Abstract
To determine the effects on gingival bleeding, dental biofilm, and salivary flow and pH in patients with gingivitis of using toothpaste with extra-virgin olive oil (EVOO), xylitol, and betaine in comparison to a placebo or commercial toothpaste. This controlled, double blinded, and multicenter randomized clinical trial included patients with gingivitis randomly assigned to one of three groups: test group (EVOO, xylitol, and betaine toothpaste), control group 1 (placebo toothpaste), or control group 2 (commercial toothpaste). Percentage supragingival biofilm and gingival bleeding were evaluated at baseline (T0), 2 months (T2), and 4 months (T4), measuring non-stimulated salivary flow and salivary pH. Comparisons were performed between and within groups. The final study sample comprised 20 in the test group, 21 in control group 1, and 20 in control group 2. In comparison to control group 1, the test group showed significantly greater decreases in gingival bleeding between T4 and T0 (p = 0.02) and in biofilm between T2 and T0 (p = 0.02) and between T4 and T0 (p = 0.01). In the test group, salivary flow significantly increased between T2 and T0 (p = 0.01), while pH alkalization was significantly greater between T4 and T0 versus control group 2 (p = 0.01) and close-to-significantly greater versus control group 1 (p = 0.06). The toothpaste with EVOO, xylitol, and betaine obtained the best outcomes in patients with gingivitis, who showed reductions in gingival bleeding and supragingival biofilm and an increase in pH at 4 months in comparison to a commercial toothpaste.
Similar content being viewed by others
Introduction
The oral microbiome comprises all commensal, symbiotic, and pathogenic microorganisms present in the oral cavity and is made up of viruses, fungi, protozoa, archaea, and bacteria together with their habitat or ecosystem. At least 750 species of bacteria remain in eubiosis, i.e., in dynamic balance with the host immune system and microenvironment1,2. Disruption of this balance generates microbiome dysbiosis, producing diseases such as caries, gingivitis, and periodontitis3,4. Gingivitis is a reversible inflammatory condition triggered by biofilm accumulation on the dental surface; it is characterized by reddening, edema, gingival bleeding, and the absence of periodontal insertion loss without involvement of cementum, periodontal ligament, or alveolar bone5. In agreement with the 2017 Workshop, a diagnosis of gingivitis is defined by ≥ 10% bleeding on probing, being considered localized when bleeding on probing is between 10 and 30% and generalized when > 30%. Gingivitis treatment includes dental prophylaxis, use of antiseptics, and appropriate oral hygiene instructions6.
Saliva contains 99.5% water, 0.3% proteins, and 0.2% inorganic substances, including sodium, chloride, calcium, potassium, bicarbonate, phosphate, fluoride, iodide, and magnesium7. It also contains proteins such as glycoproteins, mucins, immunoglobulins, lactoferrin, peroxidases, and agglutinins8. The average salivary flow rate is 0.33–0.55 mL/min at rest and 2 mL/min when stimulated with paraffin. Around 0.5 L of saliva is secreted daily8.
The salivary pH ranges from 6.2 to 7.6, with an average of pH 6.77. Saliva contributes to pH maintenance via two mechanisms: the elimination of carbohydrates that could be metabolized by bacteria, and the neutralization of acidity created by food and drink or by acids from dental biofilm, through its buffer capacity7.
Herbal toothpastes are mostly prepared with natural ingredients9 and contain essential mineral salts, sodium fluoride, sodium chloride, and extracts from plants such as lemon, rosemary, chamomile, or aloe vera, among others10. These components act as anti-inflammatory and antibacterial agents, and various studies have recommended their use to control dental biofilm9. For instance, Xerostom®, which contains olive fruit extract, can improve the oral conditions of patients with dry mouth through its capacity to stimulate salivary secretion at rest11.
Few clinical trials have evaluated the effectiveness of herbal toothpastes to treat gingivitis, and only one study has assessed the in vitro antimicrobial activity of toothpaste prepared with olive fruit extract12. Research is needed on the effectiveness of toothpaste composed of extra-virgin olive oil (EVOO), xylitol, and betaine to reduce the formation of dental biofilm and gingivitis bleeding in comparison to conventional toothpastes. Our alternative hypothesis was that toothpaste with natural products may be more effective to improve periodontal and salivary variables in comparison to a placebo and a commercial anti-gingivitis toothpaste.
The objective of this interim study in patients with gingivitis was to determine the effects on gingival bleeding, supragingival biofilm, and salivary flow and pH of toothpaste formulated with EVOO, xylitol, and betaine in comparison to a placebo toothpaste and a commercial toothpaste indicated for gingivitis.
Results
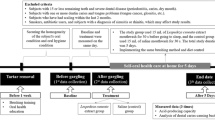
Out of 85 patients initially recruited for the study, 61 were finally included. The flowchart in Fig. 1 explains the reasons for losses. Among the 61 patients in the final study sample, 51 were examined at the Granada University School of Dentistry, 4 in the Huetor-Tajar health center, and 6 in the Loja health center. The study groups did not significantly differ in sociodemographic variables or habits, as shown in Table 1.
Within-group comparisons showed a significant reduction in bleeding (p < 0.001) between T0 and T4 in all three groups. A significant reduction in bleeding between T2 and T4 was observed in the test group (p = 0.022) and control group 1 (p = 0.018) but not in control group 2 (p = 0.474). Between-group comparisons revealed a significantly greater decrease between T0 and T4 in the test group than in control group 1 (p = 0.050), as reported in Table 2.
Table 3 shows the results regarding plaque index. Within-group comparisons showed a statistically significant reduction in plaque level (p < 0.001) in all three groups. This reduction was present in all times when paired comparison were performed. Between-group comparisons for plaque levels showed a significantly greater decrease between T0 and T2 in the test group than in control group 1 (p = 0.047). A greater reduction was also observed between T0 and T4 in the test group compared to both control group 1 (p = 0.020) and control group 2 (p = 0.030).
No significant differences in salivary flow were observed among the three groups at any time point, as shown in Table 4. However, a significant increase in salivary flow (p = 0.017) was detected between T0 and T2 in the test group. There appeared to be a trend for the salivary pH to decrease in the control groups and increase in the test group at T4. Between T4 and T0, salivary pH changes in the test group (increased pH) significantly differed (p = 0.01) from those in control group 2 (decreased pH) and were close-to-significance (p = 0.06) from those in control group 1.
Discussion
The main finding of this study was a reduction in gingival bleeding among patients with gingivitis who used a toothpaste with EVOO as main ingredient compared with those using a placebo toothpaste (control group 1). This result can be attributed to multiple biological mechanisms, with a potential role played by the combined effect of the phenolic compounds and other minority compounds present in olive oil, especially in young EVOO, as discussed below.
Hydroxytyrosol, obtained by oleuropein hydrolysis, is a phenolic compound present in olive leaves and fruit that exerts potent antioxidant, anti-inflammatory, and antibacterial effects13. Bertelli et al. recently demonstrated a promising anti-inflammatory effect of this product by reducing the synthesis of proinflammatory cytokines TNF-α, IL-1β, IL-6, and cyclooxygenase-2 (COX-2)14,15.
Oleocanthal is a phenolic compound obtained in just-pressed EVOO, and its structure was found in an in vitro study to be similar to that of ibuprofen (non-steroidal anti-inflammatory drug) and to produce a comparable stinging sensation in the throat16, as reported by several patients in the present study. Oleocanthal has a greater capacity to inhibit both cyclooxygenases (COX-1 and COX-2) in comparison to ibuprofen at the same concentrations16. Other in vitro studies found that oleocanthal can reduce the production of proinflammatory cytokines IL-1β, TNF-α, and nitric oxide14,17.
Oleacein, another phenolic compound in EVOO, structurally derives from glucoside oleuropein18 and is known to exert multiple anti-inflammatory actions at different levels. It reduces the secretion by human neutrophils of myeloperoxidases, proinflammatory mediators that can exacerbate tissue damage19. It inhibits the expression of adhesion molecules VCAM-1, ICAM-1, and E-selectin, thereby reducing immune cell migration20, and it favors the expression by macrophages of CD 163 receptor (related to inflammatory reaction regulation phase) and IL-10 (anti-inflammatory cytokine)21.
The test toothpaste also contains xylitol, which has been found to inhibit the synthesis of TNF-α and IL-1β induced by lipopolysaccharides from Porphyromonas gingivalis through NF-κB pathway activation22. The EVOO phenols and xylitol in the test toothpaste may therefore have combined effects on gingival bleeding.
In our study, dental biofilm reduction was greater with the test toothpaste than with the control toothpastes, which may also be related to the antibacterial effect of olea europaea described in in vitro studies23.
Karygianni et al. conducted an in vitro study to determine the antibacterial effect of maslinic acid, hydroxytyrosol, oleocanthal, and oleacein against eight oral bacterial species (Streptococcus mutans, S. sobrinus, S. oralis, Enterococcus faecalis, P. gingivalis, Parvimonas micra, Fusobacterium nucleatum) and Candida albicans24. Maslinic acid is a natural pentacyclic triterpenoid and damages the cell membrane of Gram-positive and Gram-negative bacteria. Lactobacillus plantarum can hydrolyze and transform oleuropein into hydroxytyrosol, which is highly effective against anaerobic Gram-negative bacteria such as P.gingivalis24.
Other documented effects of xylitol include the reduction of bacterial plaque by decreasing the adhesion of S. mutans, the main primary colonizer of dental biofilm25. Burt et al. reported that xylitol cannot be metabolized by the microorganisms in dental biofilm and inhibits the growth of S. mutans through inanition26. In addition, xylitol is transformed into xylitol-5-phosphate by phosphoenolpyruvate, resulting in the production of intracellular vacuoles and cell membrane degradation. S. mutans dephosphorylates xylitol-5-phosphate, and when this sugar molecule is expelled from the cell, bacteria generate energy expenditure in the absence of any energy supply27.
Other main component of this toothpaste is betaine (trimethylglycine.). Animal studies showed that betaine suppressed the activity of NF-κB and a wide range of inflammation-related genes, including TNF-α, VCAM-1 and ICAM-128. Betaine inhibits NF-κB through the suppression of two important activators, mitogen-activated protein kinases (MAPKs) and NF-κB-inducing kinase/Inhibitory-κB kinase (NIK/IKK)28,29.
Salivary flow was not significantly increased by the test toothpaste in comparison to the control toothpastes. However, it significantly increased in the test group alone over the first 2 months (p = 0.017) (T2), and a higher flow was observed between baseline and 4 months, although statistical significance was not reached.
The test toothpaste increased salivary pH to a slightly alkaline pH with a mean of 7.5, being more favorable for oral eubiosis30. The pH value is important for preserving correct cell biochemistry and tissue homeostasis, and it is low at sites with inflammation or cell destruction31. The response of macrophages to an acid pH environment was found to involve the activation of inflammasome NLRP3, leading to the secretion of proinflammatory cytokines IL-1β, IL-18, and IL-3332. In the same study, alkalinization of the environment was reported to inhibit the activation of proinflammatory system NLRP3, leading the authors to propose environment pH modulation as a potential novel anti-inflammatory therapy32.
It was recently demonstrated that periodontal pathogens grow in a moderately acid environment, e.g., pH of 5.0–7 for P. intermedia, pH of 5.5–7 for F. nucleatum, and pH of 6.5–7 for P. gingivalis. Also, the salivary pH of patients with periodontitis became more alkaline after scaling and root planning30. A recent study described both salivary flow and pH as markers of periodontitis severity, which was correlated with low pH values (6.25 in grade IV periodontitis) and low salivary flow (0.28 mL/min); conversely, pH and flow values were significantly increased in patients with severe periodontitis after 3 months of periodontal treatment (hygienization)33.
Study limitations include the relatively small sample size, although it was estimated to offer sufficient statistical power, and some statistically significant differences. On the other hand, this interim study is the first multicenter, parallel-group, double-blind, placebo-controlled randomized clinical trial to evaluate clinical changes in gingival bleeding, dental biofilm, salivary flow, and saliva pH after using a toothpaste with a natural product (EVOO) as the main ingredient.
Superior clinical outcomes were obtained at 4 months in patients with gingivitis who brushed their teeth three times daily with the toothpaste containing EVOO, xylitol, and betaine in comparison to a placebo toothpaste and commercial anti-gingivitis toothpaste, including a reduction in gingival bleeding as well as an increase in salivary pH, contributing to oral eubiosis. Further research is warranted to verify these findings in wider samples.
Methods
This multicenter, parallel-group, double-blind, placebo-controlled randomized clinical trial recruited patients from two primary healthcare centers (Loja and Huetor Tajar) in the province of Granada (Southern Spain) and from the Clinic of the School of Dentistry of Granada University from July 2021 until September 2022. The study complied with the principles of the 2013 revision of the Declaration of Helsinki and was approved by the Biomedical Research Ethics Committee of Andalusia (ref.2184-N-20.). Written informed consent was obtained from all participant at the time of being enrolled in the study. The trial is registered on the ClinicalTrials.gov webpage (Study on the effects of a toothpaste in the microbiome and clinical parameters in patients with oral dysbiosis. ref. NCT05463484, 19/07/2022) and reported in accordance with CONSORT guidelines34.
Inclusion criteria were age between 18 and 70 years, the presence of at least 20 teeth without counting third molars, a diagnosis of gingivitis (index of bleeding on probing ≥ 10%) according to the 2017 World Workshop6, the signing of informed consent35, and explicit commitment to a manual toothbrushing regimen of three times a day for 4 min. Exclusion criteria were the presence of periodontitis, orthodontic appliances, removable partial prostheses, soft or hard tissue tumors of the oral cavity, > 5 caries lesions requiring immediate restoration, or allergies to oral hygiene products or specific ingredient(s) of the toothpastes under study, the receipt of antibiotic therapy during the previous month, receipt of dental prophylaxis during the previous 2 weeks, and being pregnant or breastfeeding35.
Patients were randomly assigned by Mucosal Innovations S.L to one of the following three toothpaste groups using a random number generator (http://www.random.org): Test Group: toothpaste with EVOO extract, xylitol, betaine, water, hydrated silica, glycerol, sodium monofluorophosphate, titanium dioxide, sodium benzoate, aroma, potassium sorbate, and stevia (FDA 510(k) ref. number K142657), Control Group 1: placebo toothpaste with the same ingredients as the experimental toothpaste but without EVOO, xylitol, or betaine. Control Group 2: commercial anti-gingivitis toothpaste, containing water, hydrolyzed hydrogenated starch, hydrated silica, zinc citrate, sodium lauryl sulfate, aroma, cellulose gum, sodium fluoride, sodium saccharin, tocopheryl acetate, and titanium dioxide.
The researcher gave the corresponding toothpaste to each patient, with both being blinded to the type of toothpaste, which was labeled with a code. Patients were instructed to brush their teeth with the assigned toothpaste for 4 min three times a day for 4 months and to use no other oral hygiene product. Clinical evaluations were performed at baseline (T0), 2 months (T2), and 4 months (T4) with the exception of the pH measurement (at T0 and T4). Patients were asked not to eat or smoke and to drink only water during the four hours before appointments35.
Data were gathered on patient age, sex, schooling level (compulsory schooling, high school, vocational training, and university education), tobacco consumption (cigarettes/day), alcohol consumption (standard drink units [SDUs]/day), and diabetes (yes/no). Scores were also collected for the plaque index proposed by Tonetti36 and the gingival bleeding index described by Ainamo and Bay37, using a PCPUNC15 periodontal probe (Hu-friedy, Chicago, IL, EEUU) at six sites per tooth (mesio-vestibular, vestibular, disto-vestibular, disto-lingual, lingual, mesio-lingual). A sample of non-stimulated salivary flow was gathered following Navazesh38, with the patient in physiological resting position, mouth closed and head tilted slightly backwards, without speaking, and spitting into a container every 60 s for 5 min. The total volume was collected in a syringe and divided by five to calculate the saliva flow (in mL) per minute.
Salivary pH was determined in saliva gathered at rest at T0 and T4 using PH 60 DHS equipment (XS Instruments, Carpi [MO], Italy) and an electrode (ref. H-238150) with diameter of 6 mm and minimum immersion of 15 mm that scales the pH from 0 to 14.
Statistical analysis
Sample Power 2.0 (SPSS Inc., Chicago, IL) was used to calculate the sample size required to detect with 80% power and 5% alpha error, using the Student’s t-test for independent samples, a standardized difference of 0.9 on Cohen’s scale39 among the groups in study variables (gingivitis, plaque, salivary flow, and pH). A minimum sample size of 20 patients per group was estimated.
For p-value calculations, briefly, we used different descriptive and analytic methods, depending on the type of variable (qualitative or quantitative). These methods are described in table footnotes. For quantitative variables in inter-group comparisons, we used ANOVA with Bonferroni's method for post-hoc paired comparisons when the global p-value was significant, i.e., p < 0.005. For intra-group comparisons Friedman's test was applied, and Student's T-test for paired comparisons when Friedman's test was significant.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request, considering the privacy and data protection.
References
Dewhirst, F. E. et al. The human oral microbiome. J. Bacteriol. 192, 5002–5017. https://doi.org/10.1128/JB.00542-10 (2010).
Wade, W. G. The oral microbiome in health and disease. Pharmacol. Res. 69, 137–143. https://doi.org/10.1016/j.phrs.2012.11.006 (2013).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759. https://doi.org/10.1038/s41579-018-0089-x (2018).
Deo, P. N. & Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 23, 122–128. https://doi.org/10.4103/jomfp.JOMFP_304_18 (2019).
Abusleme, L., Hoare, A., Hong, B. Y. & Diaz, P. I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000(86), 57–78. https://doi.org/10.1111/prd.12362 (2021).
Trombelli, L., Farina, R., Silva, C. O. & Tatakis, D. N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodontol. 89(Suppl 1), S46–S73. https://doi.org/10.1002/JPER.17-0576 (2018).
Seethalakshmi, C., Reddy, R. C., Asifa, N. & Prabhu, S. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: A cross-sectional study. J. Clin. Diagn. Res. 10, 12–14. https://doi.org/10.7860/JCDR/2016/16310.7351 (2016).
Kubala, E. et al. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. Biomed. Res. Int. 2018, 6572381. https://doi.org/10.1155/2018/6572381 (2018).
Laleman, I. & Teughels, W. Novel natural product-based oral topical rinses and toothpastes to prevent periodontal diseases. Periodontol. 2000(84), 102–123. https://doi.org/10.1111/prd.12339 (2020).
Dhingra, K. Aloe vera herbal dentifrices for plaque and gingivitis control: A systematic review. Oral Dis. 20, 254–267. https://doi.org/10.1111/odi.12113 (2014).
Ship, J. A., McCutcheon, J. A., Spivakovsky, S. & Kerr, A. R. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J. Oral Rehabil. 34, 724–732. https://doi.org/10.1111/j.1365-2842.2006.01718.x (2007).
Pretty, I. A., Gallagher, M. J., Martin, M. V., Edgar, W. M. & Higham, S. M. A study to assess the effects of a new detergent-free, olive oil formulation dentifrice in vitro and in vivo. J. Dent. 31, 327–332. https://doi.org/10.1016/s0300-5712(03)00052-6 (2003).
Romani, A. et al. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients https://doi.org/10.3390/nu11081776 (2019).
Scotece, M. et al. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1alpha and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci 91, 1229–1235. https://doi.org/10.1016/j.lfs.2012.09.012 (2012).
Bertelli, M. et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 309, 29–33. https://doi.org/10.1016/j.jbiotec.2019.12.016 (2020).
Beauchamp, G. K. et al. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 437, 45–46. https://doi.org/10.1038/437045a (2005).
Lozano-Castellon, J. et al. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 60, 2532–2548. https://doi.org/10.1080/10408398.2019.1650715 (2020).
Alagna, F. et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 12, 162. https://doi.org/10.1186/1471-2229-12-162 (2012).
Czerwińska, M., Kiss, A. K. & Naruszewicz, M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 131, 940–947. https://doi.org/10.1016/j.foodchem.2011.09.082 (2012).
Sindona, G. et al. Anti-inflammatory effect of 3,4-DHPEA-EDA [2-(3,4 -hydroxyphenyl) ethyl (3S, 4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate] on primary human vascular endothelial cells. Curr. Med. Chem. 19, 4006–4013. https://doi.org/10.2174/092986712802002536 (2012).
Filipek, A., Czerwinska, M. E., Kiss, A. K., Wrzosek, M. & Naruszewicz, M. Oleacein enhances anti-inflammatory activity of human macrophages by increasing CD163 receptor expression. Phytomedicine 22, 1255–1261. https://doi.org/10.1016/j.phymed.2015.10.005 (2015).
Han, S. J. et al. Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis. Clin. Diagn. Lab. Immunol. 12, 1285–1291. https://doi.org/10.1128/CDLI.12.11.1285-1291.2005 (2005).
Golestannejad, Z. et al. Inhibitory effects of ethanolic, methanolic, and hydroalcoholic extracts of olive (Olea europaea) leaf on growth, acid production, and adhesion of Streptococcus mutans. Dent. Res. J. 17, 179–185 (2020).
Karygianni, L. et al. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Complement Altern. Med. 19, 51. https://doi.org/10.1186/s12906-019-2461-4 (2019).
Talib, H. J., Mousa, H. A. & Mahmood, A. A. Assessment of the plaque-induced gingivitis patient with and without hyaluronic acid and xylitol toothpaste. J. Int. Soc. Prev. Community Dent. 11, 138–143. https://doi.org/10.4103/jispcd.JISPCD_371_20 (2021).
Burt, B. A. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J. Am. Dent. Assoc. 137, 190–196. https://doi.org/10.14219/jada.archive.2006.0144 (2006).
GasmiBenahmed, A. et al. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 104, 7225–7237. https://doi.org/10.1007/s00253-020-10708-7 (2020).
Go, E. K., Jung, K. J., Kim, J. Y., Yu, B. P. & Chung, H. Y. Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1252–1264. https://doi.org/10.1093/gerona/60.10.1252 (2005).
Lee, E. K. et al. Betaine attenuates lysophosphatidylcholine-mediated adhesion molecules in aged rat aorta: Modulation of the nuclear factor-kappaB pathway. Exp. Gerontol. 48, 517–524. https://doi.org/10.1016/j.exger.2013.02.024 (2013).
Koppolu, P. et al. Correlation of blood and salivary pH levels in healthy, gingivitis, and periodontitis patients before and after non-surgical periodontal therapy. Diagnostics https://doi.org/10.3390/diagnostics12010097 (2022).
Menkin, V. Biology of inflammation; chemical mediators and cellular injury. Science 123, 527–534. https://doi.org/10.1126/science.123.3196.527 (1956).
Rajamaki, K. et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 288, 13410–13419. https://doi.org/10.1074/jbc.M112.426254 (2013).
Lazureanu, P. C. et al. Saliva pH and flow rate in patients with periodontal disease and associated cardiovascular disease. Med. Sci. Monit. 27, e931362. https://doi.org/10.12659/MSM.931362 (2021).
Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 63, 834–840. https://doi.org/10.1016/j.jclinepi.2010.02.005 (2010).
Seriwatanachai, D. et al. Effect of stannous fluoride and zinc phosphate dentifrice on dental plaque and gingivitis: A randomized clinical trial with 6-month follow-up. J. Am. Dent. Assoc. 150, S25–S31. https://doi.org/10.1016/j.adaj.2019.01.003 (2019).
Tonetti, M. S. The future of periodontology: New treatments for a new era. J. Int. Acad. Periodontol. 4, 110–114 (2002).
Ainamo, J. & Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 25, 229–235 (1975).
Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 694, 72–77. https://doi.org/10.1111/j.1749-6632.1993.tb18343.x (1993).
Cohen, J. Statistical Power Analysis for the Behavioural Sciences 2nd edn. (Lawrence Erlbaum Associates, 1988).
Acknowledgements
This study was supported with a grant (STOP ORAL DYSBIOSIS Ref. OTRI-4907) awarded by the University of Granada and Mucosal Innovations S.L. (Madrid).
Author information
Authors and Affiliations
Contributions
Each author’s contribution is detailed as follows: Conception or design of the work: F.M. and A.R.A; Data acquisition: A.R.A., R.M. and J.F.; Data analysis: M.B.; Data interpretation: A.M.F. and A.L.T; Manuscript draft: F.M., A.R.A, A.M.F. All authors have revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodríguez-Agurto, A., Bravo, M., Magán-Fernandez, A. et al. Randomized clinical trial on the clinical effects of a toothpaste containing extra virgin olive oil, xylitol, and betaine in gingivitis. Sci Rep 13, 6294 (2023). https://doi.org/10.1038/s41598-023-33521-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33521-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.