Abstract
Biological invasions represent a major threat to biodiversity, especially in cold insular environments characterized by high levels of endemism and low species diversity which are heavily impacted by global warming. Terrestrial invertebrates are very responsive to environmental changes, and native terrestrial invertebrates from cold islands tend to be naive to novel predators. Therefore, understanding the relationships between predators and prey in the context of global changes is essential for the management of these areas, particularly in the case of non-native predators. Merizodus soledadinus (Guérin-Méneville, 1830) is an invasive non-native insect species present on two subantarctic archipelagos, where it has extensive distribution and increasing impacts. While the biology of M. soledadinus has recently received attention, its trophic interactions have been less examined. We investigated how characteristics of M. soledadinus, its density, as well as prey density influence its predation rate on the Kerguelen Islands where the temporal evolution of its geographic distribution is precisely known. Our results show that M. soledadinus can have high ecological impacts on insect communities when present in high densities regardless of its residence time, consistent with the observed decline of the native fauna of the Kerguelen Islands in other studies. Special attention should be paid to limiting factors enhancing its dispersal and improving biosecurity for invasive insect species.
Similar content being viewed by others
Introduction
Invasive non-native species—those species that are introduced by humans, establish, spread in environments where they were not formerly found—represent a major threat to native biodiversity worldwide1,2,3,4. The invasion-induced threat is worsening with intensification of trade and development of new transport pathways, in addition to global warming, altogether facilitating non-native species to arrive, establish and spread in more diverse locations1,5,6.
The impacts caused by non-native species may be particularly pronounced towards the poles, due both to the ongoing potential for biological invasion as new trade routes open, and to climate warming7,8,9. Consistently, “polar amplification” refers to the fact that cold regions (alpine, Arctic, subarctic, Antarctic and subantarctic areas) experience faster and more intense effects of climate change, manifesting in the reduction of frozen surfaces, extreme winter warming events, precipitation regime changes, summer droughts and heat periods, among others10,11. These strong changes in local climatic conditions alter physiological responses of many organisms, in particular plants and ectotherms. Some native plants and insects seem to benefit from warmer conditions, but those species might suffer from increased mortality and reduced development once developmental thresholds in environmental conditions are reached, compromising ecosystem functioning12,13,14.
In a warmer world, insular communities from cold regions are also increasingly exposed to incursions of non-native species, especially when native communities are weakened due to global changes15,16. These native communities are often species poor, and characterized by a high degree of endemism, making non-native species a high risk to biodiversity conservation in the absence of trophically-analogous species17. Insular cold areas also have lower functional diversity, hence displaying vacant niches that can be readily exploited by non-native species18, 19, and in some cases even lack predatory species17. Consequently, native communities from cold regions are naive to predators, as evidenced, for example, by the fact that many native insect species are flightless; an obvious disadvantage in the face of non-native predators20,21,22,23.
Subantarctic islands are cold environments situated between 45 °S and 54 °S, and encompass most of the terrestrial biotas at mid and high latitudes of these locations24. They exhibit key characteristics—harsh conditions and geographic isolation, high sensitivity to climate change, low species diversity, limited anthropogenic influence as compared with temperate regions, and recent introductions of non-native species—making them ideal open-air laboratories to study non-native species and their impacts, many of which are relatively unknown24,25. Subantarctic islands have long been considered as relatively pristine areas in terms of non-native species, but the number of non-native species began increasing in the nineteenth century when sealing and whaling industries established, and the introduction of non-native species has dramatically risen in the last 60 years after the establishment of permanent research stations26,27. As insects are particularly responsive to environmental conditions, including climate change28, the impacts caused by non-native insect species establishment in these cold regions may be bolstered under warmer conditions, due to an increase in metabolism, fecundity, spread and survival16,29,30.
Along with competition for food and/or space, predation is a major source of demographic density dependence which greatly influences prey community structure. Predator responses to prey density can vary among species and environments, but prey consumption rates most often decrease at high prey densities, while in these conditions, insect predators often kill more prey than they eat31,32. Predator sex and body mass, as well as environmental conditions such as temperature increasing physiological activities, can also affect prey consumption33,34. For example, when prey are scarce, a greater mobility of their predators has usually been reported, which makes them more visible and increases their own predation risk if higher predators are present35. Moreover, effects of body size on predation can differ between sexes36, with interactions also mediated by sex-specific behaviors such as mate searching37,38. Interaction strengths towards prey additionally often peak at intermediate predator–prey body size ratios, with predators less efficient towards prey which are too large or too small35,39,40. Understanding the relationships between predators and prey is therefore essential as they establish the links between trophic levels, and in consequence affect species community dynamics41,42,43,44.
On Kerguelen Islands, mean annual air temperature has increased by 1.3 °C since the 1960s, and summer temperature extremes have been recorded, reaching up to 23 °C11. Non-native insect species have been introduced to the Kerguelen Islands, which now host more non-native than native arthropod species (which is also the case for other species groups, such as vascular plants, and on other subantarctic islands)11,27. One of those non-native insect species, the predatory carabid beetle Merizodus soledadinus (Guérin-Méneville, 1830) (Coleoptera: Trechidae), was accidentally introduced from the Falkland Islands in 1913 at a single site on Kerguelen Islands (Port Couvreux: 49°17′04.9″S, 69°41′41″E), where it was first identified in 193945. Its distribution and density increased rapidly in the early 1990s11,46. Nowadays, it occupies a large range, mainly constituted of coastal environments but is also expanding along hydrographic networks, where trophic resources and water are available47. In its invaded ranges, M. soledadinus predation heavily impacts native arthropods48.
While the biology of M. soledadinus has recently received attention, its ecological impacts, in particular the trophic interactions of the species, have been less examined. The continuous monitoring of the geographic expansion of the species on Kerguelen Islands makes it possible to record the time since the arrival of M. soledadinus at a specific location (hereafter referred to as “residence time”), and thus indicates the spatial sorting of the populations and along the invasion fronts. This is particularly crucial information, as it has been found that individuals from the invasion front are larger25, and hence, may be characterized by different predation behavior49,50,51. Here, we used the detailed knowledge about the invasion process of M. soledadinus on Kerguelen Islands, to investigate what impact the population density of such a non-native predatory insect has on its predation rate. The following main characteristics were also considered: mean body mass, residence time, sex ratio, the number of prey, and the duration of the experiment, which allowed observations of the prey-predator interactions, i.e. the proportion of eaten, attacked and dead prey at various observation time points during the experiment. We hypothesized that the number of predators would be positively correlated with predation rates, while an opposite pattern would occur with number of prey. Additionally, we hypothesized that the residence time would affect predation, with greater predation expected by populations with a shorter residence time owing to greater boldness at the invasion fronts49. Finally, we derived a measure of the potential population-level impact of M. soledadinus, taking into account its maximum feeding rate and its abundances (based on Dick et al. 201752), hypothesizing that longstanding populations will have a greater abundance, but that this will be offset by a lower per capita effect.
Results
The proportion of eaten, attacked and dead larvae of the fly Fucellia maritima (Haliday 1838) (Diptera: Anthomyiidae) varied in a similar way in response to the numbers of predators, the quadratic term of the predator number variable, and the duration of the experiment (Table 1). Conversely, an opposite relationship was found for the number of prey and the quadratic term of the number of prey variable (Table 1). Specifically, the number of eaten, attacked or dead prey increased with increasing numbers of M. soledadinus, and with increasing duration of the experiment, while the number of eaten, attacked or dead prey decreased with increasing prey density (Table 1, Figs. 1, 2 and 3). The proportions of eaten, attacked and dead prey were strongly correlated (Pearson coefficient of: 0.90 for the correlation between the proportion of eaten and attacked prey, − 0.94 for the correlation between the proportion of eaten and dead prey, − 0.93 for the correlation between the proportion of attacked and dead prey).
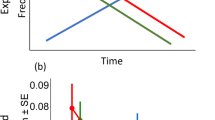
Trends for the best generalized linear mixed models testing for the drivers of the proportion of eaten, attacked and dead prey as a function of the number of predators (A, C, E, respectively). Lower and upper 95 % confidence intervals are represented by the dashed lines. Violin boxplots for the proportion of eaten, attacked or dead prey are a combination of kernel density plots and boxplots. For each predator density (n = 180 replicates), violin boxplots represent the distribution of the raw data, and the upper adjacent value, third quartile, median, first quartile and lower adjacent value from top to bottom (B,D,F, respectively).
Trends for the best generalized linear mixed models testing for the drivers of the proportion of eaten, attacked and dead prey as a function of the duration of the experiment (Experiment time, A, C, E, respectively). Lower and upper 95 % confidence intervals are represented by the dashed lines. Violin boxplots for the proportion of eaten, attacked or dead prey are a combination of kernel density plots and boxplots. For each experiment time (n = 72 replicates), violin boxplots represent the distribution of the raw data, and the upper adjacent value, third quartile, median, first quartile and lower adjacent value from top to bottom (B, D, F, respectively).
Trends for the best generalized linear mixed models testing for the drivers of the proportion of eaten, attacked and dead prey as a function of the number of prey (A, C, E, respectively). Lower and upper 95 % confidence intervals are represented by the dashed lines. Violin boxplots for the proportion of eaten, attacked or dead prey are a combination of kernel density plots and boxplots. For each prey density (n = 240 replicates), violin boxplots represent the distribution of the raw data, and the upper adjacent value, third quartile, median, first quartile and lower adjacent value from top to bottom (B, D, F, respectively).
The proportion of eaten, attacked or dead prey significantly increased at predator densities of more than five individuals, and then stabilized at maximum values when 20 and 50 predators were present (Fig. 1). The mean proportions and the variance of eaten prey for 1, 5, 20 and 50 predators were 0.45, 0.67, 0.88 and 0.96; and 1, 0.95, 0.75 and 0.40, respectively (see Supplementary material 3, Table 1 for the mean proportion and variance of attacked and dead prey as a function of the number of predators, calculated at the end of the experiment).
The proportion of eaten, attacked or dead prey steadily rose as experimental duration increased, reaching a maximum at the end of the experiment (experiment time point 10; Fig. 2). The mean proportion and the variance of eaten prey for experiment time points 1 and 10 reached 0.63 and 0.80, and 1 and 1, respectively (see Supplementary material 3, Table 2 for the mean proportion and variance of attacked and dead prey in function of the experiment time point, calculated at the end of the experiment).
Conversely, while a sharp decline occurred when the prey density was at five, the proportion of eaten, attacked or dead prey continued to decrease as prey density increased (20 prey, Fig. 3A, C and E). Specifically, the mean proportion of eaten prey when the density prey was at 1, 5 and 20 prey equaled 0.97, 0.67 and 0.58 respectively; with a variance of 1 for all prey densities (see Supplementary material 3, Table 3 for the mean proportion and variance of attacked and dead prey as a function of the number of prey, calculated at the end of the experiment).
The residence time, body mass and the sex ratio of M. soledadinus were not retained in the best models, and were not significant in the full models (Table 1, Supplementary material 4, Table 1). These variables did not significantly explain the variation in the proportion of eaten, attacked or dead prey by M. soledadinus.
Finally, the impact potential values varied considerably between the populations, but did not vary as a function of their residence time. Impact potential exhibited a ratio of 7.5 between the lowest and highest values, i.e. ranging from 196.5 to 1500, with a mean of 663 (Table 2). The highest impacts were projected for the populations at Pointe Suzanne (1500) and Ratmanoff (800) which are two of the populations with the shortest residence times (10 and 8 years, respectively). Populations of M. soledadinus with the longest residence times did not have the highest abundances, and those with the shortest residence times did not have the highest maximum feeding rates (Table 2).
Discussion
The impacts of the non-native invasive beetle M. soledadinus appeared to be independent of residence time, body mass and sex in our study, and therefore their effects on resident fauna may be widespread. The number of adult M. soledadinus and the duration of the experiment had a significant positive influence on the proportion of eaten, attacked and dead prey. The number of prey significantly negatively influenced the proportion of eaten, attacked and dead prey. Conversely, the residence time of the population, sex ratio and body mass were not significant predictors of the strength of predator–prey interaction. Moreover, population-level impacts were not formally correlated with residence time even if some of the populations with the shortest residence times had high impact potential.
Per capita feeding rates of invasive species can be linked to their ecological effects, and the impact potential metric applied here has been found to correlate with independent assessments of ecological impacts in the field52. The proportion of eaten, attacked and dead prey rapidly increased before plateauing at higher densities of M. soledadinus. However, this “saturation” in the effect of the predator density (visible by the significant negative quadratic term of the number of predators) might also partly result from progressive prey depletion. Prey were not replaced during the experiment, which represents a possible experimental bias53. The proportion of eaten, attacked and dead prey first decreased at the intermediate prey density but then increased towards the highest prey density, potentially revealing a form of “predator learning”, or an increased efficiency of foraging as the number of prey increased. Learning in foraging and prey selection have been identified in insects54,55,56. Further examination of this theory should include additional experimental treatments in order to cover a higher range of predator–prey densities.
Residence time can influence a myriad of physiological and morphological traits in invasive species. Former investigations found that the dispersal capacities of adult M. soledadinus, and their morphology, varied as a function of their residence time: heavier and larger individuals were collected in the populations having the shortest residence times in comparison with the populations having the longest residence times (3 and 11 years, 36 and 93 years, respectively)57. Since we did not find any clear residence time effect, in situ field studies and inclusion of other sites in future that have a more longstanding invasion would be beneficial. Interestingly, body mass, sex or residence time of populations of M. soledadinus had no effect on the proportion of eaten, attacked or dead prey in our study. This finding suggests a lack of effect of these context-dependencies in the predator–prey relationship among the different populations of M. soledadinus regarding the prey used in this study. By maintaining a constantly high predation rate, the impacts caused by this species of beetle on native insect communities could therefore be immediate and severe, albeit non-linear, escalating with its increasing density, especially where prey are limited.
Our study provides a new insight into predation dynamics, as we uniquely studied the predator–prey relationships of M. soledadinus with three response variables: the proportion of eaten, attacked or dead prey. While the energy consumption of M. soledadinus was visible via the proportion of eaten prey, the proportion of attacked prey gave an indication on predation, and the possible existence of wasteful killing. The proportion of dead prey provided information on a combination of the effects of M. soledadinus on prey survival and on ‘natural’ prey death. It is important to account for potential wasteful killing by predators, as this phenomenon has been often noted in insects58,59,60,61. Moreover, wasteful killing behavior in predators can have very important negative consequences for the density of prey populations62. For M. soledadinus, we found no evidence of wasteful killing; the same decreasing trend for the proportion of eaten, attacked and dead prey was measured even as prey densities increased. Yet, conclusions made from the present results must also be considered with caution, as they might vary with the type of prey used, and are not completely reflective of natural conditions53.
Our results using the non-native prey species F. maritima suggest that M. soledadinus is likely to have a much higher impact on the insect fauna, native and non-native, in areas where its population densities are very high, in line with former investigations on other non-native predators invading different environments63. Indeed, we measured an increased proportion of eaten, attacked and dead prey with increasing densities of this beetle species. On the Kerguelen Islands, M. soledadinus can be found in high densities in some areas, with up to 100 individuals found during a ten-minute count a single location48. Such densities clearly exceed the maximum predator density we applied in our experiment. Moreover, current densities recorded of M. soledadinus are considerably higher than those reported in the 1990s48, which would suggest that polar amplification boosts population dynamics and increases the impacts of this predatory non-native insect on native communities.
Habitat complexity is an important consideration when measuring predator–prey dynamics. The addition of a sand layer at the bottom of the experimental boxes is not only useful to maintain a suitable humidity for M. soledadinus which only survives in moist conditions, but also to allow F. maritima to be somewhat inconspicuous, so as not to influence predation pressure64. The sand substrate also acts as a potential refuge for F. maritima. Refuge effects have been widely recognized as prevailing in predator–prey relationships, with a higher concentration of refuges leading to reduced predator impacts due to an increased searching time for prey65,66. However, even if the relatively shallow sandy substrate we used was not entirely comparable to field conditions, it was the most realistic experimental design that could be used, as both M. soledadinus and F. maritima are found in coastal environments on Kerguelen Islands53,67.
Predation rates were prey density-dependent in the present study. We found that the prey consumption rate by M. soledadinus decreased with increasing prey density (at least with its linear term), despite the restricted prey densities we studied in our experiment. This pattern could correspond to the most common type-two functional response, whereby prey consumption rates fall as their density increases (thus following a hyperbolic form)32. However, experiments using a broader range of prey densities than assessed here would be required to test this theory. Our generalized conclusions on the impacts of M. soledadinus across the native invertebrate community on the island are restricted by our experiments using a single non-native prey species, and therefore patterns might be different when focusing on native prey species. Meanwhile, it is important to consider the predator–prey dynamics between F. maritima and M. soledadinus, as the non-native F. maritima is present in high densities in coastal areas of the Kerguelen Islands and its larvae represent an important part of the beetle’s diet27,67. Therefore, exploring greater prey and predator densities, or ideally including native prey species, despite their vulnerability, would allow us to expand our knowledge of the impacts of M. soledadinus in its invaded range on the Kerguelen Islands.
Contrary to our expectations, and to previous findings on other predator species35,36,49, the residence time of the populations, the sex or the body mass of M. soledadinus did not affect its predation rate. We found that the impact of M. soledadinus was relatively high no matter its residence time. Indeed, predator density was the most important characteristic explaining the impact of M. soledadinus overruling its other characteristics such as residence time, sex and body mass. Additionally, even if populations did not appear to have significantly increasing impact values in relation to decreasing residence times compared to some other non-native predators49, we found that some populations with short residence times had very high potential impact when using F. maritima as the model prey species. Thus, a pattern of increased impact along the invasion gradient tended to appear for these populations that combined short residence times and high potential impacts, in line with the spatial sorting concept which is often associated with increased performance of invasive organisms at the invasion front67,68. The relatively low observed potential impact of the population of M. soledadinus from Isthme Bas may be a consequence of a much faster increase in population density than in other localities. Indeed similar prey capture rates to those of a long-established population were found at Isthme Bas, ca. 3 years after the arrival of M. soledadinus48. Such prey-capture rates may have quickly increased intraspecific competition and altered the trophic behaviour of M. soledadinus. Generally, the present results would imply that M. soledadinus might have important and rapid impacts on the native insect communities, in line with the observed rapid decline of the native—and sometimes endemic—fauna of the Kerguelen Islands11,27,46. Indeed, in the presence of high numbers of M. soledadinus on the Kerguelen Islands, the densities of native arthropods and especially of native flightless insects—which are inherently naive to ground-based predators—are negatively impacted.
For example, two flightless native species display important negative impact from the presence of M. soledadinus on Kerguelen Islands. Populations of the native Anatalanta aptera (Eaton 1875, Diptera: Sphaeroceridae) are highly reduced, while the native Calycopteryx moseleyi (Eaton 1875, Diptera: Micropezidae) has been locally driven to extinction48. On Kerguelen Islands, it has to be noted that there may be competition between native prey species of M. soledadinus such as A. aptera or C. moseleyi, and non-native prey species of M. soledadinus such as F. maritima, which are not taken into account in our study. These competition impacts could play an important role in the decline of populations of native prey species in addition to the negative influence of predation by M. soledadinus. However, prey species such as C moseleyi and F. maritima strongly respond to increases in food availability. For example, C. moseleyi preferably feeds on decomposing tissues of the native plant species Pringlea antiscorbutica, while F. maritima is mainly found in seaweed. In areas where multiple food sources are found, those two species seem to have little impact on each other via competition69. For now, the sharp declines in native prey species on Kerguelen Islands are mainly attributed to the predation by M. soledadinus, although further studies on competition between native and non-native prey species should be done to elucidate how multiple impact mechanisms might combine, as well as the effects of this invader on native prey species48. Finally, some places on the Kerguelen Islands are not yet invaded by M. soledadinus, due to barrier zones such as cliffs or penguin colonies. At these sites, native insect species remain unimpacted by this non-native beetle48.
The distribution and survival of many native species from subantarctic islands are highly influenced by global changes, notably by the intense temperature warming and precipitation changes occurring in the region70,71,72. Long-term monitoring of M. soledadinus on Kerguelen Islands provides valuable insights into its distribution and range expansion since the 1990’s, and the colonization of the beetle towards higher elevations48. Native species are already migrating to higher elevations on subantarctic islands73. However, on Kerguelen Islands, such altitudinal expansion is likely to be also under pressure from the negative impacts of M. soledadinus, since this non-native predatory species progresses rapidly in areas with abundant food resources64. Additionally, M. soledadinus displays limited stress signals in warming conditions (as high as 20 °C), which would indicate that temperature warming on Kerguelen Islands might not limit individuals in their further spread, but could even stimulate them, for example by intensifying predation behavior14,48,74. However, the decreased precipitation induced by climate change on Kerguelen Islands could be problematic for M. soledadinus, if the areas where it is present become drier, as has been noted for some wetlands and ponds with the decline of native wetland plant species10,11,75,76. Indeed, it has been found in experimental settings that the survival of this beetle is reduced in drier conditions64.
M. soledadinus is a non-native predator species that already seems to have very deleterious impacts on the native fauna on subantarctic islands where it has invaded11,27. Such impacts are at risk of exacerbation in the future, as climatic changes within the region further weaken and stress native biota, and also possibly bolster the metabolism of this non-native insect predator11,20,22. Furthermore, this non-native predator species might entirely disrupt ecosystem functioning. Indeed, the native species that M. soledadinus appears to threaten play a significant role in decomposition activities12,13. Once M. soledadinus reached the scientific research station on the Kerguelen Islands, the only part of the archipelago with sustained anthropogenic influence, its spread rapidly increased, assisting its reach to isolated locations48. It is therefore essential to mitigate the risks of further anthropogenic assistance in the continued spread of M. soledadinus. The declaration of the Kerguelen Islands as a Nature Reserve in 2006 has resulted in the development and application of biosecurity protocols that may assist managers to control the further spread of M. soledadinus, and limit the risk of future non-native species introductions77,78. In the future, it will be important to limit the importation of individuals of M. soledadinus to areas on the Kerguelen Islands that are still free of this species, but also to ensure it does not invade other cold environments.
Methods
Insect collection
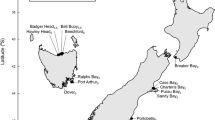
In 2015, 350 live adult M. soledadinus were hand-collected from each of the six following localities of the Kerguelen Islands: Port Elizabeth, Cataractes, Anse du Pacha, Pointe Suzanne, Ratmanoff and Isthme Bas (Fig. 4). All of these localities are coastal, and host large densities of M. soledadinus with known residence times. At the time of collection, M. soledadinus was established for 46 years at Port Elizabeth, 36 years at Cataractes, 21 years at Anse du Pacha, 10 years at Pointe Suzanne, eight years at Ratmanoff and five years at Isthme Bas (Fig. 4) (Source: project 136—SUBANTECO, French polar institute). Adults from these six localities were deemed to belong to different populations. Based on the fact that the average adult longevity of M. soledadinus is about eight months75 and may produce two generations per year, the number of generations since the establishment of the insect in each locality would vary at least from seven (for the population with the shortest residence time used in our study: Isthme Bas) to 92 generations (for the population with the longest residence time used in our study: Port Elizabeth).
Locations of the populations of M. soledadinus on the Kerguelen Islands are represented by grey dots, with increasing dot size corresponding to increasing residence time: 46 years at Port Elizabeth, 36 years at Cataractes, 21 years at Anse du Pacha, 10 years at Pointe Suzanne, eight years at Ratmanoff and five years at Isthme Bas. The red dot corresponds to the research station of Port-aux-Français. The map of the Kerguelen Islands was created by the authors. The background map comes from ©OpenStreetMap79; OpenStreetMap is open data (Open Data Commons Open Database License, ODbL, and license CC BY-SA 2.0 for the documentation). QGIS 3.28.8 LTR was used to generate the map (www.qgis.org).
Insect maintenance
All collected individuals were transported live the day after the collection, in sealed plastic boxes, to the research station laboratory (Port-aux-Français, Kerguelen Islands). Then, each population was immediately placed in separate plastic boxes (18 × 12 × 7 cm) at standardized densities of 150 individuals per box (total of 350 individuals for each population, distributed in two boxes with 150 individuals each, and a back-up box with 50 individuals). Sand collected at the Anse du Pacha locality (49°21′01.3"S, 70°11′52.6"E) was thoroughly rinsed with tap water, drained and left moist, and a 5 mm layer was placed at the bottom of each box before the transfer of the insects. Adults of M. soledadinus cannot survive in low air humidity conditions (relative humidity < 70%), or if they lack access to fresh water64. Hence, this sandy substrate provided adults with their humidity and water requirements. Rinsed wood pieces or gravel were added to provide adults with shelters. The plastic boxes containing the insects collected from the different populations were then placed in a walk-in climatic chamber at 8 °C, photoperiod 12:12 (Light:Dark).
Plastic boxes of all populations were checked once per week to control the stability of air and soil humidity. To that aim, they were opened 1 h per week for the renewal of the air, and the humidity of the substrate was readjusted with tap water to keep it appropriately moist. During the weekly checks, larvae of M. soledadinus were removed, if present. At the same time, adults of M. soledadinus were fed weekly with larvae of the fly F. maritima. This dipteran is a non-native species present in high densities in coastal environments on the Kerguelen Islands, and is a large part of the diet of M. soledadinus there27,75,80. Furthermore, F. maritima is very similar in size, habitat and diet during larval stages to A. aptera or C. moseleyi, two example native fly species also consumed by M. soledadinus67. So far as we are aware, these other fly species are naive to insect predators, and the use of F. maritima as a prey had less impact on insect communities than if we had collected a native species. One week prior to the beginning of the experiment, feeding of all M. soledadinus was stopped, so that the beetles used for the experiments were likely to have cleared guts67.
Experimental design and setup
Transparent plastic boxes (18 × 12 × 7 cm) were filled with 2 mm of tap water-rinsed sand from the aforementioned location of Anse du Pacha. The shallower depth of sand in the boxes was applied to avoid larval digging, thereby reducing their ability to hide from M. soledadinus. One plastic box was assigned to a single treatment combination: population of the predator (i.e., M. soledadinus: Port Elizabeth, Cataractes, Anse du Pacha, Pointe Suzanne, Ratmanoff and Isthme Bas) × number of predators (1, 5, 20, or 50 individuals) x number of prey (i.e., F. maritima: 1, 5, or 20 individuals), resulting in a total of 72 treatment combinations. We had one experimental replicate per treatment combination. As we needed 76 M. soledadinus individuals for each population for the predator treatment, we used the individuals collected from the two plastic boxes of 150 individuals (Insects from the box containing 50 individuals were not used). At the start of each experiment, plastic boxes were placed in a single walk-in climatic chamber at 8 °C, photoperiod 12:12 (L:D), and the designated number of predators from one out of the six studied populations of M. soledadinus was paired with the designated number of prey. The experiment lasted for five days, and began at 18 h on day 0. We defined the experiment time point as the different time points during which the prey-predator interactions were observed over the course of the experiment, ranging from observations at experiment time point 1 to 10. Thereby, the condition of the prey (i.e., analyzed response variables) was checked two times a day with a binocular magnifier installed in the walk-in climatic chamber, at 9.00 am (experiment time point 1) and 6.00 pm (experiment time point 2) at day 1, and repeated every day until 6.00 pm on day 5 (experiment time point 10) (Supplementary material 1, Table 1). At each experiment time point, prey were classified as dead or alive, attacked or non-attacked, eaten or non-eaten. At the end of the assay, M. soledadinus individuals were killed, weighed (body mass in mg), and their sex was determined.
Statistical analyses
Based on the classification of the prey, we calculated: the number of eaten, non-eaten, attacked, non-attacked, dead and alive prey. To do so, the number of eaten prey was calculated as: \(partially\, eaten\, larvae\, +\, entirely \,eaten\, larvae\); while the number of non-eaten prey was calculated as: \(alive\, larvae\, +\, dead\, larvae\, +\, alive \,wounded\, larvae\, +\, dead\, wounded \,larvae\). The number of attacked prey was calculated as: \(alive \,wounded\, larvae\, + \,dead\, wounded\, larvae\, +\, partially\, eaten\, larvae\, +\, entirely\, eaten\, larvae\); the number of non-attacked prey was calculated as: \(alive\, larvae\, + \,dead\, larvae\). The number of dead prey was calculated as: \(dead\, larvae \,+\, dead\, wounded\, larvae\, +\, partially\, eaten\, larvae\, +\, entirely \,eaten\, larvae\); while the number of alive prey was calculated as: \(alive\, larvae\, + \,alive\, wounded \,larvae\). The number of eaten or non-eaten prey informed the energy consumption of M. soledadinus. The number of attacked and non-attacked prey indicated the ecological impact of M. soledadinus on F. maritima including wasteful killing. The number of dead and alive prey gave an indication on the influence of predation, but also on death of larvae of F. maritima not related to predator attacks. Since we were interested on the effects of different numbers of M. soledadinus on F. maritima, we took into account their sex ratio and their mean body mass for each treatment combination. We thus calculated the ratio of female M. soledadinus as: \(\frac{number \,of \,female\, M. soledadinus}{total\, number\, of \,M. soledadinus}\times 100\), and the mean body mass of M. soledadinus individuals.
We analyzed three response variables: the proportion of eaten prey, the proportion of attacked prey and the proportion of dead prey. These response variables allowed us to focus on slightly different signals depicting the effects of the predator M. soledadinus on the prey F. maritima. To do so, we used a binomial denominator written as a vector: number of successes, number of failures81, corresponding in the present work to the pairs: number of eaten prey, number of non-eaten prey; number of attacked prey, number of non-attacked prey; and number of dead prey, number of alive prey, respectively. The relationships between the response variables (proportion of eaten prey, proportion of attacked prey, proportion of dead prey) and the explanatory variables (number of prey (continuous) and number of predators (continuous), mean body mass (continuous), residence time (continuous), ratio of female predators (continuous), and duration of the experiment (continuous)) were analyzed using generalized linear mixed models with a binomial family and a logit link (See Supplementary Material 2 for model structure, R package lme4, Ref.82). We included the quadratic terms for the number of prey and the number of predators. We also included the second order interactions between: the number of predators and the residence time, the residence time and the female ratio, the residence time and the mean body mass. The addition of the identity of each independent plastic box as a random intercept was necessary to account for the experimental design, and increased the fit of the mixed models for each of the response variables (Akaike Information Criterion (AIC) decreased with more than two units, Ref.83. The dredge function on the full models was used for each response variable (package MuMIn, Ref.84) to select the best model (the one with the lowest AIC and with the highest Phi coefficient (model precision)). We did not detect zero inflation for the best models for each of the response variable (DHARMa residuals diagnostic, R package DHARMa, Ref.85). For each best model, the pseudo-R-squared was calculated (R package MuMIn, Ref.84).
Following Dick et al.52, an adaptation of the “impact potential” metric was employed, and calculated as: \(abundance\, \times \,maximum\, feeding\, rate\). The rounded abundance was estimated in the field as a mean number of M. soledadinus adults at each of the population locations sampled. The maximum feeding rate was determined from the number of eaten prey at the last experiment time point for each population, from each single treatment condition: the lowest predator density (one predator) and the highest prey density (20 prey). This allowed the per capita effect and the population-level effect to be combined and compared, to gain a fuller understanding of ecological impact among beetle populations.
All statistical analyses were performed in R, version 3.5.286 and p = 0.05 was taken as threshold for significance.
Data availability
The data presented in this study are openly available in GitHub at https://github.com/davidrenault/Merizodus_predator_prey.
Code availability
R scripts from the analyses in this article were developed by CC with the help of RNC and are openly available in GitHub at https://github.com/davidrenault/Merizodus_predator_prey.
References
Dullinger, I. et al. Climate change will increase the naturalization risk from garden plants in Europe. Glob. Ecol. Biogeogr. 26, 43–53 (2017).
Bellard, C., Marino, C. & Courchamps, F. Ranking threats to biodiversity and why it doesn’t matter. Nat. Commun. 13, 1–4 (2022).
Jentsch, A. & Beierkuhnlein, C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus Géosci. 340, 621–628 (2008).
Richardson, D. M. et al. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 6, 93–107 (2000).
Seebens, H. et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 1–9 (2017).
Daly, E. Z. et al. Climate change helps polar invasives establish and flourish: Evidence from long-term monitoring of the blowfly. Biology (Basel) 12, 1–16 (2023).
McCarthy, A. H., Peck, L. S., Hughes, K. A. & Aldridge, D. C. Antarctica: The final frontier for marine biological invasions. Glob. Chang. Biol. 25, 2221–2241 (2019).
Duffy, G. A. et al. Barriers to globally invasive species are weakening across the Antarctic. Divers. Distrib. 23, 982–996 (2017).
Duffy, G. A. & Lee, J. R. Ice-free area expansion compounds the non-native species threat to Antarctic terrestrial biodiversity. Biol. Conserv. 232, 253–257 (2019).
Turner, J. & Overland, J. Contrasting climate change in the two polar regions. Polar Res. 28, 146–164 (2009).
Lebouvier, M. et al. The significance of the sub-Antarctic Kerguelen Islands for the assessment of the vulnerability of native communities to climate change, alien insect invasions and plant viruses. Biol. Invasions 13, 1195–1208 (2011).
Renault, D. et al. The rising threat of climate change for arthropods from Earth’s cold regions: Taxonomic rather than native status drives species sensitivity. Glob. Chang. Biol. 28, 5914–27 (2006).
Bokhorst, S. et al. Extreme winter warming events more negatively impact small rather than large soil fauna: Shift in community composition explained by traits not taxa. Glob. Chang. Biol. 18, 1152–1162 (2012).
Renault, D. et al. Advancing biological invasion hypothesis testing using functional diversity indices. Sci. Total Environ. 834, 155102 (2022).
Kennedy, D. Antarctic terrestrial ecosystem response to global environmental change. Annu. Rev. Ecol. Syst. 26, 683–704 (1995).
Hellmann, J. J., Byers, J. E., Bierwagen, B. G. & Dukes, J. S. Five potential consequences of climate change for invasive species. Conserv. Biol. 22, 534–543 (2008).
Cox, J. G. & Lima, S. L. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Opinion 21, 674–680 (2006).
Houghton, M., Terauds, A., Merritt, D., Driessen, M. & Shaw, J. The impacts of non-native species on the invertebrates of Southern Ocean Islands. J. Insect Conserv. 23, 435–452 (2019).
Russell, J. C., Meyer, J.-Y., Holmes, N. D. & Pagad, S. Invasive alien species on islands: Impacts, distribution, interactions and management. Environ. Conserv. 44, 359–370 (2017).
Atkinson, I. A. E. Introductions of wildlife as a cause of species extinctions T- !. Wildlife Biol. 2, 135–141 (1996).
Diamond, J. A new species of rail from the Solomon Islands and convergent evolution of insular flightlessness. Auk Q. J. Ornithol. 108, 461–470 (1991).
Salo, P., Korpimäki, E., Banks, P. B., Nordström, M. & Dickman, C. R. Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B Biol. Sci. 274, 1237–1243 (2007).
Causton, C. E., Peck, S. B., Sinclair, B. J. & Hodgson, C. J. Alien insects: Threats and implications for conservation of Galapagos Islands. Annu. Entomol. Soc. Am. 99, 121–143 (2006).
Bergstrom, D. M. & Chown, S. L. Life at the front: History, ecology and change on southern ocean islands. Trends Ecol. Evol. 14, 472–477 (1999).
Renault, D., Lebouvier, M. & Delattre, T. Is dispersal promoted at the invasion front? Morphological analysis of a ground beetle invading the Kerguelen Islands, Merizodus soledadinus (Cpleoptera, Carabidae). Biol. Invasions 15, 1641–1648 (2013).
Chown, S. L., Lee, J. E. & Shaw, J. D. Conservation of Southern Ocean Islands: Invertebrates as exemplars. J. Insect Conserv. 12, 277–291 (2008).
Frenot, Y. et al. Biological invasions in the Antarctic: Extent, impacts and implications. Biol. Rev. Cambridge Philos. Soc. 80, 45–72. https://doi.org/10.1017/S1464793104006542 (2005).
Burraco, P., Orizaola, G., Monaghan, P. & Metcalfe, N. B. Climate change and ageing in ectotherms. Glob. Chang. Biol. 26, 5371–5381 (2020).
Charnov, E. L. & Gillooly, J. F. Thermal time: Body size, food quality and the 10°C rule. Evol. Ecol. Res. 5, 43–51 (2003).
Renault, D., Laparie, M., Mccauley, S. J. & Bonte, D. Environmental adaptations, ecological filtering, and dispersal central to insect invasions. Annu. Rev. Entomol. 63, 345–368 (2018).
Beardsell, A. et al. Derivation of predator functional responses using a mechanistic approach in a natural system. Front. Ecol. Evol. 9, 1–12 (2021).
Holling, C. S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 1–14 (1959).
Parajulee, M. N., Phillips, T. W. & Hogg, D. B. Functional reponse of Lyctocoris campestris (F.) adults: Effects of predator sex, prey species, and experimental habitat. Biol. Control 4, 80–87 (1994).
Rall, B. C. et al. Universal temperature and body-mass scaling of feeding rates subject collections articles on similar topics can be found in the following collections. Philos. Trans. R. Soc. B Biol. Sci. 367, 2923–2934 (2012).
Hildrew, A. G. et al. Strong density dependence in a predatory insect: Large-scale experiments in a stream. J. Anim. Ecol. 73, 448–458 (2004).
Glen, A. S. & Dickman, C. R. Diet of the spotted-tailed quoll Dasyurus maculatus in eastern Australia: Effects of season, sex and size. J. Zool. 269, 241–248 (2006).
Nakashima, Y., Teshiba, M. & Hirose, Y. Flexible use of patch marks in an insect predator: Effect of sex, hunger state, and patch quality. Ecol. Entomol. 27, 581–587 (2002).
Honek, B. A. Activity and predation of Coccinella septempunctata adults in the field (Col., Coccinellidae). Zeitschrift für Angew. Entomol. 100, 399–409 (1985).
Cuthbert, R. N. et al. Influence of intra- and interspecific variation in predator prey body size ratios on trophic interaction strenghts. Ecol. Evol. 10, 5946–5962 (2020).
Brose, U. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34 (2010).
Mccann, K., Hastings, A. & Huxel, G. R. Weak trophic interactions and the balance of nature. Nature 395, 794–798 (1998).
Berlow, E. L. Strong effects of weak interactions in ecological communities demonstrate here that some ‘weak’ interactors may also be. Nature 398, 330–334 (1999).
Bascompte, J., Jordano, P. & Olesen, J. M. Facilitate biodiversity maintenance. Science 312, 431–433 (2014).
Tang, S., Pawar, S. & Allesina, S. Correlation between interaction strengths drives stability in large ecological networks. Ecol. Lett. 17, 1094–1100. https://doi.org/10.1111/ele.12312 (2014).
Jeannel, R. Croisière du Bougainville aux îles australes françaises. III. (1940).
Chevrier, M., Vernon, P. & Frenot, Y. Potential effects of two alien insects on a sub-Antarctic wingless fly in the Kerguelen Islands. In Antarctic Communities: Species, Structure and Survival (ed. Chevrier, M.) 424–431 (Cambridge University Press, 1997).
Ouisse, T. et al. Effects of elevational range shift on the morphology and physiology of a carabid beetle invading the sub-Antarctic Kerguelen Islands. Sci. Rep. https://doi.org/10.1038/s41598-020-57868-0 (2020).
Lebouvier, M. et al. Spotlight on the invasion of a carabid beetle on an oceanic island over a 105-year period. Sci. Rep. 10, 1–17 (2020).
Iacarella, J. C., Dick, J. T. A. & Ricciardi, A. A spatio-temporal contrast of the predatory impact of an invasive freshwater crustacean. Divers. Distrib. 21, 803–812 (2015).
Raby, G. D., Gutowsky, L. F. G. & Fox, M. G. Diet composition and consumption rate in round goby (Neogobius melanostomus) in its expansion phase in the Trent River, Ontario. Environ. Biol. Fishes 89, 143–150 (2010).
Lieven, T., Lefevre, E., Bonte, D. & Stoks, R. Increased activity and growth rate in the non-dispersive aquatic larval stage of a damselfly at an expanding range edge. Freshw. Biol. 59, 1266–1277 (2014).
Dick, J. T. A. et al. Invader Relative Impact Potential: A new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. J. Appl. Ecol. 54, 1259–1267 (2017).
Griffen, B. D. Considerations when applying the consumer functional response measured under artificial conditions. Front. Ecol. Evol. 9, 1–8 (2021).
Hollis, K. L. Ants and antlions: The impact of ecology, coevolution and learning on an insect predator-prey relationship. Behav. Processes 139, 4–11 (2017).
Guillette, L. M., Hollis, K. L. & Markarian, A. Learning in a sedentary insect predator: Antlions (Neuroptera: Myrmeleontidae) anticipate a long wait. Behav. Processes 80, 224–232 (2009).
Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160 (2008).
Laparie, M., Lebouvier, M., Lalouette, L. & Renault, D. Variation of morphometric traits in populations of an invasive carabid predator (Merizodus soledadinus) within a sub-Antarctic island. Biol. Invasions 12, 3405–3417 (2010).
Sunderland, K. Mechanisms underlying the effects of spiders on pest populations. J. Arachnol. 27, 308–316 (1999).
Trubl, P., Blackmore, V. & Johnson, J. C. Wasteful killing in urban black widows: Gluttony in response to food abundance. Ethology 117, 236–245 (2011).
Ayre, K. Effect of predator size and temperature on the predation of Deroceras reticulatum (Muller) (Mollusca) by carabid beetles. J. Appl. Entomol. 125, 389–395 (2001).
Lounibos, L. P., Makhni, S., Alto, B. W. & Kesavaraju, B. Surplus killing by predatory larvae of Corethrella appendiculata: Prepupal timing and site-specific attack on mosquito prey. J. Insect Behav. 21, 47–54 (2008).
Lang, A. & Gsödl, S. ‘Superfluous killing’ of aphids: A potentially beneficial behaviour of the predator Poecilus cupreus (L.) (Coleoptera: Carabidae)?. J. Plant Dis. Prot. 110, 583–590 (2003).
Albins, M. A. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Invasions 15, 29–43 (2013).
Ouisse, T., Bonte, D., Lebouvier, M., Hendrickx, F. & Renault, D. The importance of relative humidity and trophic resources in governing ecological niche of the invasive carabid beetle Merizodus soledadinus in the Kerguelen archipelago. J. Insect Physiol. 93–94, 42–49 (2016).
Mcnair, J. N. The effects of refuges on predator-prey interactions: A reconsideration. Theor. Popul. Biol. 29, 38–63 (1986).
Sih, A. Prey refuges and predator-prey stability. Theor. Popul. Biol. 31, 1–12 (1987).
Laparie, M., Larvor, V., Frenot, Y. & Renault, D. Starvation resistance and effects of diet on energy reserves in a predatory ground beetle (Merizodus soledadinus; Carabidae) invading the Kerguelen Islands. Comp. Biochem. Physiol. A 161, 122–129 (2012).
Phillips, B. L., Brown, G. P. & Shine, R. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627 (2010).
Lebouvier, M., Chapuis, J., Gloaguen, J. & Frenot, Y. Résilience des communautés insulaires subantarctiques: Facteurs influençant la vitesse de restauration écologique après éradication de mammifères introduits. Rev. d’Ecologie 57, 189–198 (2002).
Walther, G. et al. Ecological response to recent climate change. Nature 416, 389–395 (2002).
Cramer, M. D., Hedding, D. W., Greve, M., Midgley, G. F. & Ripley, B. S. Plant specialisation may limit climate-induced vegetation change to within topographic and edaphic niches on a sub-Antarctic island. Funct. Ecol. 36, 2636–2648 (2022).
Saucède, T. et al. THE KERGUELEN PLATEAU : MARINE ECOSYSTEM + FISHERIES Proceedings of the Second Symposium Modelling species response to climate change in sub-Antarctic islands : echinoids as a case study for the Kerguelen Plateau. in The Kerguelen Plateau: Marine Ecosystems and Fisheries 1–22 (2017).
Convey, P. Terrestrial ecosystem responses to climate changes in the Antarctic. In “Fingerprints” of Climate Change (eds Walther, R. G. et al.) 17–42 (Kluwer Academic/Plenum Publishers, 2001). https://doi.org/10.1007/978-1-4419-8692-4_2.
Sentis, A., Gémard, C., Jaugeon, B. & Boukal, D. S. Predator diversity and environmental change modify the strengths of trophic and nontrophic interactions. Glob. Chang. Biol. 23, 2629–2640 (2017).
Ouisse, T., Laparie, M., Lebouvier, M. & Renault, D. New insights into the ecology of Merizodus soledadinus, a predatory carabid beetle invading the sub-Antarctic Kerguelen Islands. Polar Biol. 40, 2201–2209 (2017).
Chapuis, J., Frenot, Y. & Lebouvier, M. Recovery of native plant communities after eradication of rabbits from the subantarctic Kerguelen Islands, and influence of climate change. Biol. Conserv. 117, 167–179 (2004).
Bartlett, J. C., Convey, P., Pertierra, L. R. & Hayward, S. A. L. An insect invasion of Antarctica: The past, present and future distribution of Eretmoptera murphyi (Diptera, Chironomidae) on Signy Island. Insect Conserv. Divers. 13, 77–90 (2020).
Laparie, M. & Renault, D. Physiological responses to temperature in Merizodus soledadinus (Col., Carabidae), a subpolar carabid beetle invading sub-Antarctic islands. Polar Biol. 39, 35–45 (2016).
OpenStreetMap Contributors. OpenStreetMap Contributors. Planet Dump https://www.openstreetmap.org (2023).
Hidalgo, K. et al. Metabolic fingerprinting of the responses to salinity in the invasive ground beetle Merizodus soledadinus at the Kerguelen Islands. J. Insect Physiol. 59, 91–100 (2013).
Crawley, M. J. The R Book (Wiley, 2013).
Bates, D. et al. Linear Mixed-Effects Models using ‘Eigen’ and S4. https://cran.r-project.org/web/packages/lme4/lme4.pdf (2020).
Galwey, N. W. Introduction to Mixed Modelling: Beyond Regression and Analysis of Variance (Wiley, 2006). https://doi.org/10.1002/9781118861769.
Barton, K. MuMIn : multi-model inference. http://r-forge.r-project.org/projects/mumin/ at http://ci.nii.ac.jp/naid/20001420752/en/ (2009).
Hartig, F. & Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. http://florianhartig.github.io/DHARMa/ (2020).
R Core Team. R: A Language and Environment for Statistical Computing. Language and envionment for statistical computing at https://cran.r-project.org/ (2018).
Acknowledgements
We thank four anonymous reviewers for their constructive comments on the previous version of the manuscript.
Funding
The salary of Charly Géron was funded by the ASICS project (ANR-20-EBI5-0004, BiodivERsA, BiodivClim call 2019–2020). The research was funded by the French Polar Institute (Project 136-SUBANTECO). Ross N. Cuthbert is funded by the Leverhulme Trust Early Career Fellowship (ECF-2021-001).
Author information
Authors and Affiliations
Contributions
Data were collected by H.H. and D.R. All authors designed the analyses, C.G. led them with the help of R.N.C. C.G., D.R. and R.N.C. wrote, structured, and edited the manuscript. All authors gave constructive feedback. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Géron, C., Cuthbert, R.N., Hotte, H. et al. Density-dependent predatory impacts of an invasive beetle across a subantarctic archipelago. Sci Rep 13, 14456 (2023). https://doi.org/10.1038/s41598-023-41089-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41089-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.