Abstract
The association between plasma lipids and breast cancer (BC) has been extensively explored although results are still conflicting especially regarding the relationship with high-density lipoprotein cholesterol (HDLc) levels. HDL mediates cholesterol and oxysterol removal from cells limiting sterols necessary for tumor growth, inflammation, and metastasis and this may not be reflected by measuring HDLc. We addressed recently diagnosed, treatment-naïve BC women (n = 163), classified according to molecular types of tumors and clinical stages of the disease, in comparison to control women (CTR; n = 150) regarding plasma lipids and lipoproteins, HDL functionality and composition in lipids, oxysterols, and apo A-I. HDL was isolated by plasma discontinuous density gradient ultracentrifugation. Lipids (total cholesterol, TC; triglycerides, TG; and phospholipids, PL) were determined by enzymatic assays, apo A-I by immunoturbidimetry, and oxysterols (27, 25, and 24-hydroxycholesterol), by gas chromatography coupled with mass spectrometry. HDL-mediated cell cholesterol removal was determined in macrophages previously overloaded with cholesterol and 14C-cholesterol. Lipid profile was similar between CTR and BC groups after adjustment per age. In the BC group, lower concentrations of TC (84%), TG (93%), PL (89%), and 27-hydroxicholesterol (61%) were observed in HDL, although the lipoprotein ability in removing cell cholesterol was similar to HDL from CRT. Triple-negative (TN) BC cases presented higher levels of TC, TG, apoB, and non-HDLc when compared to other molecular types. Impaired HDL functionality was observed in more advanced BC cases (stages III and IV), as cholesterol efflux was around 28% lower as compared to stages I and II. The altered lipid profile in TN cases may contribute to channeling lipids to tumor development in a hystotype with a more aggressive clinical history. Moreover, findings reinforce the dissociation between plasma levels of HDLc and HDL functionality in determining BC outcomes.
Similar content being viewed by others
Introduction
Female breast cancer (BC) is the most commonly diagnosed cancer worldwide, accounting for 11.7% of all cancer cases, and is the fifth leading cause of cancer-related deaths, representing 6.9% of all cancer deaths1. Breast cancer is considered a heterogeneous disease, and its molecular classification is widely used to determine treatment options and prognosis. This classification is based on the expression of estrogen and progesterone receptors (luminal A; LA, and luminal B; LB), human epidermal growth factor receptor 2 (HER2), or the absence of these receptors (triple-negative; TN)2,3.
In recent decades, evidence has emerged linking plasma lipid levels to the development and worse outcomes of BC4,5,6,7. This is related to tumor cell reprogramming that enables greater lipid uptake for cell division8. However, clinical and epidemiological data have shown mixed results, with some studies suggesting a positive or negative impact of hyperlipidemia on BC incidence, while others suggest no impact9,10,11,12,13. Similarly, the association between statins and the risk of BC is still controversial14,15.
Much attention has been focused on the role of cholesterol in high-density lipoproteins (HDLc) in relation to BC risk and prevention, with most studies suggesting a protective role of plasma HDLc4,10,11. These results may be due to the beneficial activities of HDL in removing excess cholesterol and oxysterols from tumor cells16. Furthermore, HDL has antioxidant and anti-inflammatory activities and acts as a carrier for bioactive lipids, proteins, and microRNAs that may modulate tumor growth and progression17,18. However, there is still controversy surrounding this topic, with some studies showing only a weak association between HDLc and BC, while others show a positive or no association at all9,12,13,19.
Similar to the role of HDL in atherosclerosis and other chronic non-communicable diseases, it is possible that the HDLc metric may not be the best predictor variable for determining BC incidence and progression20. Therefore, alterations in HDL functionality should be considered, particularly when taking into account the modulation of this lipoprotein's composition and function by the tumor microenvironment21.
HDL is well known for its ability to remove cellular lipids, allowing cholesterol for uptake by the liver, secretion into bile, and excretion in feces through reverse cholesterol transport. Similarly, in the case of tumor cells, reverse cholesterol transport can be considered a defensive mechanism that prevents cholesterol accumulation that supports cell proliferation and metastasis. The expression of HDL receptors, such as ATP-binding cassette transporter A-1 (ABCA-1) and scavenger receptor class B type 1 (SR-B1), that mediated cholesterol exportation is altered in tumors and is related to epithelial-mesenchymal transition, tumor growth, and metastasis22,23,24,25. HDL also mediates the transport of oxysterols produced intracellularly by the oxidation of the cholesterol molecule. Due to its higher hydrophobicity compared to other oxysterols and cholesterol, 27 hydroxycholesterol (27HC) can easily diffuse across the plasma membrane26. Additionally, its transfer to HDL is facilitated by the ATP-binding cassette transporters G-1 (ABCG-1) located in the cell membrane of tumor cells and macrophages that infiltrate the tumor area27. Then, 27HC flux outside cells is considered an additional route for reverse cholesterol transport, limiting sterol content that is associated with cell inflammation, oxidative stress, and proliferation18,28. 27 hydroxycholesterol also acts as a selective estrogen receptor modulator (SERM), promoting tumor growth and metastasis in BC. In human BC samples, the expression of the enzymes involved in the production of 27HC (CYP27A1) and metabolism (CYP7B1) is respectively increased and decreased and is associated with poor tumor prognosis29. Furthermore, tumor metastasis is related to 27HC-dependent activation of the liver X receptor (LXR)29,30,31,32.
HDL removes cholesterol and oxysterols from cells, making acceptable the idea that it limits the intracellular accumulation of sterols and their negative impact on BC. In this way, it was addressed in newly diagnosed women with BC categorized according to the clinical stage of the disease and molecular classification of the tumor, without pharmacological or surgical intervention, compared to control women (CTR): (1) plasma lipid profile [total cholesterol (TC), HDLc, apolipoprotein B (apoB) and triglycerides (TG)]; (2) the composition of isolated HDL in lipids, main species of oxysterols and apolipoprotein A-I (apoA-I); and (3) the ability of HDL to mediate cholesterol removal from macrophages.
Plasma lipids were similar between controls (CTR) and all women with BC, but when categorized according to tumor molecular type, triple-negative (TN) BC cases had higher plasma TC, TG, and apoB values compared to other molecular types. Despite having lower levels of TC, phospholipids (PL), and 27HC, HDL from women with BC maintained its ability to remove cellular cholesterol when compared to HDL from CTR. In advanced BC (covering clinical stages III and IV), despite similar composition in apoA-I and lipids, HDL had an impaired ability to remove cholesterol from macrophages.
Material and methods
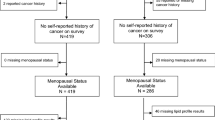
Two hundred and one women newly diagnosed with BC between 18 and 80 years old in any clinical stage and with the molecular classification of the tumor were recruited at Hospital Pérola Byington. A hundred and fifty-seven women without any type of cancer were recruited at Universidade de São Paulo and at Unidade Básica de Saúde Dra. Ilza Weltman Hutzler (control group; CTR). Exclusion criteria were diabetes mellitus, chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73m2), autoimmune diseases, smokers, alcoholics, use of contraceptives, and hormone replacement therapy, pregnancy, previous history of any cancer, and in situ breast disease. After excluding those who did not meet the eligibility criteria, 150 CTR and 163 women with BC remained in the study. All participants were informed about the study and signed an informed written consent previously approved by institutional Ethics Committees, in accordance with the Declaration of Helsinki, including approval for publication (Universidade Nove de Julho, # 3.139.460; Centro de Referência da Saúde da Mulher, Hospital Pérola Byington, #3.225.220; and Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, #3.317.909).
The molecular classification of tumors was obtained from medical records being accessed immunohistochemically after percutaneous biopsy, according to the American College of Pathologists33. Samples that were positive for hormone receptors (estrogen and progesterone) were those in which > 1% of the tumor cells showed positive nuclear staining of moderate to strong intensity on immunohistochemistry and were classified as Luminal A and B neoplasia if they presented the Ki67 index below or above 14%, respectively. Samples that exhibited > 10% of invasive tumor cells with strong HER2 staining in the plasma membrane were considered HER2 positive. In case of moderate staining in > 10% of the cells or strong staining in < 10% of the cells, the sample was re-evaluated by in situ hybridization and was considered positive if a HER2/centromere ratio > 2.0; or if a HER2/centromere ratio < 2.0 with mean HER2 > 6 signals per cell (greater than 120 signals in 20 nuclei). Tumor samples that did not express either hormonal or HER2 receptors were classified as TN BC34.
For sample calculation, the number of new cases of BC included for treatment at Hospital Pérola Byington during 2018 (2,985 cases) was taken into account; also the study design, with the comparison of outcome variables between two main groups (CTR vs. BC); the main outcome variables; the effect size of the variables according to the main studies published in the area; and the probability of committing a type 1 error (0.05) and type 2 error (0.20), with 80% power, resulted in 144 patients in each group (pairing 1:1).
Blood collection
Venous blood was drawn after 12 h fasting and plasma was immediately isolated after centrifugation (3,000 rpm, 4 °C, 15 min). Plasma TC, TG, and HDLc were determined by enzymatic techniques. Low-density lipoprotein cholesterol (LDLc) was determined by the Friedewald formula35, and VLDLc as TG/5. ApoB was quantified by immunoturbidimetry (Randox Lab. Ltd. Crumlin, UK).
Isolation of plasma lipoproteins
High-density lipoproteins (HDL; D = 1.063–1.21 g/mL) were isolated from BC and CTR women´s plasma by discontinuous density ultracentrifugation and immediately frozen at − 80 °C in a 5% saccharose solution. HDL composition in lipids (TC, TG, and PL) was determined by enzymatic techniques. ApoA-I was determined by the immunoturbidimetric method (Randox Lab. Ltd. Crumlin, UK).
Low-density lipoprotein isolation and acetylation
LDL (D = 1.019–1.063 g/mL) was obtained by sequential ultracentrifugation of plasma from healthy volunteers and was purified by discontinuous density ultracentrifugation. After protein quantification by the Lowry technique36, LDL was incubated with acetic anhydride as previously described37. Acetylated LDL was dialyzed and utilized to load macrophages with cholesterol.
HDL-mediated cholesterol efflux from bone marrow-derived macrophages
The Institutional Animal Care and Research Advisory Committee (Universidade Nove de Julho # 7,070,120,821, 08/23/2021) approved the study according to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. All the methods were performed in accordance with ARRIVE guidelines (Supplementary file S1). Thirty male and female C57BL/6 J mice, aged 2–48 weeks, were housed with free access to commercial chow (Nuvilab CR1, São Paulo, Brazil) and drinking water in a conventional animal facility at 22 ± 2 °C under a 12 h light/dark cycle. Intraperitoneal overdose of ketamine hydrochloride (Ketalar) (300 mg/kg of body weight) and xylazine hydrochloride (Rompun) (30 mg/kg of body weight) were used to euthanize the animals, in accordance with the norms of the National Council for the Control of Animal Experimentation (CONCEA) of the Ministry of Science, Technology and Innovation (MCTI). Undifferentiated bone marrow cells were obtained from C57BL/6 wild-type mouse´s tibia and femora, as previously described38. Cells were differentiated into macrophages by incubating with L929 cells-conditioned medium (ATCC, American Tissue Culture Collection) and plated in culture dishes for 5 days at 37 °C, under 5% (v/v) CO2. The medium was changed for a new one and after 6 days replaced by DMEM (low glucose, containing 1% penicillin/streptomycin and 10% heat-inactivated fetal calf serum). Macrophages were overloaded with acetylated LDL (50 µg/mL) and 14C-cholesterol (0.3 µCi/mL) for 48 h, and after washing incubated with DMEM containing fatty acid-free albumin for equilibration of intracellular cholesterol pools. Cells were incubated for 6 h with HDL (50 µg/mL) from BC or control women; control incubations were performed in the absence of HDL (basal efflux). After incubation, the radioactivity in the medium was determined as well as in the cell lipid extract. The cholesterol efflux was determined as 14C-cholesterol in the medium / 14C-cholesterol in the medium + 14C-cholesterol in cells × 100. Values from basal efflux were subtracted from those obtained after incubation with HDL, in order to express the specific ability of HDL in removing cell cholesterol.
Oxysterols quantification in isolated HDL
Oxysterols (24-hydroxycholesterol, 25-hydroxycholesterol, and 27-hydroxycholesterol) were measured from 1 mL of extracted HDL as previously described39,40. Briefly, a mixture of 100 ng of oxysterol deuterium-labeled (7α-hydroxycholesterol-d7, 7β-hydroxycholesterol-d7, 25-hydroxycholesterol-d7, 27-hydroxycholesterol-d7) (Avanti Polar Lipids, Alabaster, USA) was added as internal standard. Alkaline saponification was done by adding 10 mL of ethanolic KOH (0.4 M) solution for 2 h, at room temperature with adjustment to pH 7 with phosphoric acid. Then 20 mL of chloroform and 6 mL of water were added to the sample. After strong shaking and centrifugation at 4 °C, the aqueous phase was eliminated and the organic phase evaporated. The lipid extract was dissolved in toluene (1 mL). Oxysterols were separated from cholesterol by solid-phase extraction (Sigma- Aldrich Supelclean LC-Si SPE Tubes SUPELCO, Bellefonte, USA). The sample (1 mL in toluene) was placed in the column previously conditioned with 2 mL of hexane, following washing with 1 mL of hexane. Sterols were eluted with 1.5% isopropanol in hexane (8 mL), and oxysterols were further eluted with 30% isopropanol in hexane (6 mL). Then, the solvent was evaporated and samples were derivatized (100 μL of pyridine and 100 μL of N, O-bis (trimethylsilyl) trifluoroacetamide with trimethylchlorosilane (BSTFA; Sigma- Aldrich, St. Louis, USA), for 1 h at 60 °C). One microliter of the derivatized sample (1 µL) was injected into a gas chromatograph coupled to a mass spectrometer (Shimadzu GCMS-QP2010, Kyoto, Japan) by an automatic injector and analyzed in selected ion monitoring. The separation was carried out on a Restek capillary column (100% dimethyl polysiloxane-RxiR -1 ms. Cat. #13,323), 30 m, internal diameter 0.25 mm, for 30 min, using helium as mobile phase, with a constant linear velocity of 44.1 cm/sec. The oven started at 240 °C with an increment of 5◦C/min, for 7 min up to 290 °C. The mass spectrometer operated in impact electron mode at an ionization voltage of 70 eV with the temperature of the ion source at 300 °C40. Comparisons from standard curves peak areas to internal standards were made in order to quantify the oxysterols, as previously described41.
Statistical analysis
The Shapiro–Wilk test was used to analyze normality; parametric data were represented by the mean and standard deviation of the sample and compared by Student's t-test, with or without Welch correction, depending on the performance of Levene's Test regarding sample sphericity. When analyzing more than two samples, analysis of variance of non-repeated measures was used with Tukey or Games-Howell post-test, according to the homo or heteroscedasticity, respectively; with bootstrap when necessary. Covariate analyses were performed to adjust the outcome variables with Sidak's post-test. Non-parametric data were represented by the median, lower and upper quartiles, and compared with each other using the Mann–Whitney test for two samples, and when comparing more than two samples, the Kruskal–Wallis test was used. If normalization was necessary, variables were log-transformed. Frequencies were compared using the Chi-square test. A value of P < 0.05 was considered statistically significant. IBM® SPSS Statistics (version 27.0), GraphPad Prisma (version 5.04) for Windows, and Microsoft® Excel for Mac (version 16.52) software were used for data tabulation and analysis.
Ethical approval and informed consent
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Universidade Nove de Julho (# 3.139.460; February 2019); Centro de Referência da Saúde da Mulher (Hospital Pérola Byington; #3.225.220; March 2019) and Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (#3.317.909; March 2019).
Results
In the BC group, the median age and the frequency of postmenopausal status, dyslipidemia and hypertension were higher in comparison to the CTR, although BMI, overweight (BMI \(\ge\) 25 kg/m2) and reported statin use were similar between groups. Among tumors, the frequency (%) of histological types was: ductal (87.7), lobular (7.4), mucinous (4.3), and metaplastic (0.6). As expected, a higher frequency of LA and LB tumors was observed. 70.8% of BC women were categorized in clinical stages I and II, and 29.2% in advanced stages of disease (stages III and IV) (Table 1). The postmenopausal status was similar among clinical stages (stage I = 66.7%; stage II = 59.6%; stage III = 74.2%; and stage IV = 68.8%; χ2 = 2.011; P = 0.570). The luminal types (LA and LB) accounted for the majority of tumors (68%), with most cases classified as stages I and II (70.8%). Women with TN tumors had the highest frequency of advanced disease, with 60% being classified as stages III and IV, while those with LA tumors had the lowest percentage of advanced disease (8.9%) (Supplementary file S2).
Plasma lipid profile adjusted per age and the ratios TC/apoB and TG/HDLc were similar between CTR and BC groups (Table 2). In isolated HDL, the concentrations of TC, TG, and PL were lower in the BC group, while apoA-I was similar in both groups. The 27HC content in HDL was lower in BC as compared to CTR even after adjustment for age, but the concentrations of 24HC and 25HC in HDL were similar between groups. Despite some alterations in composition, HDL particles from BC and CTR presented similar abilities in mediating macrophage cholesterol removal (Table 2).
When comparing the molecular types of BC, age and BMI were similar, but TC was higher in TN as compared to LA, LB, and HER2 tumors. Besides, plasma levels of TG, apoB, and non-HDLc were greater in TN as compared to LB and HER2 (Table 3). Although plasma lipids were different in TN tumors, the ratios TC/apoB (P = 0.065) and TG/HDLc (P = 0.091) did not reach statistical difference. Oxysterols in HDL were similar among LA, LB, HER2, and TN as well as the ability of cell cholesterol removal (Table 3).
When subjects were categorized according to the stage of the disease reflected by increased levels of Ki67, it was observed that plasma lipids and their ratios were similar among groups. The composition of HDL in TC, PL, apoA-I, and oxysterols was similar among stages. Only HDL-TG was increased on stage III as compared to I, II, and IV. Reduced cholesterol efflux was observed with HDL isolated from BC women in more advanced stages of the disease (III and IV) as compared to stages I and II. (Table 4).
Discussion
The present study investigated plasma lipid levels, HDL composition, and functionality in newly diagnosed women with BC compared to CTR women, taking into account the molecular classification of the tumor and the clinical stage of the disease. The results showed that (1) BC cases (including all molecular types) had similar concentrations of plasma lipids adjusted for age but HDL particles less enriched in TC, PL, TG, and 27HC compared to CTR; (2) TN cases had a higher concentration of plasma lipids as compared to other molecular types of tumor; (3) HDL functionality along the first step of reverse cholesterol transport was impaired in advanced stages of BC, meaning less cholesterol removal from macrophages.
Plasma lipids are considered contributing factors for many types of cancer, including BC42,43,44,45,46, although the interpretation and comparison of studies are limited by the heterogeneity of BC, disease duration and staging, ethnic diversity, age, menopausal status, lifestyle, and oncological treatments, which are confounding factors. Furthermore, diabetes mellitus, insulin resistance, and obesity, which can alter lipoprotein profiles, are also present in many cases of BC as potential contributors to the disease development and progression. The current study exclusively included newly diagnosed, treatment-naïve participants, excluding smoking habit and women with diabetes mellitus and comorbidities that affect lipids. Moreover, the data were adjusted for age, which was higher in the BC group. As a result, many factors contributing to dyslipidemia were minimized, and the strict matching between the CTR and BC groups likely accounted for the absence of variation in the plasma lipid profile between all BC cases and CTR. Even with the exclusion data of women informing dylipidemia and/or statin use, results were similar (data not shown).
In the casuistic of the present investigation, as anticipated, the majority of tumors were of the luminal types in in stages I and II. Triple-negative cases had the highest frequency of advanced disease and higher levels of TC, apoB, nonHDLc, VLDLc, and TG compared to LA, LB, and HER-2. However, HDLc, composition, and functionality of the HDL particle were similar among all types. These alterations were independent of BMI and may represent a distinct characteristic of TN tumors, directing lipids as an energy source and structural components for tumor growth in a histotype with a more aggressive clinical course, lower survival rate, and a higher metastasis rate. These factors should be considered when exploring the association between plasma lipids and BC and deserves further investigation.
Also, findings are consistent with previous studies that have reported increased levels of VLDL-TG in TN BC. However, changes in CT/apoB and TG/HDLc did not reach statistical significance. A retrospective cohort study demonstrated that a high pretreatment TG/HDLc ratio was an independent predictor of the overall survival rate in TN tumors47. The enhanced expression of the LDL receptor and LDLR-related proteins 5 and 6 were found in TN tumors and associated with the higher ability of tumor growth and invasion; while the knockdown of LDLR-5 and 6 decreased tumorigenesis48,49,50,51.
A syngeneic tumor graft model demonstrated that tumor growth leads to changes in host lipid metabolism, promoting the synthesis of very low-density lipoproteins while inhibiting their metabolism, ultimately providing more energy to the tumor52. Furthermore, a targeted plasma liquid chromatography-tandem mass spectrometry (LC–MS/MS) approach identified potential lipid biomarkers in TN BC cases, such as ceramides, phosphatidylcholine, lysophosphatidylcholine, and diacylglycerol, thus confirming the alteration of the lipid profile in this molecular subtype53.
Unlike other studies, it was not found in the present investigation any changes in plasma lipids in cases of HER2-positive BC45,54,55. However, in individuals with HER2-positive BC, the analysis of the lipoprotein profile using nuclear magnetic resonance demonstrated an enhanced level of specific VLDL subfractions, which served as a marker of plasma lipid alteration compared to control women, even with similar BMI between the groups. Reductions in HDL subfractions were surrogate markers for the response to neoadjuvant chemotherapy follow-up56.
Although the ability of HDL to remove cell cholesterol was preserved in all women with BC when compared to CTR women, the reduced content of TC, PL, and 27HC in the HDL particles from BC cases may be a consequence of alterations in the tumor cells that hinder sterol exportation to HDL. In addition to the intrinsic ability of HDL to receive cholesterol, it is important to consider that lipid efflux is modulated by cellular components, such as the bioavailability of free or unesterified cholesterol and the content and functionality of HDL receptors sch as ABCA-1, ABCG-1, and SR-B157. ABCA-1, which is expressed in the mammary gland, is indicated to be reduced in BC and associated with positive lymph nodes58. Its expression negatively affects the therapeutic efficiency of chemotherapy59 and is considered by some authors as a marker of TN tumors60. Conversely, its deficiency contributes to an increase in cellular and mitochondrial cholesterol content, which hinders cell death processes mediated by this organelle, thereby favoring tumor cell survival24.
Solid tumors are known to accumulate large amounts of cholesterol48,61,62, which is mainly due to the increased synthesis and uptake of lipoproteins48,63. The scavenger receptor class B type 1 (SR-B1) mediates the efflux of free cholesterol from cells and promotes its transfer to HDL. However, SR-B1 can also facilitate the uptake of modified lipoproteins, leading to the supply of cholesterol to cells and tumor progression64. Higher expression of SR-B1 is associated with increased aggressiveness and a worse prognosis of tumors23,65,66, while mutations in the Scarb1 gene have been shown to inhibit tumor proliferation67.
Moreover, the reduced lipid content in HDL could be associated with the decreased detachment of surface components of TG-rich lipoproteins during lipolysis mediated by lipoprotein lipase, which is now recognized as a process of reverse transport of remnant cholesterol68. However, it is difficult to determine which mechanisms are involved in shaping the composition of HDL based on the present findings. These events cannot be captured by a simple determination of HDLc in plasma, which may explain some of the controversies surrounding the association between HDLc and BC.
According to the clinical stage of the disease, a diminished intrinsic capacity of the HDL particle to remove cell cholesterol was observed in stages III and IV in comparison to stages I and II. This observation was independent of changes in HDL composition and plasma lipids, highlighting the dissociation between plasma levels of HDLc and the overall functionality of HDL as a particle. Increasing levels of intracellular cholesterol promote the formation of oxysterols, which suggests an increase in the concentration of 27HC in the tumor microenvironment that negatively influences BC progression. The underlying mechanisms of the reduced HDL ability in removing cell cholesterol in advanced stages of BC were not investigated in the present study. HDL functionality may be impaired by its chemical modification resulting from glycoxidative and inflammatory stress that often accompanies cancer69. Consequently, a vicious circle may be established, which compromises tumor cholesterol homeostasis, exacerbating cancer progression and impairing HDL function. In this sense, HDL may even promote tumor growth, as experimental studies have previously shown that modified HDL was oncogenic42,69. Once again, these findings support the idea that the tumor's energy demand adapts systemic metabolism to its advantage.
The concept of reverse causation suggests that the tumor may modulate HDL, which may disassociate HDL from being a predictor of tumor risk. Therefore, HDL would serve more as a marker of tumor evolution rather than a protector or inducer of tumor genesis. Bone marrow-derived macrophages were used in this investigation to minimize cellular effects on HDL functionality measurement. However, it is crucial to consider that in vivo, the interplay between lipoprotein subsets and tumor cell biology determines HDL functionality and cell cholesterol content. Additional experiments employing BC cell lineages are necessary to enhance our comprehension of the interplay between plasma lipids, HDL, and BC.
To our knowledge, this is the first study demonstrating the loss of HDL function along the first step of reverse cholesterol transport according to disease burden, independent of plasma lipids. However, the study has certain limitations, such as the absence of detailed clinical data, including components of metabolic syndrome and visceral adiposity, as well as lifestyle information. Nevertheless, BMI was similar between the groups, and comorbidities that were associated with changes in lipid metabolism were excluded. Moreover, it was not identified the specific HDL structure components that were responsible for the impairment of cholesterol efflux, independently of lipids and apo A-I. A more detailed analysis of HDL proteomics, lipidomics, and microRNAs may provide additional information. A subanalysis of TN cases from this casuistic (n = 27) showed an enhanced antioxidant role of the HDL particle—reflected by retardation in LDL oxidation—that was positively associated to the apoA-I but independent of HDLc70.
The results of this investigation confirmed that TN tumors have increased levels of plasma lipids, potentially driving the more aggressive type of BC. This study also contributes to a better understanding of the role of HDL in BC, particularly in patients with a worse prognosis, where HDL's ability to remove excess cellular cholesterol is impaired. In comparison to the CTR group, only the composition of HDL was distinctive in subjects with BC. A prospective cohort study will aid in determining the prognostic value of HDL composition and functionality in predicting BC outcomes.
Finally, results contribute to the growing understanding of HDL's role in breast cancer pathophysiology. Since routine laboratory metrics cannot directly indicate this, it is essential to recognize the impact of HDL functionality on BC and develop new metrics accordingly.
Data availability
All data reported are included in the manuscript and raw data can be kindly shared upon personal request to the corresponding author MP (m.passarelli@uni9.pro.br).
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3), 209–249 (2021).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol [Internet] 38(12), 1346–1366 (2020).
Hamilton, E., Shastry, M., Shiller, S. M. & Ren, R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat. Rev. 100, 102286 (2021).
Munir, M. T. et al. The contribution of cholesterol and epigenetic changes to the pathophysiology of breast cancer. J. Steroid Biochem. Mol Biol. 183, 1–9 (2018).
McDonnell, D. P. et al. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 74(18), 4976–4982 (2014).
Wei, Y. et al. The significances and clinical implications of cholesterol components in human breast cancer. Sci. Progr. 104(3), 003685042110283 (2021).
Guan, X. et al. Emerging roles of low-density lipoprotein in the development and treatment of breast cancer. Lipids Health Dis. 18, 137 (2019).
Beloribi-Djefaflia, S., Vasseur, S. & Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 5(1), e189 (2016).
Nowak, C. & Ärnlöv, J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat. Commun. 9(1), 3957 (2018).
Li, X. et al. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast 32, 1–6 (2017).
Fan, Y. et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int. J. Biol. Markers 30(2), e200–e207 (2015).
His, M. et al. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control. 28, 77–88 (2017).
Chandler, P. D. et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am. J. Clin. Nutr. 103, 1397–1407 (2016).
Undela, K., Srikanth, V. & Bansal, D. Statin use and risk of breast cancer: A meta-analysis of observational studies. Breast Cancer Res. Treat. 135(1), 261–269 (2012).
Mansourian, M. et al. Statins use and risk of breast cancer recurrence and death: A systematic review and meta-analysis of observational studies. J. Pharm. Pharm. Sci. 19(1), 72–81 (2016).
Huang, X. et al. High-density lipoprotein of patients with breast cancer complicated with type 2 diabetes mellitus promotes cancer cells adhesion to vascular endothelium via ICAM-1 and VCAM-1 upregulation. Breast Cancer Res. Treat. 155(3), 441–455 (2016).
Rohatgi, A., Westerterp, M., von Eckardstein, A., Remaley, A. & Rye, K. A. HDL in the twenty first century: A multifunctional roadmap for future HDL research. Circulation 143(23), 2293–2309 (2021).
Yamauchi, Y. & Rogers, M. A. Sterol metabolism and transport in atherosclerosis and cancer. Front. Endocrinol. (Lausanne) 19(9), 509 (2018).
Kucharska-Newton, A. M. et al. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 18, 671–677 (2008).
Samadi, S. et al. High-density lipoprotein functionality and breast cancer: A potential therapeutic target. J. Cell Biochem. 120(4), 5756–5765 (2019).
Kopecka, J., Godel, M. & Riganti, C. Cholesterol metabolism: At the cross road between cancer cells and immune environment. Int. J. Biochem. Cell Biol. 129, 105876 (2020).
Pussinen, P. J. et al. The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem. J. 349(Pt 2), 559–566 (2000).
Danilo, C. et al. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 15(5), R87 (2013).
Smith, B. & Land, H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2(3), 580–590 (2012).
Sag, D., Cekic, C., Wu, R., Linden, J. & Hedrick, C. C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 6, 6354 (2015).
Meaney, S., Bodin, K., Diczfalusy, U. & Björkhem, I. J. Lipid Res. 43, 2130–2135 (2002).
Sag, D., Cekic, C., Wu, R., Linden, J. & Hedrick, C. C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 27(6), 6354 (2015).
Nunes, V. S. et al. Increased 27-hydroxycholesterol plasma level in men with low high density lipoprotein-cholesterol may circumvent their reduced cell cholesterol efflux rate. Clin. Chim. Acta. 433, 169–173 (2014).
Wu, Q. et al. 27-Hydroxycholesterol promotes cell-autonomous ER-positive breast cancer growth. Cell Rep. 5(3), 637–645 (2013).
Sharma, B. & Agnihotri, N. Role of cholesterol homeostasis and its efflux pathways in cancer progression. J. Steroid Biochem. Mol. Biol. 191, 105377 (2019).
El Roz, A., Bard, J. M., Huvelin, J. M. & Nazih, H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: Relation to proliferation and apoptosis. Anticancer Res. 32(7), 3007–3013 (2012).
Dalenc, F. et al. Circulating oxysterol metabolites as potential new surrogate markers in patients with hormone receptor-positive breast cancer: Results of the OXYTAM study. J. Steroid Biochem. Mol. Biol. 169, 210–218 (2017).
Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 134(7), e48–e72 (2010).
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ; Panel Members. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 22(8), 1736–47 (2011).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18(6), 499–502 (1972).
Lowry, O. H., Rosenbrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Basu, S. K., Goldstein, J. L., Anderson, R. W. & Brown, M. S. Degradation of cationized low-density lipoprotein and regulation of cho-lesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 73, 3178–318210 (1976).
Minanni, C. A. et al. Persistent effect of advanced glycated albumin driving inflammation and disturbances in cholesterol efflux in macrophages. Nutrients 13(10), 3633 (2021).
Pinto, R. S. et al. Plasma advanced glycation end products and soluble receptor for advanced glycation end products as indicators of sterol content in human carotid atherosclerotic plaques. Diabetes Vasc. Dis. Res. 19(2), 147916412210852 (2022).
Ferreira, G. S. et al. Aerobic exercise training selectively changes oxysterol levels and metabolism reducing cholesterol accumulation in the aorta of dyslipidemic mice. Front. Physiol. 5(8), 644 (2017).
Dzeletovic, S., Breuer, O., Lund, E. & Diczfalusy, U. Determination of cholesterol oxidation products in human plasma by isotope dilution mass spectrometry. Anal. Biochem. 225, 73–80 (1995).
Cedo, L., Reddy, S. T., Mato, E., Blanco-Vaca, F. & Escolà-Gil, J. C. HDL and LDL: Potential new players in breast cancer development. J. Clin. Med. 8(6), 853 (2019).
Ghahremanfard, F., Mirmohammadkhani, M., Shahnazari, B., Gholami, G. & Mehdizadeh, J. The valuable role of measuring serum lipid profile in cancer progression. Oman Med. J. 30, 353–357 (2015).
Kumie, G., Melak, T. & Baynes, H. W. The association of serum lipid levels with breast cancer risks among women with breast cancer at Felege Hiwot comprehensive specialized hospital Northwest Ethiopia. Breast Cancer Targets Ther. 12, 279–287 (2020).
Lu, C. W. et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 388, 130–138 (2017).
Michalaki, V., Koutroulis, G., Syrigos, K., Piperi, C. & Kalofoutis, A. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2, HDL3) in postmenopausal patients with breast cancer. Mol. Cell. Biochem. 268, 19–24 (2005).
Dai, D. et al. Pretreatment TG/HDL-C ratio is superior to triacylglycerol level as an independent prognostic factor for the survival of triple negative breast cancer patients. J. Cancer. 7(12), 1747–1754 (2016).
De Gonzalo-Calvo, D. et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 15, 460 (2015).
Catasus, L. et al. Low-density lipoprotein receptor-related protein 1 is associated with proliferation and invasiveness in Her-2/Neu and triple-negative breast carcinomas. Hum. Pathol. 42, 1581–1588 (2011).
Gallagher, E. J. et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 36, 6462–6471 (2017).
Maubant, S. et al. LRP5 regulates the expression of STK40, a new potential target in triple-negative breast cancers. Oncotarget 9, 22586–22604 (2018).
Huang, J. et al. Tumor-induced hyperlipidemia contributes to tumor growth. Cell Rep. 15(2), 336–348 (2016).
Eghlimi, R., Shi, X., Hrovat, J., Xi, B. & Gu, H. Triple negative breast cancer detection using LC–MS/MS lipidomic profiling. J. Proteome Res. 19(6), 2367–2378 (2020).
Flote, V. G. et al. Lipoprotein subfractions by nuclear magnetic resonance are associated with tumor characteristics in breast cancer. Lipids Health Dis. 15, 1–12 (2016).
Jung, S. M. et al. Impact of serum lipid on breast cancer recurrence. J. Clin. Med. 9, 2846 (2020).
Corona, G. et al. 1H-NMR plasma lipoproteins profile analysis reveals lipid metabolism alterations in HER2-positive breast cancer patients. Cancers (Basel) 13(22), 5845 (2021).
Adorni, M. P., Ronda, N., Bernini, F. & Zimetti, F. High density lipoprotein cholesterol efflux capacity and atherosclerosis in cardiovascular disease: Pathophysiological aspects and pharmacological perspectives. Cells 10(3), 574 (2021).
Schimanski, S. et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm. Metab. Res. 42(2), 102–109 (2010).
Wang, W. et al. ABCA1 is associated with the development of acquired chemotherapy resistance and predicts poor ovarian cancer outcome. Cancer Drug Resist. 4, 485–502 (2021).
Pan, H. et al. Expression of LXR-β, ABCA1 and ABCG1 in human triple-negative breast cancer tissues. Oncol. Rep. 42(5), 1869–1877 (2019).
Freeman, M. R. & Solomon, K. R. Cholesterol and prostate cancer. J. Cell Biochem. 91(1), 54–69 (2004).
Krycer, J. R. & Brown, A. J. Cholesterol accumulation in prostate cancer: A classic observation from a modern perspective. Biochim. Biophys. Acta 1835(2), 219–29 (2013).
Murai, T. Cholesterol lowering: role in cancer prevention and treatment. Biol. Chem. 396(1), 1–11 (2015).
Gutierrez-Pajares, J. L., Ben Hassen, C., Chevalier, S. & Frank, P. G. SR-BI: Linking cholesterol and lipoprotein metabolism with breast and prostate cancer. Front. Pharmacol. 7, 338 (2016).
Yuan, B. et al. High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumour Biol. 37, 3581–3588 (2016).
Llaverias, G. et al. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 178, 402–412 (2011).
Cao, W. M. et al. A mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cells. Cancer Res. 64, 1515–1521 (2004).
Kontush, A. HDL and reverse remnant-cholesterol transport (RRT): Relevance to cardiovascular disease. Trends Mol. Med. 26, 1086–1100 (2020).
Mazzuferi, G., Bacchetti, T., Islam, M. O. & Ferretti, G. High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 20(1), 143 (2021).
Campos, A. L. et al. The increased antioxidant action of HDL is independent of HDL cholesterol plasma levels in triple-negative breast cancer. Front. Oncol. 13, 1111094 (2023).
Acknowledgements
The authors are thankful to Rafaela V Coutinho, Karina CLB Lima, Jessica AV Siqueira, Julia HC Dezotti, Raiana C Novaes, Jessica Mosello, Karina Cezar, and Jacira X Carvalho for helping in blood collection and obtaining clinical data; to Fernanda Faccioli Schober and Nurya Bustamante de Araújo, from Universidade Nove de Julho Animal Facility Unity for animal caring.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (Grants # 2019/18431-4 to MP; #2022/11186-7 to DSP; #2021/04989-3 to SISA; 2021/02401-9 to MFMS). MP is a recipient of a research award from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.P., L.H.G.; casuistic selection: M.I.B.A.C.S., M.R.; methodology, M.R., M.F.M.S., S.I.S.A., D.S.P., L.A.P., V.S.N.; formal analysis, M.I.B.A.C.S., M.L.C.C.G., M.P.; investigation; data curation, M.I.B.A.C.S., M.P.; writing—original draft preparation, M.I.B.A.C.S., M.P.; writing—review and editing, M.P.; resources, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sawada, M.I.B.A.C., de Fátima Mello Santana, M., Reis, M. et al. Increased plasma lipids in triple-negative breast cancer and impairment in HDL functionality in advanced stages of tumors. Sci Rep 13, 8998 (2023). https://doi.org/10.1038/s41598-023-35764-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35764-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.