Abstract
Systemic inflammatory responses caused by tumor cells play an important role in the occurrence and development of tumors. The aim of this study was to identify biomarkers that most accurately predict prognoses in patients with non-metastatic cancer and to evaluate their clinical significance when combined with muscle markers. This study retrospectively evaluated 2,797 cancer patients diagnosed with cancer at TNM stages I, II, and III. Lymphocyte-C-reactive protein ratio (LCR) in conjunction with calf circumference (CC) were used (or chosed) after evaluating the predictive value of 13 inflammatory marker combinations and five anthropometric indicators for patient outcomes using the C-index. The Kaplan–Meier method and Cox’s proportional hazards regression modeling were used to analyze the individual and combined effects of these two potential biomarkers on overall survival. This study enrolled 1,604 men (57.3%) and 1,193 women (42.7%) with a mean age of 58.75 years. Among the 13 inflammatory nutritional indicators, the LCR was the most accurate predictor of prognoses in patients with non-metastatic cancer. After multifactorial adjustment, we found that low LCR had an adverse effect on overall survival (hazard ratio [HR]: 2.50; 95% confidence interval [CI]: 2.17, 2.88; P < 0.001). Low LCR combined with low CC was also shown to be an independent risk factor for poor overall survival (HR: 2.26; 95% CI: 1.80, 2.83; P < 0.001). Compared with LCR or CC alone, the combination of the two had greater prognostic value for patients with non-metastatic cancer. The LCR can be implemented as a useful biomarker to predict prognoses in patients with non-metastatic cancer. CC is the best anthropometric indicator of muscle loss in patients with non-metastatic cancer. The combination of LCR and CC can better predict the prognosis of patients with non-metastatic cancer, and can provide important information for clinicians to formulate diagnosis and treatment plans.
Similar content being viewed by others
Introduction
Cancer is the leading cause of death in all countries worldwide, imposing a severe health and economic burden1. The number of cancer cases and deaths is growing rapidly given a growing and aging population and an increase in the prevalence of lifestyle risk factors2. As in the rest of the world, cancer has become a serious public health problem in China that has been attracting increasing attention3. Although cancer treatments have become more diversified in recent years, prognoses for many cancer patients remain poor. Generally, patients with early non-metastatic cancer have improved prognoses and longer survival times. Cancer patients with more complications are more likely to present with a high inflammatory state accompanied by muscle loss4. Therefore, it is important to identify more accurate and valuable prognostic parameters for patients with non-metastatic cancers. This could help clinicians identify problems and intervene earlier for patients with early-stage cancer.
Inflammation has become a recognized hallmark of cancer progression, leading to a series of cancer-associated symptoms, including fever, sweating, and weight loss5. The interaction of inflammation with the host tumor is now considered the seventh hallmark of cancer6. After tumor cells enter the body's blood circulation, they activate a series of inflammatory responses and stimulate the release of inflammatory factors and immune cells, thereby promoting cancer development7. Currently, some serological indicators are used to reflect the body's inflammation and nutritional status, and studies have shown that the prognostic nutritional index (PNI)8, the modified Glasgow prognostic score (mGPS)9, the lymphocyte-c-reactive protein ratio score (LCS)10, the geriatric nutritional risk index (GNRI)11, the nutritional risk index (NRI)12, the neutrophil to lymphocyte ratio (NLR)13, the platelet to lymphocyte ratio (PLR)14, the glucose to lymphocyte ratio (GLR)15, the advanced lung cancer inflammation index (ALI)16, the Systemic Immune-Inflammation Index (SII)17, the controlling nutritional status score (CONUT score)18, the lymphocyte-to-C-reactive protein ratio (LCR)19, the albumin–globulin ratio (AGR)20, and other inflammatory nutrition-associated indicators can be used as independent prognostic factors in cancer patients. Studies have shown that inflammation can promote the loss of muscle mass as well as decrease strength and muscle function in cancer patients21. Reductions of skeletal muscle in cancer patients indicate an increase in the toxic effects of cancer treatments22 and are associated with a decrease in survival rates23. Therefore, assessing the inflammatory state combined with muscle indicators in patients with non-metastatic cancer may predict prognoses effectively. Although the prognostic value of inflammatory nutritional indicators in cancer patients has been reported in prior studies, to the best of our knowledge, few studies have examined whether systemic inflammation and muscle mass predict prognoses in patients with non-metastatic cancer.
Hence, this study aimed to explore the best indicators for predicting prognoses for non-metastatic cancers. We evaluated 13 inflammatory nutrition-associated indicators and identified commonly used anthropometric indicators that were most valuable for predicting patient outcomes in 2,797 patients diagnosed with stage I-III cancer. We then assessed the independent effects as well as the joint associations between these indicators and patient survival.
Methods
Study population
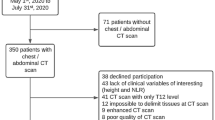
This multicenter observational study investigated nutritional status and clinical results within the Chinese Common Cancer (INSCOC) cohort (registration number: ChiCTR1800020329; http://www.chictr.org.cn) from May 2013 to June 202124. This prospective cohort collects data from multiple centers in China; the study design, methods, and study development process have been described earlier25. All patients enrolled in the INSCOC cohort were aged 18 years or older, were diagnosed with solid tumors, and received surgery, chemotherapy, radiotherapy, or other anti-cancer treatments; we enrolled hospitalized patients with a length of stay > 48 h. All patients included in the study were diagnosed with oncology at the time of their first hospitalization from May 2013 to June 2021. If patients experienced multiple hospitalizations, only the data from the first hospitalization were used for analysis. Patients with clinical evidence of active infection, patients presenting with immune disease, and patients lacking specific data on age, height, albumin levels, globulin levels, cholesterol levels, C-reactive protein (CRP) levels, blood glucose levels, neutrophil counts, lymphocyte counts, and platelets (PLT) counts were excluded from the current study. In addition, we excluded patients with TNM stage IV and those who were lost to follow-up during the follow-up process. The study followed the principles outlined in the Declaration of Helsinki and was approved by the ethics committees of all local study centers. Written informed consent for the use clinical data (without disclosing personal information) was obtained from all participants. Figure 1 shows a flow chart for the research object screening process.
Patient and public involvement
Patients and members of the public were not involved in the design, conduct, reporting or dissemination of this research as it was an observational study focusing on the incidence of insulin resistance and inflammation and prognostic factors in women with cancer of the reproductive system.
Patient characteristics
Patient age, sex, primary tumor type, tumor stage, smoking history, and drinking history were obtained from the electronic medical record system at the participating medical centers. Body mass index (BMI) was calculated for all patients, and was defined as weight (kg) divided by height (m) squared. Patients were divided into three groups: underweight (< 18.5 kg/m2) and normal weight (≥ 18.5 kg/m2). Clinical staging was evaluated based on the TNM staging system delineated in the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Patient-generated subjective nutritional assessments (PG-SGA) and Karnofsky performance status (KPS) were assessed and recorded at baseline by trained staff. Serum albumin levels, CRP levels, hemoglobin (Hb) levels, PLT levels, and other serological indicators were obtained after the patients fasted overnight within 24 h following admission and were analyzed and standardized in the central laboratory to eliminate differences caused by different laboratory equipment.
Inflammatory nutrition and muscle state
Patients’ inflammatory nutritional status (based on PNI, mGPS, LCS, GNRI, NRI, NLR, PLR, GLR, ALI, SII, CONUT score, LCR, and AGR readings) were reassessed according to data collected at baseline. The calculation formula for each index is shown in Supplementary Table 1.
In addition, anthropometric indicators were measured for all patients. Calf circumference (CC) was measured with a standard tape measure in 0.1 cm increments with knees bent to 90 degrees and relaxed feet and ankles. Mid-arm circumference (MAC) and triceps skinfold thickness (TSF) were measured in 0.1 cm and 1 mm increments, respectively. MAC was measured with a plastic meter, and TSF was measured with a skinfold caliper. Mid-arm muscle circumference (MAMC) was calculated using the following formula: MAMC (mm) = MAC (mm)—[3.14 × TSF (mm)]. Hand grip strength (HGS) was measured using a Jamar dynamometer to evaluate the strength of the patient’s non-dominant hand. The patients were asked to recall their weight six months prior and to compare it with their weight as measured at admission. We classified each index using maximally selected rank statistics to obtain the optimal cut-off point values.
Outcomes
The primary endpoint of this study was all-cause mortality. The overall survival (OS) period was defined as the time from the date of admission to death or the last follow-up. Secondary endpoint events included length of stay (LOS), cost, and Karnofsky scores (KPS; a self-scoring of health status with a total score of 100 points and 10 points per level).
Statistical analysis
Demographic characteristics of the study population were calculated, with continuous variables expressed as either means ± standard deviations or medians and interquartile ranges (IQR). Categorical variables were presented as numbers and percentages (n, %). Comparisons of differences between groups were conducted via independent Student’s t-tests or non-parametric tests for comparing continuous variables and via Chi-square tests or Fisher’s exact tests for comparing categorical variables. The optimal cut-off point values for all inflammatory nutritional indicators were obtained using maximally selected rank statistics. We selected covariates and potential confounders based on previous knowledge. Univariate and multivariate Cox regression analyses were used to evaluate hazard ratios (HRs) and 95% confidence intervals (CIs) for important prognostic factors based on OS. A sensitivity analysis excluding patients who died within six months of enrollment was performed. Kaplan–Meier (K-M) curves and log-rank tests were presented to evaluate time-patient survival trends and to compare survival between groups. The Harrell C index and the area under time-dependent curve (AUC) were calculated to evaluate and compare the predictive ability of inflammatory nutritional and anthropometric indicators for patient survival. Differences were considered statistically significant given two-sided P values of < 0.05. All statistical analyses were performed using R software, version 4.1.1 (The R Project for Statistical Computing, Vienna, Austria).
Ethics approval and consent to participate
The study followed the principles outlined in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University. Written informed consent was obtained from all the participants to use clinical data without disclosing personal information.
Results
Predicting prognoses in patients with non-metastatic cancer
When calculating the C-index (Supplementary Table 2) and AUC (Supplementary Fig. 1) for 13 inflammatory nutritional indicators and five anthropometric indicators, we found that the LCR (C-index = 0.65252) was the strongest predictor of survival in patients with non-metastatic cancer, although almost all research indicators statistically significantly predicted OS (Supplementary Table 3). The anthropometric index with the strongest prognostic ability was CC; this result was verified by evaluating the corresponding the time-dependent receiver operating characteristic (ROC) curve (Supplementary Fig. 2). Based on these findings, we decided to focus the analyses of this study on LCR in order to comprehensively evaluate its clinical impact and potential as a prognostic biomarker.
Patient characteristics
A total of 2,797 cancer patients diagnosed with TNM stages I-III were enrolled in the current study. We enrolled 1,604 men (57.3%) and 1,193 women (42.7%), presenting with a mean age of 58.75 years. We classified each index using maximally selected rank statistics to obtain the optimal cut-off point values (Supplementary Fig. 3). Table 1 shows the characteristics of the 2,797 enrolled patients with non-metastatic cancer, classified according to LCR values. Compared with the high LCR group (> 2,500), patients in the low LCR group (≤ 2,500) were more likely to be male, elderly, and to present with lung cancer diagnoses, low BMI, poor tumor stage. These patients were also more likely to have low albumin levels, low Hb levels, high CRP levels, high PLT levels, and high PG-SGA scores.
LCR, CC, and OS
We found that the LCR was negatively correlated with OS, regardless of whether the LCR was analyzed as a continuous (Fig. 2A; Supplementary Fig. 4A) or categorical variable (Supplementary Fig. 4B). Patients with a lower LCR tended to have poorer prognoses. In various adjusted models, we demonstrated that patients’ OS consistently improved with an increase in the LCR. This conclusion remained true even after patients with non-metastatic cancer were divided into early (stage I, II) and stage III cancer patients in secondary analyses (Table 2).
Survival curves via Kaplan–Meier analysis of LCR and CC. (A) Survival curves via Kaplan–Meier analysis of LCR. (B) Survival curves via Kaplan–Meier analysis of CC. (C) Survival curves for total tumor patients with LCR and CC. (D) Survival curves of TNM stage I and II tumor patients with LCR and CC E. Survival curves of TNM stage III tumor patients with LCR and CC.
We evaluated maximally selected rank statistics to determine the optimal cut-off values for gender classifications with regard to CC (male: 34.5 cm; female: 29.8 cm). Patients with high CC values had improved prognoses as compared with those with low CC in various adjusted models (Fig. 2B; Supplementary Table 4).
Predictions of OS
In survival analyses, we cross-classified the LCR and CC into four categories (high, high; low, low; high, low; and low, high). We calculated the mean survival time (in months), assessed K-M survival curves and performed Cox proportional survival analysis in different groups. In a multivariate adjusted Cox proportional risk model, we examined the combined effects of LCR and CC as independent predictors of survival.
It was found that in the group with high LCR combined high CC, the mean survival time was 52.12 (95% CI: 51.11–53.13) months, followed by the group with high LCR and low CC, and the group with low LCR and high CC, with 45.32 (95% CI: 43.58–47.06) and 41.17 (95% CI: 38.86–43.48) months, respectively. In contrast, the mean survival time in the group with both low was only 34.15 (95% CI: 31.58–36.72) months, and the difference was statistically significant (Supplementary Table 5, P < 0.001). In addition, we calculated the mean survival time in different subgroups and the results were consistent with the total population and the differences were significant through continuing significance (Supplementary Table 5).
Cox univariate proportional hazard analyses demonstrated that age, sex, smoking, drinking, tumor stage, surgery history, KPS, PG-SGA, BMI, and LCR combined with CC were associated with OS (Table 3). After adjusting for age, sex, smoking, drinking, tumor stage, surgery, KPS, BMI, and PG-SGA, multivariate analysis identified low LCR combined with low CC as an adverse prognostic factor affecting the survival of patients with non-metastatic cancer (HR: 2.26; 95% CI: 1.80, 2.83; P < 0.001, Table 3). In a sensitivity analysis excluding patients who died within six months of enrollment, low LCR combined with low CC remained an adverse prognostic factor for survival in patients with non-metastatic cancer (Supplementary Table 6).
Among the 2,797 eligible patients, 15.02% had low LCRs and CCs. The K-M curve showed that patients with low LCR and CC values had the lowest survival time, while patients with high LCR and CC values had the longest survival time (log-rank P < 0.020, Fig. 2C). After dividing the non-metastatic patients into early stage and stage III patients, the prognoses of patients with low levels of each putative biomarker were statistically significantly worse as compared with that of the other groups (log-rank P < 0.020, Fig. 2D, E).
Non-metastatic cancer patients with high LCR and CC values had shorter lengths of hospital stay (LOS) and higher Cartesian scores (KPS); these differences were statistically significant. However, LCR and CC values had no statistically significant correlations with costs (Supplementary Table 7).
Subgroup analyses of potential confounders
To clarify the potential impact of LCR combined with CC on patient outcomes more comprehensively, we performed subgroup analysis based on several potential confounders. The results showed that low LCR combined with low CC could be used as an independent risk factor for predicting prognoses in stage I-III patients of different ages and presenting with different tumor stages, tumor types (upper gastrointestinal cancer, colorectal cancer, lung cancer), and surgery histories (Table 4).
Prognostic value verification
Compared with the prognostic ability of LCR or CC alone, LCR combined with CC has a stronger ability to predict the prognosis of patients with non-metastatic cancer (Supplementary Fig. 5).
Discussion
Accurately predicting prognoses for patients with non-metastatic cancers is very important for clinicians. Previous studies have shown that biomarkers of systemic inflammation are considered cancer markers and are cost-effective prognostic factors6,26. However, the optimal profile of systemic inflammation biomarkers and anthropometric indicators for predicting prognoses in patients with non-metastatic cancer remains unclear. A total of 2,797 patients with TNM stage I-III tumors were enrolled in this study. We first assessed the prognostic power of 13 inflammatory nutrition-associated indicators and five anthropometric indicators in this population. We found that LCR alone was a relatively good predictor of patient outcomes. Subsequently, we analyzed the synergistic effects of LCR and CC and found that patients with low LCR and CC had the worst prognoses, closely followed by prognoses when one of the indicators was high; patients with high LCR and CC values had the best prognoses. Multivariate analyses showed that low LCR combined with low CC values was independent risk factors for OS in patients with non-metastatic cancer.
A single-center prospective cohort study showed that the LCR can be used as a biomarker for predicting prognoses in patients with non-metastatic colorectal cancer27. He et al. found that LCR is a valuable biomarker for survival in patients with lung cancer28. Other studies have shown that LCR is associated with the immune status of the tumor microenvironment and can be used as a prognostic indicator for patients with liver cancer29. These results are consistent with our findings, and both studies showed that the LCR can be a useful biomarker for predicting prognoses in various cancers. Our study was innovative in that it enrolled a much larger sample size, evaluated a variety of cancers, and was the first to explore the relationship between LCR values and prognoses in patients with non-metastatic cancer.
LCR is directly related to lymphocyte and CRP levels. Lymphocytes are the key cells in the host cytotoxic immune response and play a vital role in the cell-mediated anti-tumor microenvironment30,31. Tumor-infiltrating lymphopenia is considered a predictor of poor host anti-tumor immunity and of poor prognoses32. CRP is an acute reactive protein regulated by interleukin-633, and is a clinically recognized marker of inflammatory response.
Hart et al. found that CRP is closely associated with disease severity in cancer patients34. The growth of tumor cells stimulates the host to secrete interleukin-6 and other inflammatory factors, thereby increasing the synthesis of CRP in the liver35,36. Based on the above findings as well as the new findings of our research, we conclude that the LCR may reflect the immune status of the body as well as the systemic inflammatory response in a range of populations. Low LCR represents an impaired immune response and/or an enhanced systemic inflammatory response in cancer patients, leading to tumor progression and worse prognoses.
Abbass et al. found that inflammation plays a role in the loss of muscle mass, strength, and muscle function in cancer patients21. In addition, muscle loss is a diagnostic criterion for cancer cachexia37. CC is a simple, non-invasive, and practical indicator that can replace muscle mass. Studies have shown that CC can predict the nutritional status of hospitalized patients as well as the risk of death38. Our research combined LCR and CC values to concurrently evaluate inflammation levels and nutritional status. Both biomarkers are easy to measure, commonly used, safe, and have strong stability and repeatability.
In Cox multivariate analyses, LCR combined with CC was an independent prognostic factor for patients with non-metastatic cancer. Sex, TNM stage, surgery, KPS scores, BMI, and PG-SGA also independently predicted patient prognoses. Prognoses for male patients were statistically significantly worse than that of females. This may be associated with differentially expressed or Y-linked genes in males as well as unhealthy lifestyle habits such as smoking39. TNM staging, KPS scores, and PG-SGA scores are recognized as indicators for evaluating the condition of cancer patients and are statistically significantly related to prognosis40,41,42. Surgery is currently the preferred treatment for most patients with stage I-III tumors and can greatly improve patient prognoses. BMI is an anthropometric indicator that can reflect the nutritional status of patients with cancer and is independently associated with patient mortality43.
A strength of this study is that this is a multicenter observational study with a large enrolled sample size and is more representative of the overall population of patients with non-metastatic cancer as compared with prior research. This study had several limitations. First, due to its retrospective design, this study may be subject to selection bias. Second, the optimal thresholds for the LCR and CC have not yet been unified, and different datasets may lead to the determinaiton of different cut-off values. Third, the enrolled study participants were all Chinese, and the generalizabilty of our finding needs to be verified. Fourth, although this study adjusted for potential confounders, there are still certain factors that have not been considered. In addition, some of the currently known somatic mutations and molecular markers (TP53, EGFR, HER2 mutations) are poor prognostic factors, which have not been collected in our data and therefore need to be further investigated.
Conclusions
In conclusion, our study showed that low LCR levels were statistically significantly associated with poor survival outcomes in patients with non-metastatic cancer and that this biomarker was more effective than the 12 other evaluated inflammatory markers in terms of predicting accuracy. The combined analyses of LCR and CC as potential biomarkers of overall survival provide a new basis for patients to assess prognoses. It is helpful for clinicians to classify patients according to their immune inflammatory nutritional status and to develop treatment and follow-up plans for patients accordingly. Our results thus inform research directions and will ultimately inform medical guidelines.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because they are private databases but are available from the corresponding author on reasonable request.
Abbreviations
- AGR:
-
Albumin–globulin ratio
- AJCC:
-
American Joint Committee on Cancer
- ALI:
-
Advanced lung cancer inflammation index
- AUC:
-
Area under the time-dependent curve
- CC:
-
Calf circumference
- CI:
-
Confidence interval
- CONUT:
-
Controlling nutritional status score
- CRP:
-
C-reactive protein
- GLR:
-
Glucose to lymphocyte ratio
- GNRI:
-
Geriatric nutritional risk index
- Hb:
-
Hemoglobin
- HGS:
-
Hand grip strength
- HR:
-
Hazard ratio
- INSCOC:
-
Chinese Common Cancer
- IQR:
-
Interquartile range
- K-M curve:
-
Kaplan-Meier curve
- KPS:
-
Karnofsky performance status
- LCR:
-
Lymphocyte-to-C-reactive protein ratio
- LCS:
-
Lymphocyte-c-reactive protein ratio score
- LOS:
-
Length of stay
- MAC:
-
Mid-arm circumference
- MAMC:
-
Mid-arm muscle circumference
- mGPS:
-
Modified Glasgow prognostic score
- NLR:
-
Neutrophil to lymphocyte ratio
- NRI:
-
Nutritional risk index
- PG-SGA:
-
Patient-generated subjective nutritional assessment
- PLR:
-
Platelet to lymphocyte ratio
- PLT:
-
Platelets
- PNI:
-
Prognostic nutritional index
- ROC curve:
-
Receiver operating characteristic curve
- QLQ-C30:
-
Core Quality of Life questionnaire
- SII:
-
Systemic Immune-Inflammation Index
- TSF:
-
Triceps skinfold thickness
References
Luengo-Fernandez, R. et al. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 14(12), 1165–1174 (2013).
Torre, L. A. et al. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 25(1), 16–27 (2016).
Wu, C. et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 62(5), 640–647 (2019).
Peixoto da Silva, S. et al. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 11(3), 619–635 (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144(5), 646–674 (2011).
Diakos, C. I. et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15(11), e493-503 (2014).
Fang, T. et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res 2020, 9146042 (2020).
Shoji, F. et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 98, 15–21 (2016).
Kishi, T. et al. Pretreatment modified glasgow prognostic score predicts clinical outcomes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 92(3), 619–626 (2015).
Chen, Y. R. et al. Prognostic efficacy of preoperative mGPS, SIS and LCS in patients with gastric cancer. Clin. Chim. Acta 511, 81–89 (2020).
Lidoriki, I. et al. GNRI as a prognostic factor for outcomes in cancer patients: A systematic review of the literature. Nutr. Cancer 73(3), 391–403 (2021).
Oh, J. et al. Association between nutritional risk index and outcomes for head and neck cancer patients receiving concurrent chemo-radiotherapy. Head Neck 42(9), 2560–2570 (2020).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 106(6), dju124 (2014).
Stojkovic Lalosevic, M. et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers 2019, 6036979 (2019).
Zhong, A. et al. Clinical significance of glucose to lymphocyte ratio (GLR) as a prognostic marker for patients with pancreatic cancer. Front. Oncol. 10, 520330 (2020).
Hua, X. et al. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J. Surg. Oncol. 17(1), 177 (2019).
Alifano, M. Systemic immune-inflammation index and prognosis of advanced non-small cell lung cancer. Ann. Transl. Med. 8(11), 667 (2020).
Kheirouri, S. & Alizadeh, M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: A systematic review. Adv. Nutr. 12(1), 234–250 (2021).
Okugawa, Y. et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin. Nutr. 39(4), 1209–1217 (2020).
Chi, J. et al. Prognostic value of albumin/globulin ratio in survival and lymph node metastasis in patients with cancer: A systematic review and meta-analysis. J Cancer 9(13), 2341–2348 (2018).
Abbass, T. et al. The relationship between imaging-based body composition analysis and the systemic inflammatory response in patients with cancer: A systematic review. Cancers (Basel) 11(9), 1304 (2019).
Van Vugt, J. L. et al. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 112(6), 681–682 (2015).
Jung, H. W. et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 23(3), 687–694 (2015).
Xu, H., & Yin, L., et al. Extension protocol for the Investigation on Nutrition Status and Clinical Outcome of Patients with Common Cancers in China (INSCOC) study: 2021 update. Precision Nutrition, 2022.
Song, C. et al. Nutritional risk assessment by scored patient-generated subjective global assessment associated with demographic characteristics in 23,904 common malignant tumors patients. Nutr. Cancer 71(1), 50–60 (2019).
Crusz, S. M. & Balkwill, F. R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 12(10), 584–596 (2015).
Ou, W. et al. Prognostic significance of preoperative lymphocyte-to-C-reactive protein ratio in patients with non-metastatic colorectal cancer. Onco Targets Ther. 14, 337–346 (2021).
He, Y. et al. Lymphocyte-to-C-reactive protein ratio is a potential new prognostic biomarker for patients with lung cancer. Biomark Med 14(9), 717–726 (2020).
Iseda, N. et al. Lymphocyte-to-C-reactive protein ratio as a prognostic factor for hepatocellular carcinoma. Int. J. Clin. Oncol. 26(10), 1890–1900 (2021).
Wu, E. S. et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol. Oncol. 140(1), 76–82 (2016).
Imai, D. et al. Prognostic nutritional index is superior as a predictor of prognosis among various inflammation-based prognostic scores in patients with hepatocellular carcinoma after curative resection. Hepatol. Res. 50(1), 101–109 (2020).
Stanton, S. E. & Disis, M. L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother Cancer 4, 59 (2016).
Hara, M. et al. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Ann. Thorac. Cardiovasc. Surg. 16(2), 85–90 (2010).
Hart, P. C. et al. C-reactive protein and cancer-diagnostic and therapeutic insights. Front. Immunol. 11, 595835 (2020).
Ishizuka, M. et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann. Surg. Oncol. 23(3), 900–907 (2016).
Allin, K. H. & Nordestgaard, B. G. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab. Sci. 48(4), 155–170 (2011).
Fearon, K. et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 12(5), 489–495 (2011).
Tsai, A. C., Lai, M. C. & Chang, T. L. Mid-arm and calf circumferences (MAC and CC) are better than body mass index (BMI) in predicting health status and mortality risk in institutionalized elderly Taiwanese. Arch. Gerontol. Geriatr. 54(3), 443–447 (2012).
Costa, A. R. et al. The Sex Bias of Cancer. Trends Endocrinol. Metab. 31(10), 785–799 (2020).
Sano, T. et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 20(2), 217–225 (2017).
Firat, S. et al. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 52(4), 1047–1057 (2002).
Bauer, J., Capra, S. & Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 56(8), 779–785 (2002).
Taghizadeh, N. et al. BMI and lifetime changes in BMI and cancer mortality risk. PLoS ONE 10(4), e0125261 (2015).
Acknowledgements
We would like to express our sincere thanks to the INSCOC project members for their substantial work on data collection and patient follow-up.
Funding
This work was supported by the National Key Research and Development Program (2017YFC1309200).
Author information
Authors and Affiliations
Contributions
H.-P.S. contributed to the design of the research; X.Z., Q.Z., G.-T.R., and L.D. contributed to the interpretation of the data; X.-Y.L., H.-L.X., Y.-Z.G., and M.-M.S. contributed to data acquisition and analysis. X.-Y.L. and T.L. drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, XY., Zhang, X., Zhang, Q. et al. Lymphocyte-C-reactive protein ratio with calf circumference could better predict survival of patients with non-metastatic cancer. Sci Rep 13, 7217 (2023). https://doi.org/10.1038/s41598-023-34096-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34096-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.