Abstract
Subthreshold depression (StD) is associated an increased risk of developing major depressive disorder (MDD) and suicidality. Suicidality could be linked to distress intolerance and use of context-dependent strategies. We identified neural correlates of executive functioning among the hubs in the resting-state functional connectome (rs-FCN) and examined associations with recent suicidality in StD and MDD. In total, 79 young adults [27 StD, 30 MDD, and 23 healthy controls (HC)] were scanned using magnetic resonance imaging. Neurocognitive measures of the mean latency to correct five moves in the One Touch Stockings of Cambridge (OTSMLC5), spatial working memory between errors (SWMBE), rapid visual information processing A′ (RVPA′), and the stop signal reaction time in the stop signal test (SSTSSRT) were obtained. Global graph metrics were calculated to measure the network integration, segregation, and their balance in the rs-FCN. Regional graph metrics reflecting the number of neighbors (degree centrality; DC), participation in the shortcuts (betweenness centrality; BC), and accessibility to intersections (eigenvector centrality; EC) in the rs-FCN defined group-level hubs for StD, HC, and MDD, separately. Global network metrics were comparable among the groups (all P > 0.05). Among the group-level hubs, regional graph metrics of left dorsal anterior insula (dAI), right dorsomedial prefrontal cortex (dmPFC), right rostral temporal thalamus, right precuneus, and left postcentral/middle temporal/anterior subgenual cingulate cortices were different among the groups. Further, significant associations with neurocognitive measures were found in the right dmPFC with SWMBE, and left dAI with SSTSSRT and RVPA′. Shorter OTSMLC5 was related to the lower centralities of right thalamus and suffer of recent 1-year suicidal ideation (all Ps < 0.05 in ≥ 2 centralities out of DC, BC, and EC). Collectively, salience and thalamic networks underlie spatial strategy and planning, response inhibition, and suicidality in StD and MDD. Anti-suicidal therapies targeting executive function and modulation of salience-thalamic network in StD and MDD are required.
Similar content being viewed by others
Introduction
Subthreshold depression (StD) is defined as a clinical syndrome characterized by the presence of two or more depressive symptoms that have been consistently present for at least 2 weeks in individuals who do not meet the criteria for diagnosis of minor depression, major depressive disorder (MDD), or dysthymia1. StD has a lifetime prevalence as high as 26%2. Likewise in MDD, individuals with StD also show impairment in social functioning3 and suffer suicidal ideation4. Therefore, individuals with StD require health care utilization similar to that required by MDD5. Regarding the structural and functional neural foundation of StD compared to HC and MDD, first, gray matter morphology and/or cortical thickness of the superior-middle temporal gyri, hippocampus, amygdala, anterior cingulate gyrus, medial orbitofrontal and superior frontal gyri, and cuneus could differentiate StD in their mid-teens from both HC and MDD6. Second, StD in their early 20 s demonstrate significantly reduced microstructural integrity in specific white matter tracts including the pontine crossing tract, genu of the corpus callosum, posterior limb of the internal capsule, and anterior/posterior/superior corona radiata compared with HC7. Notably, the resting-state functional connectivity (rs-FC) between the left amygdala and the middle frontal gyri and insula8, and between the dorsolateral prefrontal cortex and the temporoparietal junction, precuneus, and anterior insula, is weaker in StD in their early 20 s to early 30 s than in HC9. Conversely, stronger rs-FC of StD is found between the left amygdala and left precuneus8, and between the default mode network and the ventral striatum10, than in HC. Moreover, the degree of depressive symptom reduction in patients in their early 20 s with StD after attentional bias modification training was proportional to reduced rs-FC between the right anterior insula and right frontoinsular and supramarginal cortices11. In another study, enhanced health-related quality of life in patients in their late teens with StD after behavioral activation sessions showed a negative correlation with reduced rs-FC between the anterior default mode subnetwork and the dorsal anterior cingulate cortex12. In short, structural–functional characteristics of brain network could not only distinguish StD from HC and MDD but also underlie the treatment-related improvement of depressive symptoms in StD.
Although more severe depressive symptoms exhibit increased concurrent suicidal ideation13,14, StD and anxiety are also associated with an increased prevalence of current suicidal ideation14,15 and lifetime suicidal attempts4. First, suicide attempts are positively and significantly associated with a history of suicide planning and/or intention to act, difficulty controlling suicidal thoughts during the worst week of ideation, and non-suicidal self-injury16. Second, cognitive-affective styles of distress intolerance, heightened emotional reactivity, a propensity for risk-taking in decision-making, and impaired reward/punishment-based learning are associated with an increased risk of suicidal ideation and attempts17,18,19. A negative association between daily ratings of psychological pain and functional activation of the orbitofrontal cortex during social exclusion of euthymic females with a history of suicide attempts and previous major depressive episodes20 suggests a possible role of tolerance of emotional distress as a mediator between stressful life events and suicide risk in MDD21. MDD who have a history of suicide attempts may struggle to improve their problem-solving strategies. This difficulty could be attributed to their impaired ability in probabilistic reversal learning and their tendency to base decisions on feedback from the most recent trial rather than considering the overall context19. Regarding decision-making with conflict control, suicidal ideation in StD is reflected in the functional activation of the thalamus during response generation against a possible larger monetary loss22. In addition, a greater overall proportion of betting and risk-taking in decision-making during the Cambridge Gamble Task has been found in suicidal ideation and anxiety and/or mood disorders18. Third, characteristics of resilience23 such as personal competence, tenacity, tolerance of negative affect, positive acceptance of change, and secure relationships are inversely correlated with depressive symptoms and suicidality24. Collectively, associations of suicidal risk with suicidal ideation, cognitive and affective style, and task-related functional brain activation in depression have been reported.
On the contrary, few information is available regarding the neural correlates of associations between suicidal ideation versus executive functioning of distress tolerance (response inhibition and sustained attention) and strategy-based decision-making (visuospatial strategy and planning) not only in StD5,25 but also in MDD3,26,27. Of note, clinical symptoms of and neurocognitive functioning in psychiatric disorders could be explained by alterations in, or abnormal integration of, spatially distributed brain regions that would normally comprise a large-scale network subserving function28,29. Therefore, this study derived global and local graph metrics of graph theory30, and firstly aimed to find the group-level hubs having numerous neighbors (degree centrality; DC) and more access to intersections (eigenvector centrality; EC), and frequently participating in the shortcuts (betweenness centrality; BC) in the rs-FCN of StD, MDD, and HC separately. Second, this study aimed to uncover the possible neural correlates of neurocognitive functioning among the group-level hubs with significant between-group differences in 2 ≥ centralities (out of DC, BC, and EC). Third, this study also examined possible associations of executive functioning (visuospatial working memory and strategy, spatial planning, sustained attention, and response inhibition) with recent 1-year suicidal ideation.
We firstly hypothesized that the rs-FCN of StD would show centrality different from that of HC and MDD in hub regions comprising the dorsal attention network31,32, ventral attention or salience network33, default mode network34,35, thalamus36, and somatomotor network32,37. Second, among these hubs, we expected to find neural correlates of executive function in rs-FCN regions of the prefrontal, anterior, and posterior cingulate, insula, and thalamus of StD, HC, and MDD38,39. Third, considering the associations among executive dysfunction, hopelessness, and suicidality40,41, we also expected to elucidate the association between recent (past year) suicidal ideation versus executive function in StD and MDD. In regards of the possible staging concept of psychiatric disorders including mood disorder42, we hypothesized that neural underpinning of suicidality-executive functioning association would be shared between the StD and MDD.
Methods
Participants and clinical measurements
A total of 79 undergraduate or graduate students (HC, n = 23; MDD, n = 30; StD, n = 26; Table 1) participated in this study between October 2017 and September 2020 at Seoul National University (SNU), Seoul, Republic of Korea. For recruiting the study participants among the total student population of N = 42,603 (https://diversity.snu.ac.kr/page/dashboard.php?lang=en), promotional flyers were distributed using the mass e-mails through the portal system of SNU, were displayed on campus bulletin boards, and were provided to participants of annual health examination program and public lectures on campus. All participants provided written informed consent prior to enrollment. Participants’ anonymity has been preserved. All participants satisfied the following inclusion criteria: (1) 18–35 years of age; (2) no lifetime diagnosis of psychotic disorder, substance use disorder, or loss of consciousness due to head injury; and (3) no use of psychotropic medication within 8 weeks before study participation. Diagnosis of psychiatric disorders (either MDD or StD) or exclusion of lifetime history or current morbidity of psychiatric disorders (for HC) were made based on semi-structured interviews using the MINI-International Neuropsychiatric Interview43,44 and clinical decision by licensed psychiatrists. At the time of study participation, current experience of a depressive mood and/or loss of interest or pleasure during the previous 2 weeks was reported by all MDD (n = 30) and StD (n = 26). Further, they also satisfied five or more components (MDD) or two to four components (StD) of item A of the diagnostic criteria for MDD in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders45. For all participants of StD, MDD, and HC, self-reporting measures of the 9-question Patient Health Questionnaire (PHQ-9)46,47 and the 7-item General Anxiety Disorder questionnaire (GAD-7)48,49 were applied to measure the depressive symptoms [sadness/hopelessness, anhedonia, insomnia, appetite change, psychomotor changes, fatigue, self-reproach, concentration difficulty, suicidality (thoughts of self-harm or suicide)] and anxiety, respectively. The internal consistency measured by way of the Cronbach’s alpha were 0.95 for the PHQ-9 and 0.88 for the GAD-747,49. This study was approved by the Institutional Review Board of Seoul National University College of Medicine and Hospital (Seoul, Republic of Korea; No. 1608–079-785) and was performed in accordance with the ethical standards of the 1975 Declaration of Helsinki and its later amendments in 2013.
Magnetic resonance imaging (MRI)
All participants underwent MRI using a 3-Tesla scanner (MAGNETOM TrioTim syngo MR B17; Siemens Healthineers, Erlangen, Germany) to evaluate the whole-brain anatomy and rs-FC. The whole-brain anatomy was acquired with a high-resolution T1-weighted, three-dimensional magnetization-prepared rapid acquisition gradient echo sequence (repetition time = 1670 ms, echo time = 1.89 ms, field of view = 250 mm, flip angle = 9°, number of slices = 208, and voxel size = 1.0 × 1.0 × 1.0 mm3). During the acquisition of resting-state functional MRI data, the participants were asked to relax with their eyes closed but to remain awake (repetition time = 2000 ms, echo time = 30 ms, flip angle = 80°, number of slices = 34, and voxel size = 3.4 × 3.4 × 3.4 mm3).
Neuropsychological tests
Four tasks from the Cambridge Neuropsychological Test Automated Battery (CANTAB) were applied in this study: rapid visual information processing (RVP), spatial working memory (SWM), the One Touch Stockings of Cambridge (OTS) task, and the stop signal test (SST). Among several outcome measures, four (one from each task) were selected as representative of higher neurocognitive function (Table 1): (1) sustained attention [RVPA′ (RVPA′): a measure of sensitivity to a target (regardless of response tendency), calculated as 0.5 + [(h − f) + (h − f)2] / [4 × h × (1 − f)]), where h = probability of hits (correct responses) and f = probability of false alarms (inappropriate responses) divided by the sum of total false alarms and total correct rejections]50,51, (2) visuospatial working memory and strategy [SWM between errors (SWMBE): number of times the participant revisited a box where a token had previously been found]52, (3) spatial planning [mean latency to make five correct moves in the OTS (OTSMLC5): the mean latency between the appearance of the balls and the choice of the correct box via five moves]53, and (4) response inhibition [stop signal reaction time in the SST (SSTSSRT): the delay between the instructions and the stop signal, adjusted for individual performance (i.e., the 50% response inhibition failure rate)54.
Resting-state functional MRI: Preprocessing and construction of the rs-FCN
The first four volumes (corresponding to 8 s) were removed to allow for signal stabilization. Preprocessing of anatomical and functional MRI data was performed using ENIGMA HALFpipe software (version 1.2.1)55, which implements fMRIPrep56,57. Afterwards, for each participant and time point, average resting-state time series data were extracted from 210 cortical and 36 subcortical brain regions (5-mm-radius spheres) comprising the Human Brainnetome Atlas58,59, which is a probabilistic tractography-based finer-grained version of the Desikan–Killiany Atlas60. In accordance with consensus partitioning of the rs-FCN reported by Yeo et al61. in 2011, each brain region or node was assigned to the seven cortical subnetworks (default-mode, frontoparietal, limbic, ventral attention, dorsal attention, somatomotor, and visual) and one subcortical network. For each participant, the FCN was created by calculating Pearson’s correlation coefficients between the time series of the 226 nodes (20 regions of interest were excluded from among the initial 246 regions of interest because they were not covered by the resting-state functional MRI scan of one or more participants), followed by Fisher’s r-to-z transformation [= \(0.5\times [ln\left(1+r\right)-ln\left(1-r\right)]\)]59,62. Considering their ambiguous interpretation63 and detrimental effects on test–retest reliability64, the negative-edge weight values (Fisher’s z-transformed correlation coefficients) were set to zero65,66.
Graph theory approach to rs-FCNs: Global network metrics
For examining the global network topology of rs-FCN, the following five global graph metrics of network segregation (γ, Q), integration (λ, GE), and their balance (σ) were calculated using the binary version of rs-FCNs across the network sparsity range of 0.05–0.30 in steps of 0.01 (in connectomes with a sparsity level of 0.05, only the top 5% ranked edges in terms of the edge values survived)67,68.
-
Normalized clustering coefficient (γ): a measure of the tendency of brain regions within a network to form interconnected groups of three neighboring nodes, normalized by comparing it to the average value of the same measure calculated from 1000 random networks; these random networks are essentially randomized versions of the original network, but they preserve the distribution of connections between nodes69,70
-
Normalized characteristic path length (λ): average shortest path length between different nodes within a network, normalized by the averaged value of the same variable derived from the 1000 random networks69,71
-
Small-worldness (σ = γ/λ): a measure of the balance between segregation and integration in the brain network71
-
Normalized global efficiency (GE): a measure of network integration based on the minimum number of steps separating nodes from each other, normalized by the averaged value of the same variable derived from the 1000 random networks69,71,72,73
-
Modularity (Q): the degree to which the network is subdivided into specific modules with denser intra- than inter-modular connections74,75,76
Using the analysis of variance (ANOVA) and independent t-tests, the area under the curve values of these global graph metrics calculated for the subset of network sparsity ranges satisfying the (1) network connectedness [presence of ≥ 1 connections with other brain regions in > 98% of nodes], (2) small-worldness (σ > 1), and (3) modular organization (Q > 0.3) in rs-FCNs of all HC, were compared among the HC, MDD, and StD [Fig. 1; statistical threshold of significance P < 0.05/5(= number of global graph metrics) = 0.01).
Global graph metrics of the resting-state functional connectome. Five global graph metrics of normalized clustering coefficient (gamma), normalized characteristic path length (lambda), small-worldness (Sigma), normalized global efficiency (GE), and modularity (Q) were calculated. Ranges of network sparsity satisfying the (1) network connectedness (presence of ≥ 1 connections with other brain regions in > 98% of nodes), (2) small-worldness (sigma > 1), and (3) modular organization (Q > 0.3) in all HC (N = 23) were found for the rs-FCN (K = 0.11–0.15). Mean and standard deviation values of each global graph metrics (y-axis) were depicted per group [blue for healthy controls (HC), yellow for major depressive disorder (MDD), and red for sub-threshold depression (StD)] and per network sparsity level (x-axis). The area under the curve (AUC) of each global graph metrics were calculated and compared among the HC, MDD, and StD (statistical threshold of significance P < 0.05).
Graph theory approach to rs-FCNs: Regional graph metrics
For finding principal brain regions in the rs-FCN, the following three regional graph metrics reflecting differential facets of principal nodes such as (1) participation in the shortcuts (BC), (2) connection with intersections (EC), and (3) number of neighbors (DC) were calculated71,77.
-
Betweenness centrality (BC): fraction of all shortest paths in the network that contain a given node; nodes with high BC values participate in a large number of shortest paths
-
Eigenvector centrality (EC): self-referential measure of centrality that reflects the presence of connectedness between a given node and other nodes with high EC values
-
Degree centrality (DC): the number of connections or edges incident upon a node)
Because the distribution of centrality values is not normal in a scale-free network, these regional graph metrics were z-score-normalized (using the mean and standard deviation of each centralities for HC, MDD, and StD separately) at network sparsity ranges satisfying the network connectedness, small-worldness, and modular organization in rs-FCN of all HC (refer to the ‘Graph theory approach to rs-FCNs: Global network metrics’ in the ‘Methods’), and then averaged for each participant to derive the participant-level centrality values68. Brain regions ranked in the top 12% (= 226 nodes × 0.12 ≈ 26–27 nodes) for two or three centralities (out of BC, EC, and DC) in terms of the group-averaged participant-level centrality values were defined as group-level hubs68,78 for HC, MDD, and StD separately (Fig. 2). Between-group comparisons of z-transformed centralities (BC, EC, and DC) using the ANOVA and post-hoc independent t-tests were performed only for these group-level hubs; those with significantly different z-transformed centralities (P < 0.05) for ≥ 2 centralities (out of BC, EC, and DC) were defined as brain regions having significant between-group differences in hubness (Table 2 and Fig. 2).
Group-level hubs of resting-state functional connectome (rs-FCN). Group-level hubs most frequently ranked as top 12% in each group of (A) HC, (B) StD, or (C) MDD in 2–3 centralities [among the degree, betweenness, and eigenvector; in the network sparsity range of K = 0.11–0.15 that satisfied three criteria of network connectedness, small-worldness, and modular organization in the rs-FCN of all HC (n = 23)] are displayed. Nodes (circles) are colored to display the 7 cortical and 1 subcortical sub-networks or modules. Among these 47 group-level hubs (comprised of 23 HC hubs, 27 StD hubs, and 26 MDD hubs), seven nodes that showed significant between-group differences in the z-transformed centralities (P < 0.05 in 2–3 centralities) are marked with thicker orange-colored rims. First, right precuneus (BA 5) was one of the 9 hubs shared by all three groups; compared to MDD, z-transformed of centralities were higher in StD. Second, among the 7 hubs shared in both MDD and StD, centralities of left postcentral gyrus (BA 1/2/3) were higher in MDD than StD and HC. Third, among the 9 regions ranked as hubs only in StD, centrality values of left anterior dorsal dysgranular insula and right dorsomedial prefrontal cortex (BA 6) were higher in StD compared to both MDD and HC. In addition, centralities of left middle temporal gyrus (BA 37) was higher in StD compared to MDD. Fourth, among the 12 regions ranked as hubs only in HC, centralities of right rostral temporal thalamus were lower in MDD than HC; also, centrality values of left anterior sub-genual cingulate (BA 32) was higher in HC compared to both MDD and StD.
Further, to elucidate the graph-based neural foundation of higher neurocognitive functioning in the StD, MDD, and HC groups, Pearson’s correlation coefficients of z-transformed centralities in these group-level hubs having significant between-group differences in hubness with four higher-neurocognitive function measures including RVPA′ (sustained attention)50,51, SWMBE (visuospatial working memory and strategy)52, OTSMLC5 (spatial planning)53, and SSTSSRT (response inhibition) were calculated (Fig. 3; threshold of statistical significance set as P < 0.05 for ≥ 2 centralities out of BC, EC, and DC)54. All graph theory measures were calculated using the Brain Connectivity Toolbox71 and MATLAB R2022a software (https://kr.mathworks.com).
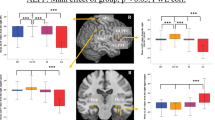
Associations of centralities in hub brain regions with higher neurocognitive performance. (A) A positive association was found between the OTSMLC5 (Mean Latency to Correct 5 moves in the One touch stocking of Cambridge task; spatial planning) and z-transformed centralities of right rostral temporal thalamus (r = 0.262 and P = 0.020 for degree centrality; r = 0.277 and P = 0.014 for eigenvector centrality). (B) A negative association between the SWMBE (Between Errors of the Spatial working memory task; visuospatial working memory and strategy) and z-transformed centralities of right dorsomedial prefrontal cortex (BA 6) (r = − 0.317 and P = 0.004 for degree centrality; r = − 0.243 and P = 0.031 for eigenvector centrality). (C) Positive correlation of z-transformed centralities in the left anterior dorsal dysgranular insula with the SSTSSRT (Stop Signal Reaction Time of the Stop signal test; response inhibition; r = 0.228 and P = 0.044 for degree centrality, r = 0.242 and P = 0.031 for eigenvector centrality). (D) Positive correlation of z-transformed centralities in the left anterior dorsal dysgranular insula with the and RVPA (detection sensitivity in the Rapid visual information processing task; sustained attention; r = 0.245 and P = 0.030 for degree centrality, r = 0.263 and P = 0.019 for eigenvector centrality). (E) Seven group-level hubs that showed significant between-group differences of z-transformed centralities (degree, betweenness, and eigenvector) averaged in the network sparsity levels of K = 0.11–0.15 [that satisfied all three criteria of network connectedness, small-worldness, and modular organization in the rs-FCN of all HC individuals (n = 23)]. Only 47 brain regions (out of the 226 brain regions comprising the rs-FCN) ranked as group-level hubs either in HC (23 hubs), StD (27 hubs) or MDD (26 hubs) hubs underwent between-group comparisons. Threshold of statistical significance was set as P < 0.05 in 2–3 centralities. Three brain regions that demonstrated significant associations of 2–3 centralities with higher neuropsychological functioning (as shown in A–D) are tagged with thicker rims.
Statistical analyses
Between-group comparisons of age, years of education, and total PHQ-9 and GAD-7 scores (Table 1) were performed by way of ANOVA and independent t-tests. The chi-square test was used for between-group comparisons of sex (male/female). The threshold of statistical significance was set at P < 0.05. Additionally, neurocognitive measures were compared in terms of the presence or absence of recent 1-year suicidal ideation (Fig. 4). All statistical analyses were performed using MATLAB R2022a software.
Neurocognitive performance in young adults with [n = 23; subthreshold depression (StD) or major depressive disorder (MDD)] or without [n = 56; StD, MDD, or healthy controls (HC)] suicidal ideation within last year. (A) OTSMLC5 (Mean Latency to Correct 5 moves in the One touch stocking of Cambridge task; spatial planning). (B) SWMBE (Between Errors of the Spatial working memory task; visuospatial working memory and strategy). (C) SSTSSRT (Stop Signal Reaction Time of the Stop signal test; response inhibition). (D) RVPA (detection sensitivity in the Rapid visual information processing task; sustained attention).
Results
Participants’ demographic, clinical, and neurocognitive characteristics
Table 1 shows the demographic, clinical, and neurocognitive characteristics of the HC (n = 23), MDD (n = 30), and StD (n = 26). All participants (N = 79) were in their mid-20 s. The study population comprised 42 men and 37 women with an average of 16.7 years of education, and all had remained free of psychotropic medication for > 8 weeks prior to study participation. No statistically significant between-group differences in age, sex, or years of education were found among the HC, MDD, and StD groups (all P > 0.05). The four neurocognitive variables (OTSMLC5, SWMBE, SSTSSRT, and RVPA′) showed no significant differences among the three groups (all P > 0.05, ANOVA).
However, the severity of depressive and anxiety symptoms differed significantly among the HC, MDD, and StD groups (all P < 0.05). Post-hoc analyses also revealed significant between-group differences in depressive symptoms (P < 0.001 for HC vs. StD, HC vs. MDD, and StD vs. MDD) and anxiety symptoms (P = 0.030 for HC vs. StD, P < 0.001 for HC vs. MDD, and P = 0.004 for StD vs. MDD). First, MDD experienced moderate depressive symptoms (mean PHQ-9 total score = 12) and mild anxiety symptoms (mean GAD-7 total score = 8.6). Second, StD reported mild depressive symptoms (mean PHQ-9 total score = 7.8) and mild anxiety symptoms (mean GAD-7 total score = 5.2). Third, depressive symptoms (mean PHQ-9 total score = 3.2) and anxiety symptoms (mean GAD-7 total score = 2.3) were mild in HC. Finally, the number of participants with recent (past year) suicidal ideation was zero in the HC group and was similar between the StD and MDD groups (P > 0.05).
Global graph metrics of rs-FCN
All three criteria of network connectedness, small-worldness, and modular organization were satisfied in the network sparsity range of K = 0.11–0.15 for the rs-FCN of all HC. The five global graph metrics [normalized clustering coefficient (γ), normalized characteristic path length (λ), small-worldness (σ = γ/λ), normalized global efficiency (GE), and modularity (Q)] summed in these network sparsity range of K = 0.11–0.15 were comparable among the HC, MDD, and StD groups [F(2) = 0.66 and P = 0.521 for γ; F(2) = 0.41 and P = 0.664 for λ; F(2) = 0.4 and P = 0.672 for σ; F(2) = 0.26 and P = 0.769 for GE; and F(2) = 0.15 and P = 0.858 for Q] (Fig. 1).
Regional graph metrics of rs-FCN
Among the 47 group-level hubs (23 HC hubs, 27 StD hubs, and 26 MDD hubs; Fig. 2), 7 nodes showed significant between-group differences in hubness (Table 2). First, the right precuneus [Brodmann area (BA) 5; dorsal attention network] was one of the nine hubs shared by all three groups; the z-transformed values of EC (P = 0.024) and DC (P = 0.003) were higher in StD than in MDD. Second, among the seven hubs shared by both MDD and StD, the centralities of the left postcentral gyrus (BA 1/2/3; somatomotor network) were higher in MDD than in StD (BC, P = 0.002) and HC (DC, P = 0.004). Third, among the nine regions ranked as hubs only for StD, the centrality values of the left anterior dorsal dysgranular insula (ventral attention network) [EC, P = 0.005 (StD vs. MDD) and P = 0.011 (StD vs. HC); DC, P = 0.008 (StD vs. MDD) and P = 0.005 (StD vs. HC)] and right dorsomedial prefrontal cortex (dmPFC) (BA 6; somatomotor network) [EC, P = 0.010 (StD vs. MDD) and P = 0.031 (StD vs. HC); DC, P = 0.020 (StD vs. MDD) and P = 0.043 (StD vs. HC)] were higher in StD than in both MDD and HC. In addition, EC (P = 0.012) and DC (P = 0.005) of the left middle temporal gyrus (BA 37; dorsal attention network) were higher in StD than in MDD. Fourth, among the 12 regions ranked as hubs only for HC, the centralities of the right rostral temporal thalamus [EC (P = 0.006) and DC (P = 0.005)] were lower in MDD than in HC. Additionally, the centrality values of the left anterior subgenual cingulate (BA 32; default mode network) were higher in HC than in both MDD [EC (P = 0.040) and DC (P = 0.022)] and StD [EC (P = 0.005), BC (P = 0.010), and DC (P = 0.005)].
Associations of hub regions with neuropsychological functioning
Pearson’s correlation coefficients were calculated between the four CANTAB neurocognitive measures (RVPA′, SWMBE, OTSMLC5, and SSTSSRT) and z-transformed centralities in seven group-level hubs with significant between-group differences in hubness (Fig. 3). Notably, a positive association was found between the OTSMLC5 (spatial planning) and z-transformed centralities of the right rostral temporal thalamus [r = 0.262 (P = 0.020) for DC, r = 0.277 (P = 0.014) for EC]. Moreover, a negative association was found between the SWMBE (visuospatial working memory and strategy) and z-transformed centralities of the right dmPFC (BA 6) [r = − 0.317 (P = 0.004) for DC, r = − 0.243 (P = 0.031) for EC]. Finally, positive correlations were seen between the z-transformed centralities of the left anterior dorsal dysgranular insula and the SSTSSRT (response inhibition) [r = 0.228 (P = 0.044) for DC, r = 0.242 (P = 0.031) for EC] and RVPA′ (sustained attention) [r = 0.245 (P = 0.030) for DC, r = 0.263 (P = 0.01) for EC].
Regarding the associations of four executive functioning with suicidal ideation, those with recent 1-year suicidal ideation revealed shorter values of OTSMLC5 [T = 3.270 (P = 0.016); Fig. 4A] than those without recent 1-year suicidal ideation. On the contrary, neither the SWMBE [T = − 0.254 (P = 0.800); Fig. 4B], the SSTSSRT [T = − 0.295 (P = 0.769); Fig. 4C], nor the RVPA′ [T = − 0.309 (P = 0.759); Fig. 4D] demonstrated significant differences regarding the presence/absence of recent 1-year suicidal ideation.
Discussion
Associations of right rostral temporal thalamus rs-FCN with spatial planning and suicidality
Among the 47 group-level hubs (23 HC hubs, 27 StD hubs, and 26 MDD hubs), the centralities of the right rostral temporal thalamus were lower in MDD than in HC and StD (Fig. 2 and Table 2). This is in accordance with a previous study showing weaker rs-FC of the rostral temporal thalamus with the left lingual gyrus in bipolar disorder (either depressive or remitted status) than in HC79. After a body–mind relaxation meditation, both MDD and HC showed decreased FC between the left rostral temporal thalamus and the left inferior occipital cortex80. The rostral temporal thalamus, as defined in the Brainnetome Atlas58, covers parts of the anterior thalamic complex, which projects to limbic areas in the medial temporal lobe and parts of the dorsomedial thalamic nucleus (related to emotional behavior, memory, attention, and higher cognitive functioning of organization and planning), which receives inputs from the temporal lobe79 and projects to the prefrontal cortex and limbic system.
The current study revealed associations of spatial planning (OTSMLC5) with the z-transformed centralities of the right rostral temporal thalamus [r = 0.262 (P = 0.020) for DC, r = 0.277 (P = 0.014) for EC] (Fig. 3A) and suicidal ideation in StD and MDD within the previous year (Fig. 4A). Impaired executive function in domains such as decision-making, planning, response inhibition, selective attention, working memory, and verbal fluency54,81 is a key feature of MDD that has been linked to the risk of suicidal ideation and attempts82. First, impaired spatial planning measured using the OTS outcome measure “problems solved on first choice” was associated with a higher risk of suicide attempts within the upcoming year54. Second, adolescents with depression and a history of suicidal attempts within the last year showed impaired problem-solving and set-shifting (measured using the Wisconsin Card Sorting Test) and working memory (assessed using the digit span subtest of the Wechsler Adult Intelligence Scale, Fourth Edition) than HC and a history of non-suicidal self-injury83. Cognitive inflexibility poses additional difficulties in terms of interpreting and managing life events84 and could be a risk factor for suicide in depression85.
Visuospatial strategy with rs-FCN centrality of the right dmPFC
Among all 47 group-level hubs, the centralities of the right dmPFC (BA 6) were higher in the StD group than in both the MDD and HC groups (Fig. 2 and Table 2). Focal brain lesions of the left dmPFC are associated with more severe depressive symptoms86. Conditional knockout of lactate dehydrogenase A from the mouse brain reduces L-lactate levels and neuronal excitability in the dmPFC, promoting depressive-like behaviors87. Additionally, the depressive symptoms anhedonia, avolition88,89,90, rumination91, and apathy92 have been linked to dmPFC dysfunction. In one study, hypoactivation of the left dmPFC was related to poorer emotional regulation and an increased likelihood of subsequent mood episode relapse during a 1.5-year follow-up of bipolar disorder compared with HC93. Additionally, in adolescents with preschool-onset depression, recognition of fear and surprise during emotional regulation of sadness was accompanied by greater functional activation of the dAI and dmPFC94. In another study, rs-FC between the dmPFC and insula was weaker in MDD than in HC95. By contrast, increased rs-FC of the dmPFC with the rostral anterior cingulate cortex in response to mindfulness induction was related to emotion regulation96. The dmPFC has served as a target of repetitive transcranial magnetic stimulation97,98 and intermittent theta burst stimulation98,99,100 for the treatment of anhedonia, avolition, and blunted affect in depression.
The present study revealed a negative association between the SWMBE (visuospatial working memory and strategy) and z-transformed centralities of the right dmPFC (BA 6) [r = − 0.317 (P = 0.004) for DC, r = − 0.243 (P = 0.031) for EC] (Fig. 3B). The dmPFC is the site of convergence for the cognitive control and affect-triggering networks and thereby plays a critical role in both the generation and regulation of emotion101. Variance in the rs-FC strength of the dmPFC with other regions, including dorsal and ventral attention networks and the subcortical network, could explain the severity of depressive rumination in both the subclinical population and MDD35. Reduced rs-FC strength of the dmPFC with the basolateral amygdala, and of the node strength of the basolateral amygdala, was found in HC after acute stress, but not in MDD102. In another study, treatment-resistant depression exhibited significantly reduced oxyhemoglobin activation changes in the dmPFC when performing a verbal fluency task assessed with near-infrared spectroscopy103. Further, functional activation of the dmPFC during response inhibition in the SST, which reflects both the severity of depressive symptoms and the resilience of young adults without symptoms, was influenced by the interplay between childhood maltreatment and a family history of alcohol use disorder104. In terms of vulnerability to depressive symptoms, a lower level of social trust (which is related to depressive symptoms) was associated with lower gray matter density of the dmPFC in community-dwelling adults105.
Response inhibition and sustained attention: association with rs-FCN centrality of the left dAI
Among all 47 group-level hubs in this study, the centrality of the left dAI in the rs-FCN was higher in the StD group than in both the MDD and HC groups (Fig. 2 and Table 2). This is in line with previous studies that showed associations of the dAI with depressive symptom severity and improvement. First, focal brain lesions in the bilateral AI were associated with more severe depressive symptoms86. Second, higher EC of the left dAI in the rs-FCN was associated with more severe depressive symptoms in Parkinson’s disease106. Third, depressive symptoms in a diagnosis of either MDD or bipolar disorder were related to inhibitory rs-FC between the AI and the precuneus107. Fourth, a significant reduction of depressive symptoms in MDD after sertraline pharmacotherapy was accompanied by decreased rs-FC of the insula with the nucleus accumbens108. Fifth, a change in the rs-FC of the left AI with the dorsal anterior cingulate cortex after treatment with repetitive transcranial magnetic stimulation explained the improved emotional health seen in traumatic brain injury109.
This study also showed a positive correlation of z-transformed centralities in the left dAI with the SSTSSRT (response inhibition) [r = 0.228 (P = 0.044) for DC, r = 0.242 (P = 0.031) for EC] (Fig. 3C) and a positive correlation of the z-transformed centralities of the left dAI with the RVPA′ (detection sensitivity in the RVP; sustained attention) [r = 0.245 (P = 0.030) for DC, r = 0.263 (P = 0.019) for EC] (Fig. 3D). The dAI has functional connections to frontal, anterior cingulate, and parietal areas and is involved in cognitive control processes110. Stronger rs-FC of the bilateral insula, supramarginal gyrus, and dorsal anterior cingulate cortex in MDD than in HC was sustained even after the remission of depressive symptoms111. Dynamic functional network connectivity analyses have revealed more diverse time-varying FC patterns of the dAI with other brain regions112 across multiple task domains113. Compared with HC, StD show blunted activity of the bilateral AI when choosing to exert effort to obtain rewards but increased willingness and greater activation of the bilateral AI when choosing to exert effort on the behalf of others114.
Limitations
The current study had some limitations. First, the study used a cross-sectional design. Therefore, the longitudinal trajectories of clinical symptoms115,116 and executive functioning in StD must be examined in further studies. Second, the static rs-FCN estimated in the current study cannot be used to explore the possibility of multiple time-varying phases of dynamic rs-FCN in StD. Future studies acquiring brain MRI data with higher temporal resolution117 may yield more information. Third, detailed information on how insufficient visuospatial planning contributes to hopelessness and suicidal ideation in StD and MDD was lacking in the current study. Additional studies using projective psychological tests118,119 could provide more data.
Conclusions
Taken together, the results suggest that the suicidality of StD is associated with the executive functions of spatial planning and problem-solving. The executive function of StD is associated with the centralities of the thalamus, dmPFC, and dAI in the rs-FCN. Treatment approaches to improve the executive function, hopelessness, and suicidality of StD must be developed in future studies.
Data availability
Participant-level data is not publicly available, but can be accessed by researchers who meet the criteria for access to de-identified sensitive data via request to the corresponding author (jhjang602@naver.com), under the term of clinical research ethics committee of Seoul National University College of Medicine and Hospital.
References
Judd, L. L., Rapaport, M. H., Paulus, M. P. & Brown, J. L. Subsyndromal symptomatic depression: A new mood disorder?. J. Clin. Psychiatry 55, 18–28 (1994).
Fergusson, D. M., Horwood, L. J., Ridder, E. M. & Beautrais, A. L. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch. Gen. Psychiatry 62, 66–72. https://doi.org/10.1001/archpsyc.62.1.66 (2005).
Balázs, J. et al. Adolescent subthreshold-depression and anxiety: Psychopathology, functional impairment and increased suicide risk. J. Child Psychol. Psychiatry 54, 670–677. https://doi.org/10.1111/jcpp.12016 (2013).
An, J. H. et al. Subthreshold lifetime depression and anxiety are associated with increased lifetime suicide attempts: A Korean nationwide study. J. Affect. Disord. 302, 170–176. https://doi.org/10.1016/j.jad.2022.01.046 (2022).
Noyes, B. K., Munoz, D. P., Khalid-Khan, S., Brietzke, E. & Booij, L. Is subthreshold depression in adolescence clinically relevant?. J. Affect. Disord. 309, 123–130. https://doi.org/10.1016/j.jad.2022.04.067 (2022).
Ma, H., Zhang, D., Sun, D., Wang, H. & Yang, J. Gray and white matter structural examination for diagnosis of major depressive disorder and subthreshold depression in adolescents and young adults: A preliminary radiomics analysis. BMC Med. Imaging 22, 164. https://doi.org/10.1186/s12880-022-00892-5 (2022).
Ghazi Sherbaf, F., Same, K., Ashraf-Ganjouei, A. & Aarabi, M. H. Altered white matter microstructure associated with mild and moderate depressive symptoms in young adults, a diffusion tensor imaging study. Neuroreport 29, 685–689. https://doi.org/10.1097/wnr.0000000000001017 (2018).
Peng, X., Lau, W. K. W., Wang, C., Ning, L. & Zhang, R. Impaired left amygdala resting state functional connectivity in subthreshold depression individuals. Sci. Rep. 10, 17207. https://doi.org/10.1038/s41598-020-74166-x (2020).
Hwang, J. W. et al. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry 5, e683. https://doi.org/10.1038/tp.2015.174 (2015).
Hwang, J. W. et al. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J. Psychiatr. Res. 76, 111–120. https://doi.org/10.1016/j.jpsychires.2016.02.005 (2016).
Li, H. et al. Attentional bias modification (ABM) training induces spontaneous brain activity changes in young women with subthreshold depression: A randomized controlled trial. Psychol. Med. 46, 909–920. https://doi.org/10.1017/s003329171500238x (2016).
Yokoyama, S. et al. Effects of behavioral activation on default mode network connectivity in subthreshold depression: A preliminary resting-state fMRI study. J. Affect. Disord. 227, 156–163. https://doi.org/10.1016/j.jad.2017.10.021 (2018).
Augenstein, T. M. et al. Multi-informant reports of depressive symptoms and suicidal ideation among adolescent inpatients. Suicide Life Threat Behav. 52, 99–109. https://doi.org/10.1111/sltb.12803 (2022).
Sahimi, H. M. S. et al. Depression and suicidal ideation in a sample of Malaysian healthcare workers: A preliminary study during the COVID-19 pandemic. Front. Psychiatry 12, 658174. https://doi.org/10.3389/fpsyt.2021.658174 (2021).
Yurgil, K. A., Barkauskas, D. A. & Baker, D. G. Deployment and psychological correlates of suicide ideation: A prospective, longitudinal study of risk and resilience among combat veterans. Mil. Med. 186, e58–e66. https://doi.org/10.1093/milmed/usaa450 (2021).
Naifeh, J. A. et al. Self-injurious thoughts and behaviors that differentiate soldiers who attempt suicide from those with recent suicide ideation. Depress. Anxiety 37, 738–746. https://doi.org/10.1002/da.23016 (2020).
Naifeh, J. A. et al. Association of emotion reactivity and distress intolerance with suicide attempts in U.S. Army soldiers. Suicide Life Threat Behav. 52, 289–302. https://doi.org/10.1111/sltb.12821 (2022).
Liaugaudaite, V. et al. Neurocognitive markers of suicidal ideation in patients with anxiety and mood disorders. Int. J. Psychiatry Clin. Pract. 24, 116–119. https://doi.org/10.1080/13651501.2019.1666148 (2020).
Dombrovski, A. Y. et al. Reward/Punishment reversal learning in older suicide attempters. Am. J. Psychiatry 167, 699–707. https://doi.org/10.1176/appi.ajp.2009.09030407 (2010).
Olié, E. et al. Prefrontal activity during experimental ostracism and daily psychache in suicide attempters. J. Affect. Disord. 285, 63–68. https://doi.org/10.1016/j.jad.2021.01.087 (2021).
Zhong, J., Huang, X. J., Wang, X. M. & Xu, M. Z. The mediating effect of distress tolerance on the relationship between stressful life events and suicide risk in patients with major depressive disorder. BMC Psychiatry 23, 118. https://doi.org/10.1186/s12888-023-04600-7 (2023).
Yun, J. Y. et al. Functional activation of insula and dorsal anterior cingulate for conflict control against larger monetary loss in young adults with subthreshold depression: A preliminary study. Sci. Rep. 12, 6956. https://doi.org/10.1038/s41598-022-10989-0 (2022).
Connor, K. M. & Davidson, J. R. T. Development of a new resilience scale: The connor-davidson resilience scale (CD-RISC). Depress. Anxiety 18, 76–82. https://doi.org/10.1002/da.10113 (2003).
Favale, D. et al. Hope and resilience among patients affected by unipolar and bipolar depression. Int. J. Soc. Psychiatry 69, 967–975. https://doi.org/10.1177/00207640221147166 (2023).
Carrellas, N. W., Biederman, J. & Uchida, M. How prevalent and morbid are subthreshold manifestations of major depression in adolescents? A literature review. J. Affect. Disord. 210, 166–173. https://doi.org/10.1016/j.jad.2016.12.037 (2017).
Pan, F. et al. Neuronavigation-guided rTMS for the treatment of depressive patients with suicidal ideation: A double-blind, randomized, sham-controlled trial. Clin. Pharmacol. Ther. 108, 826–832. https://doi.org/10.1002/cpt.1858 (2020).
Malhi, G. S. et al. Default mode dysfunction underpins suicidal activity in mood disorders. Psychol. Med. 50, 1214–1223. https://doi.org/10.1017/s0033291719001132 (2020).
Catani, M. & Ffytche, D. H. The rises and falls of disconnection syndromes. Brain J. Neurol. 128, 2224–2239. https://doi.org/10.1093/brain/awh622 (2005).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. https://doi.org/10.1038/nrn2575 (2009).
Yun, J. Y. & Kim, Y. K. Graph theory approach for the structural-functional brain connectome of depression. Progress Neuro-Psychopharmacol. Biol. Psychiatry 111, 110401. https://doi.org/10.1016/j.pnpbp.2021.110401 (2021).
Zhukovsky, P. et al. Coordinate-based network mapping of brain structure in major depressive disorder in younger and older adults: A Systematic review and meta-analysis. Am. J. Psychiatry 178, 1119–1128. https://doi.org/10.1176/appi.ajp.2021.21010088 (2021).
Liu, J. et al. The neuroprogressive nature of major depressive disorder: Evidence from an intrinsic connectome analysis. Transl. Psychiatry 11, 102. https://doi.org/10.1038/s41398-021-01227-8 (2021).
Peters, S. K., Dunlop, K. & Downar, J. Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10, 104. https://doi.org/10.3389/fnsys.2016.00104 (2016).
Sinha, P., Joshi, H. & Ithal, D. Resting state functional connectivity of brain with electroconvulsive therapy in depression: Meta-analysis to understand its mechanisms. Front. Hum. Neurosci. 14, 616054. https://doi.org/10.3389/fnhum.2020.616054 (2020).
Kim, J. et al. A dorsomedial prefrontal cortex-based dynamic functional connectivity model of rumination. Nat. Commun. 14, 3540. https://doi.org/10.1038/s41467-023-39142-9 (2023).
Greicius, M. D. et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437. https://doi.org/10.1016/j.biopsych.2006.09.020 (2007).
Martens, M. A. G., Filippini, N., Harmer, C. J. & Godlewska, B. R. Resting state functional connectivity patterns as biomarkers of treatment response to escitalopram in patients with major depressive disorder. Psychopharmacology 239, 3447–3460. https://doi.org/10.1007/s00213-021-05915-7 (2022).
Makovac, E., Fagioli, S., Rae, C. L., Critchley, H. D. & Ottaviani, C. Can’t get it off my brain: Meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Res. Neuroimaging 295, 111020. https://doi.org/10.1016/j.pscychresns.2019.111020 (2020).
Liu, Y. et al. Altered resting-state functional connectivity of multiple networks and disrupted correlation with executive function in major depressive disorder. Front. Neurol. 11, 272. https://doi.org/10.3389/fneur.2020.00272 (2020).
Gujral, S. et al. Impaired executive function in contemplated and attempted suicide in late life. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 22, 811–819. https://doi.org/10.1016/j.jagp.2013.01.025 (2014).
Lima, I. M. M., Peckham, A. D. & Johnson, S. L. Cognitive deficits in bipolar disorders: Implications for emotion. Clin. Psychol. Rev. 59, 126–136. https://doi.org/10.1016/j.cpr.2017.11.006 (2018).
van Os, J. et al. Context v. algorithm: Evidence that a transdiagnostic framework of contextual clinical characterization is of more clinical value than categorical diagnosis. Psychol. Med. 53, 1825–1833. https://doi.org/10.1017/s0033291721003445 (2023).
Sheehan, D. V. et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(20), 22–33 (1998).
Yoo, S.-W. et al. Validity of Korean version of the mini-international neuropsychiatric interview. Anxiety Mood 2, 50–55 (2006).
Association, A. P. Diagnostic and Statistical Manual of MEntal Disorders, 5th Edition: DSM-5 5th edn. (American Psychiatric Publishing, 2013).
Spitzer, R. L., Kroenke, K. & Williams, J. B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. Jama 282, 1737–1744. https://doi.org/10.1001/jama.282.18.1737 (1999).
An, J. Y., Seo, E. R., Lim, K. H., Shin, J. H. & Kim, J. B. Standardization of the Korean version of screening tool for depression (Patient Health Questionnaire-9, PHQ-9). J. Korean Soc. Biol. Ther. Psychiatry 19, 47–56 (2013).
Spitzer, R. L., Kroenke, K., Williams, J. B. & Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166, 1092–1097. https://doi.org/10.1001/archinte.166.10.1092 (2006).
Lee, S. H. et al. Validation of the Korean version of the generalized anxiety disorder 7 self-rating Scale. Asia-Pacific Psychiatry Off. J. Pac. Rim Coll. Psychiatr. 14, e12421. https://doi.org/10.1111/appy.12421 (2022).
Gau, S. S. & Huang, W. L. Rapid visual information processing as a cognitive endophenotype of attention deficit hyperactivity disorder. Psychol. Med. 44, 435–446. https://doi.org/10.1017/s0033291713000640 (2014).
Sahgal, A. Some limitations of indices derived from signal detection theory: Evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology 91, 517–520. https://doi.org/10.1007/bf00216022 (1987).
Cacciamani, F. et al. Evidence of practice effect in CANTAB spatial working memory test in a cohort of patients with mild cognitive impairment. Appl. Neuropsychol. Adult 25, 237–248. https://doi.org/10.1080/23279095.2017.1286346 (2018).
Bauer, O. et al. Association of chemokine (C-C Motif) receptor 5 and ligand 5 with recovery from major depressive disorder and related neurocognitive impairment. Neuroimmunomodulation 27, 152–162. https://doi.org/10.1159/000513093 (2020).
Riera-Serra, P. et al. Longitudinal associations between executive function impairments and suicide risk in patients with major depressive disorder: A 1-year follow-up study. Psychiatry Res. 325, 115235. https://doi.org/10.1016/j.psychres.2023.115235 (2023).
Waller, L. et al. ENIGMA HALFpipe: Interactive, reproducible, and efficient analysis for resting-state and task-based fMRI data. Hum. Brain Mapp. 43, 2727–2742. https://doi.org/10.1002/hbm.25829 (2022).
Esteban, O. et al. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat. Protoc. 15, 2186–2202. https://doi.org/10.1038/s41596-020-0327-3 (2020).
Esteban, O. et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116. https://doi.org/10.1038/s41592-018-0235-4 (2019).
Fan, L. et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526. https://doi.org/10.1093/cercor/bhw157 (2016).
Sintini, I. et al. Tau and amyloid relationships with resting-state functional connectivity in atypical alzheimer’s disease. Cereb. Cortex 31, 1693–1706. https://doi.org/10.1093/cercor/bhaa319 (2021).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021 (2006).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. https://doi.org/10.1152/jn.00338.2011 (2011).
Pedersen, R. et al. When functional blurring becomes deleterious: Reduced system segregation is associated with less white matter integrity and cognitive decline in aging. NeuroImage 242, 118449. https://doi.org/10.1016/j.neuroimage.2021.118449 (2021).
Fox, M. D., Zhang, D., Snyder, A. Z. & Raichle, M. E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101, 3270–3283. https://doi.org/10.1152/jn.90777.2008 (2009).
Wang, J. H. et al. Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PloS One 6, e21976. https://doi.org/10.1371/journal.pone.0021976 (2011).
Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E. & Wig, G. S. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. USA 111, E4997-5006. https://doi.org/10.1073/pnas.1415122111 (2014).
Malagurski, B., Liem, F., Oschwald, J., Mérillat, S. & Jäncke, L. Functional dedifferentiation of associative resting state networks in older adults—A longitudinal study. NeuroImage 214, 116680. https://doi.org/10.1016/j.neuroimage.2020.116680 (2020).
Suo, X. et al. Brain Functional Network Abnormalities in Parkinson's Disease with Mild Cognitive Impairment. Cerebral cortex (New York, N.Y, 1991), https://doi.org/10.1093/cercor/bhab520 (2022).
Yun, J. Y. et al. Brain structural covariance networks in obsessive-compulsive disorder: A graph analysis from the ENIGMA Consortium. Brain J. Neurol. 143, 684–700. https://doi.org/10.1093/brain/awaa001 (2020).
Maslov, S. & Sneppen, K. Specificity and stability in topology of protein networks. Science 296, 910–913. https://doi.org/10.1126/science.1065103 (2002).
Watts, D. J. & Strogatz, S. H. Collective dynamics of “small-world” networks. Nature 393, 440–442. https://doi.org/10.1038/30918 (1998).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52, 1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003 (2010).
Latora, V. & Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 87, 198701. https://doi.org/10.1103/PhysRevLett.87.198701 (2001).
Fagiolo, G. Clustering in complex directed networks. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 76, 026107. https://doi.org/10.1103/PhysRevE.76.026107 (2007).
Newman, M. E. Finding community structure in networks using the eigenvectors of matrices. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 74, 036104. https://doi.org/10.1103/PhysRevE.74.036104 (2006).
Newman, M. E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 103, 8577–8582. https://doi.org/10.1073/pnas.0601602103 (2006).
Reichardt, J. & Bornholdt, S. Statistical mechanics of community detection. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 74, 016110. https://doi.org/10.1103/PhysRevE.74.016110 (2006).
Kintali, S. Betweenness Centrality: Algorithms and Lower Bounds. arXiv 0809, 1906v1902 (2008).
Jolly, A. E., Scott, G. T., Sharp, D. J. & Hampshire, A. H. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain J. Neurol. 143, 1158–1176. https://doi.org/10.1093/brain/awaa067 (2020).
Zeng, C., Liao, S. & Pu, W. Trait and state-related characteristics of thalamo-cortical circuit disruption in bipolar disorder: A prospective cross-sectional study. Front. Psychiatry 14, 1067819. https://doi.org/10.3389/fpsyt.2023.1067819 (2023).
Chen, F. et al. Body-mind relaxation meditation modulates the thalamocortical functional connectivity in major depressive disorder: A preliminary resting-state fMRI study. Transl. Psychiatry 11, 546. https://doi.org/10.1038/s41398-021-01637-8 (2021).
Snyder, H. R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 139, 81–132. https://doi.org/10.1037/a0028727 (2013).
Lalovic, A. et al. A qualitative systematic review of neurocognition in suicide ideators and attempters: Implications for cognitive-based psychotherapeutic interventions. Neurosci. Biobehav. Rev. 132, 92–109. https://doi.org/10.1016/j.neubiorev.2021.11.007 (2022).
Chen, H. et al. Cognitive impairment and factors influencing depression in adolescents with suicidal and self-injury behaviors: A cross-sectional study. BMC Psychiatry 23, 247. https://doi.org/10.1186/s12888-023-04726-8 (2023).
Ho, M. C., Hsu, Y. C., Lu, M. L., Gossop, M. & Chen, V. C. “Cool” and “Hot” executive functions in suicide attempters with major depressive disorder. J. Affect. Disord. 235, 332–340. https://doi.org/10.1016/j.jad.2018.04.057 (2018).
Bredemeier, K. & Miller, I. W. Executive function and suicidality: A systematic qualitative review. Clin. Psychol. Rev. 40, 170–183. https://doi.org/10.1016/j.cpr.2015.06.005 (2015).
Trapp, N. T. et al. Large-scale lesion symptom mapping of depression identifies brain regions for risk and resilience. Brain J. Neurol. 146, 1672–1685. https://doi.org/10.1093/brain/awac361 (2023).
Yao, S. et al. Astrocytic lactate dehydrogenase A regulates neuronal excitability and depressive-like behaviors through lactate homeostasis in mice. Nat. Commun. 14, 729. https://doi.org/10.1038/s41467-023-36209-5 (2023).
Chai, X. J. et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 36, 2009–2017. https://doi.org/10.1038/npp.2011.88 (2011).
Kinou, M. et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophrenia Res. 150, 459–467. https://doi.org/10.1016/j.schres.2013.08.026 (2013).
Salomons, T. V. et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 39, 488–498. https://doi.org/10.1038/npp.2013.222 (2014).
Sheline, Y. I., Price, J. L., Yan, Z. & Mintun, M. A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 107, 11020–11025. https://doi.org/10.1073/pnas.1000446107 (2010).
Steffens, D. C., Fahed, M., Manning, K. J. & Wang, L. The neurobiology of apathy in depression and neurocognitive impairment in older adults: A review of epidemiological, clinical, neuropsychological and biological research. Transl. Psychiatry 12, 525. https://doi.org/10.1038/s41398-022-02292-3 (2022).
Kjaerstad, H. L. et al. The longitudinal trajectory of emotion regulation and associated neural activity in patients with bipolar disorder: A prospective fMRI study. Acta Psychiatrica Scandinavica 146, 568–582. https://doi.org/10.1111/acps.13488 (2022).
Elsayed, N. M., Vogel, A. C., Luby, J. L. & Barch, D. M. Labeling emotional stimuli in early childhood predicts neural and behavioral indicators of emotion regulation in late adolescence. Biological psychiatry. Cogn. Neurosci. Neuroimaging 6, 89–98. https://doi.org/10.1016/j.bpsc.2020.08.018 (2021).
He, C. et al. Insula network connectivity mediates the association between childhood maltreatment and depressive symptoms in major depressive disorder patients. Transl. Psychiatry 12, 89. https://doi.org/10.1038/s41398-022-01829-w (2022).
Sezer, I., Pizzagalli, D. A. & Sacchet, M. D. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci. Biobehav. Rev. 135, 104583. https://doi.org/10.1016/j.neubiorev.2022.104583 (2022).
Downar, J. & Daskalakis, Z. J. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 6, 231–240. https://doi.org/10.1016/j.brs.2012.08.006 (2013).
Bodén, R., Bengtsson, J., Thörnblom, E., Struckmann, W. & Persson, J. Dorsomedial prefrontal theta burst stimulation to treat anhedonia, avolition, and blunted affect in schizophrenia or depression—A randomized controlled trial. J. Affect. Disord. 290, 308–315. https://doi.org/10.1016/j.jad.2021.04.053 (2021).
Bengtsson, J., Frick, A. & Gingnell, M. Blinding integrity of dorsomedial prefrontal intermittent theta burst stimulation in depression. Int. J. Clin. Health Psychol. 23, 100390. https://doi.org/10.1016/j.ijchp.2023.100390 (2023).
Bengtsson, J., Olsson, E., Persson, J. & Bodén, R. No effects on heart rate variability in depression after treatment with dorsomedial prefrontal intermittent theta burst stimulation. Ups. J. Med. Sci. https://doi.org/10.48101/ujms.v128.8949 (2023).
Kober, H. et al. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage 42, 998–1031. https://doi.org/10.1016/j.neuroimage.2008.03.059 (2008).
Hossein, S. et al. Effects of acute stress and depression on functional connectivity between prefrontal cortex and the amygdala. Mol. Psychiatry https://doi.org/10.1038/s41380-023-02056-5 (2023).
Sun, J. J., Shen, C. Y., Liu, X. M. & Liu, P. Z. Abnormal prefrontal brain activation during a verbal fluency task in treatment-resistant depression using near-infrared spectroscopy. Psychiatry Investig. 20, 84–92. https://doi.org/10.30773/pi.2021.0372 (2023).
Elton, A. et al. Sex moderates family history of alcohol use disorder and childhood maltreatment effects on an fMRI stop-signal task. Hum. Brain Mapp. 44, 2436–2450. https://doi.org/10.1002/hbm.26221 (2023).
Fermin, A. S. R. et al. The neuroanatomy of social trust predicts depression vulnerability. Sci. Rep. 12, 16724. https://doi.org/10.1038/s41598-022-20443-w (2022).
Jonkman, L. E., Fathy, Y. Y., Berendse, H. W., Schoonheim, M. M. & van de Berg, W. D. J. Structural network topology and microstructural alterations of the anterior insula associate with cognitive and affective impairment in Parkinson’s disease. Sci. Rep. 11, 16021. https://doi.org/10.1038/s41598-021-95638-8 (2021).
Aryutova, K. et al. Differential aberrant connectivity of precuneus and anterior insula may underpin the diagnosis of schizophrenia and mood disorders. World J. Psychiatry 11, 1274–1287. https://doi.org/10.5498/wjp.v11.i12.1274 (2021).
Fischer, A. S. et al. Intrinsic reward circuit connectivity profiles underlying symptom and quality of life outcomes following antidepressant medication: A report from the iSPOT-D trial. Neuropsychopharmacol. Off. Pub. Am. Coll. Neuropsychopharmacol. 46, 809–819. https://doi.org/10.1038/s41386-020-00905-3 (2021).
Sultana, T. et al. Neural mechanisms of emotional health in traumatic brain injury patients undergoing rTMS treatment. Mol. Psychiatry https://doi.org/10.1038/s41380-023-02159-z (2023).
Uddin, L. Q., Nomi, J. S., Hébert-Seropian, B., Ghaziri, J. & Boucher, O. Structure and function of the human Insula. J. Clin. Neurophysiol. 34, 300–306. https://doi.org/10.1097/wnp.0000000000000377 (2017).
Liu, G. et al. The alteration of cognitive function networks in remitted patients with major depressive disorder: An independent component analysis. Behav. Brain Res. 400, 113018. https://doi.org/10.1016/j.bbr.2020.113018 (2021).
Penfield, W. & Faulk, M. E. Jr. The insula; further observations on its function. Brain J. Neurol. 78, 445–470. https://doi.org/10.1093/brain/78.4.445 (1955).
Cereda, C., Ghika, J., Maeder, P. & Bogousslavsky, J. Strokes restricted to the insular cortex. Neurology 59, 1950–1955. https://doi.org/10.1212/01.wnl.0000038905.75660.bd (2002).
Bi, R., Dong, W., Zheng, Z., Li, S. & Zhang, D. Altered motivation of effortful decision-making for self and others in subthreshold depression. Depress. Anxiety 39, 633–645. https://doi.org/10.1002/da.23267 (2022).
Salazar de Pablo, G. et al. Predicting bipolar disorder I/II in individuals at clinical high-risk: Results from a systematic review. J. Affect. Disord. 325, 778–786. https://doi.org/10.1016/j.jad.2023.01.045 (2023).
Liao, Y. et al. Impact of cognitive-affective and somatic symptoms in subthreshold depression transition in adults: Evidence from depression Cohort in China (DCC). J. Affect. Disord. 315, 274–281. https://doi.org/10.1016/j.jad.2022.08.009 (2022).
Kaiser, R. H. et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 41, 1822–1830. https://doi.org/10.1038/npp.2015.352 (2016).
Palmieri, A. et al. Rorschach assessment in suicide survivors: Focus on suicidal ideation. Front. Public Health 6, 382. https://doi.org/10.3389/fpubh.2018.00382 (2018).
Bishop, J., Martin, A., Costanza, S. & Lane, R. C. Suicide signs on the rorschach. J. Contemp. Psychother. 30, 289–305. https://doi.org/10.1023/A:1004146800590 (2000).
Funding
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (JHJ, grant No. NRF-2016M3C7A1914449) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (JYY, grant No. 2023R1A2C1006783).
Author information
Authors and Affiliations
Contributions
J.Y.Y., S.H.C., and J.H.J. generated the ideas for the study and contributed to the study design. S.H.C., S.P., and J.H.J. conducted the experiments and collected the data. J.Y.Y. performed the formal analysis and drafted the manuscript. J.Y.Y., S.H.C., and J.H. provided edits and revisions. All of the authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yun, JY., Choi, SH., Park, S. et al. Association of executive function with suicidality based on resting-state functional connectivity in young adults with subthreshold depression. Sci Rep 13, 20690 (2023). https://doi.org/10.1038/s41598-023-48160-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48160-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.