).
Mehrvar, M. & Abdi, M. Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal. Sci. 20(8), 1113–1126 (2004).
Strong, M. E., Richards, J. R., Torres, M., Beck, C. M. & La Belle, J. T. Faradaic electrochemical impedance spectroscopy for enhanced analyte detection in diagnostics. Biosens. Bioelectron. 177, 112949 (2021).
Soares, J. C. et al. Diagnostics of SARS-CoV-2 infection using electrical impedance spectroscopy with an immunosensor to detect the spike protein. Talanta 239, 123076 (2022).
Soares, J. C. et al. Detection of Staphylococcus aureus in milk samples using impedance spectroscopy and data processing with information visualization techniques and multidimensional calibration space. Sens. Actuators Rep. 4, 100083 (2022).
Ozer, T., Geiss, B. J. & Henry, C. S. Chemical and biological sensors for viral detection. J. Electrochem. Soc. 167(3), 037523 (2019).
Kaushik, A. K. et al. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 3(11), 7306–7325 (2020).
K’Owino, I. O. & Sadik, O. A. Impedance spectroscopy: A powerful tool for rapid biomolecular screening and cell culture monitoring. Electroanalysis 17(23), 2101–2113 (2005).
Mattioli, I. A., Hassan, A., Oliveira, O. N. Jr. & Crespilho, F. N. On the challenges for the diagnosis of SARS-CoV-2 based on a review of current methodologies. ACS Sens. 5(12), 3655–3677 (2020).
Alatraktchi, F. A. A., Svendsen, W. E. & Molin, S. Electrochemical detection of pyocyanin as a biomarker for Pseudomonas aeruginosa: A focused review. Sensors 20(18), 5218 (2020).
Manring, N., Ahmed, M. M., Tenhoff, N., Smeltz, J. L. & Pathirathna, P. Recent advances in electrochemical tools for virus detection. Anal. Chem. 94(20), 7149–7157 (2022).
Ozdalgic, B., Gul, M., Uygun, Z. O., Atçeken, N. & Tasoglu, S. Emerging applications of electrochemical impedance spectroscopy in tear film analysis. Biosensors 12(10), 827 (2022).
Hadj Hassine, I. Covid-19 vaccines and variants of concern: A review. Rev. Med. Virol. 32(4), e2313 (2022).
Lim, R. R. X. & Bonanni, A. The potential of electrochemistry for the detection of coronavirus-induced infections. TrAC Trends Anal. Chem. 133, 116081 (2020).
Mavrikou, S., Moschopoulou, G., Tsekouras, V. & Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors 20(11), 3121 (2020).
Ahmadivand, A., Gerislioglu, B., Ramezani, Z., Kaushik, A., Manickam, P. & Ghoreishi, S. A. Femtomolar-level detection of SARS-CoV-2 spike proteins using toroidal plasmonic metasensors. arXiv:2006.08536 (2020).
Eftekhari, A. et al. A comprehensive review of detection methods for SARS-CoV-2. Microorganisms 9(2), 232 (2021).
Falzone, L., Gattuso, G., Tsatsakis, A., Spandidos, D. A. & Libra, M. Current and innovative methods for the diagnosis of COVID-19 infection. Int. J. Mol. Med. 47(6), 1–23 (2021).
Zhang, J. et al. Real-time, selective, and low-cost detection of trace level SARS-CoV-2 spike-protein for cold-chain food quarantine. npj Sci. Food 5(1), 12 (2021).
Mojsoska, B. et al. Rapid SARS-CoV-2 detection using electrochemical immunosensor. Sensors 21(2), 390 (2021).
Eissa, S. & Zourob, M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 93(3), 1826–1833 (2020).
Mahari, S., Roberts, A., Shahdeo, D. & Gandhi, S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19. BioRxiv 2020, 059204 (2020).
Seo, G. et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14(4), 5135–5142 (2020).
Ahmed, S. F., Quadeer, A. A. & McKay, M. R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 12(3), 254 (2020).
Pauly, D., Chacana, P. A., Calzado, E. G., Brembs, B. & Schade, R. IgY technology: Extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. JoVE (J. Vis. Exp.) 51, e3084 (2011).
Schade, R. et al. Chicken egg yolk antibodies (IgY-technology): A review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 33(2), 129–154 (2005).
Chen, C. J. et al. Affordable IgY-based antiviral prophylaxis for resource-limited settings to address epidemic and pandemic risks. J. Glob. Health 12, 05009 (2022).
Bizanov, G. IgY extraction and purification from chicken egg yolk. J. Hell. Vet. Med. Soc. 68(3), 265–272. https://doi.org/10.12681/jhvms.15466 (2018).
Amro, W. M., Al-Qaisi, W. & Al-Razem, F. Production and purification of IgY antibodies from chicken egg yolk. J. Genet. Eng. Biotechnol. 16(1), 99–103. https://doi.org/10.1016/j.jgeb.2017.10.003 (2018).
Layqah, L. A. & Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 186, 1–10 (2019).
Liu, F., Choi, K. S., Park, T. J., Lee, S. Y. & Seo, T. S. Graphene-based electrochemical biosensor for pathogenic virus detection. BioChip J. 5, 123–128 (2011).
Liv, L. Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchem. J. 168, 106445 (2021).
Alonaizan, F. et al. Sensitivity and specificity of rapid SARS-CoV-2 antigen detection using different sampling methods: A clinical unicentral study. Int. J. Environ. Res. Public Health 19(11), 6836. https://doi.org/10.3390/ijerph19116836 (2022).
Murakami, M. et al. Sensitivity of rapid antigen tests for COVID-19 during the Omicron variant outbreak among players and staff members of the Japan Professional Football League and clubs: A retrospective observational study. BMJ Open 13(1), e067591. https://doi.org/10.1136/bmjopen-2022-067591 (2023).
Acknowledgements
This work was supported by the Scientific Research and Innovation Fund (SRIF) project No. MPH/2/14/2020.
Author information
Authors and Affiliations
Contributions
Authors K.M.A. and Y.M.O.: conceptualization, experiments, data collection, data analysis, writing and reviewing the manuscript. Author T.A.: experiments, data collection, data analysis, writing the manuscript. Author M.O.: experiments, data collection. Author M.M.A.: experiments, conceptualization. Author M.I.A.: experiments. Author R.A.: experiments. Author A.M.R.: conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Qaoud, K.M., Obeidat, Y.M., Al-Omari, T. et al. The development of an electrochemical immunosensor utilizing chicken IgY anti-spike antibody for the detection of SARS-CoV-2. Sci Rep 14, 748 (2024). https://doi.org/10.1038/s41598-023-50501-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50501-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.