Abstract
Nonalcoholic fatty liver disease (NAFLD) is a condition that affects about 24% of people worldwide. Increased liver fat, inflammation, and, in the most severe cases, cell death are all characteristics of NAFLD. However, NAFLD pathogenesis and therapy are still not clear enough. Thus, this study aimed to determine the effect of a high-cholesterol diet (HCD) inducing NAFLD on lipolytic gene expression, liver function, lipid profile, and antioxidant enzymes in rabbits and the modulatory effects of probiotic Lactobacillus acidophilus (L. acidophilus) on it. A total of 45 male New Zealand white rabbits, eight weeks old, were randomly divided into three groups of three replicates (5 rabbits/replicate). Rabbits in group I were given a basal diet; rabbits in group II were given a high-cholesterol diet that caused NAFLD; and rabbits in group III were given a high-cholesterol diet as well as probiotics in water for 8 weeks. The results showed that a high-cholesterol diet caused hepatic vacuolation and upregulated the genes for lipoprotein lipase (LPL), hepatic lipase (HL), and cholesteryl ester transfer protein (CETP). Downregulated low-density lipoprotein receptor (LDLr) gene, increased liver enzymes [alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH)], cholesterol, triglycerides (TG), low-density lipoprotein (LDL), glucose, and total bilirubin. On the other hand, it decreased high-density lipoprotein (HDL), total protein, albumin, and liver antioxidants [glutathione peroxidase (GPx), catalase (CAT), reduced glutathione (GSH), and superoxide dismutase (SOD)]. Supplementing with probiotics helped to return all parameters to normal levels. In conclusion, probiotic supplementation, especially L. acidophilus, protected against NAFLD, and restored lipolytic gene expression, liver functions, and antioxidants to normal levels.
Similar content being viewed by others
Introduction
The percentage of nonalcoholic fatty liver disease (NAFLD) patients who also have nonalcoholic steatohepatitis (NASH) is expected to increase during the coming ten years. One modelling study predicts that by 2030, there will be an increase in NAFLD prevalence of 18%. There will be 27 million NASH patients in the US, a 56 percent increase from the current number1. NAFLD is associated with an increased chance of passing away, particularly from heart disease, hepatocellular cancer, and liver-related incidents2. Moreover, NAFLD is consistently seen in bladder cancer patients3. According to the increase in frequency in recent decades, it has emerged as the second most common reason for liver transplantation in the United States4. Hepatic steatosis, NASH, liver cirrhosis, and hepatocellular cancer are all considered to be part of the nonalcoholic fatty liver disease group of liver illnesses5.
The development and progression of NAFLD have been linked to a number of genes. NAFLD has been most strongly associated with the single nucleotide polymorphism (SNP) causing isoleucine to methionine substitution at position 148 in the patatin-like phospholipase domain-containing 3 (PNPLA3). Triacylglycerol, diacylglycerol, and monoacylglycerol are hydrolyzed by PNPLA3, but the I148M mutation renders the enzyme inactive6. This genetic variant is associated with increased NASH activity, increased liver lipid content, and an increased chance of developing hepatocellular cancer and liver fibrosis7,8. Three more well-researched genetic variants include glucokinase regulator (GCKR) P446L, membrane-bound O-acyltransferase domain-containing 7 (MBOAT7), and transmembrane 6 superfamily member 2 (TM6SF2), rs58542926 C > T. They also raise the risk of fibrosis and increase the severity of NAFLD9,10.
The lipase gene family includes lipoprotein lipase (LPL), pancreatic lipase, hepatic lipase, and endothelial lipase. The gene converts lipoprotein triglycerides into one monoacylglycerol molecule and two free fatty acids. Chylomicrons and very low density lipoprotein (VLDL) contain it. Additionally, it facilitates the uptake of free fatty acids, cholesterol-rich lipoproteins, and leftover chylomicrons into cells11. The LPL gene encodes lipoprotein lipase, which is expressed in the heart, muscles, and adipose tissue12. Low-density lipoprotein receptors are crucial for regulating blood cholesterol levels. They are particularly prevalent in the liver, which is capable of getting rid of the majority of the extra cholesterol in the body. The number of low density lipoprotein receptors (LDLr) on the surface of liver cells controls how rapidly cholesterol is removed from the bloodstream13,14,15. The LDLr gene has 18 exons and is located in band 19p13.2 on chromosome 1916. The hydrolysis of triacylglycerides is catalyzed by hepatic lipase (HL). Other names for it include hepatic triglyceride lipase (HTGL)17. Chromosome 15 contains the HL gene18. Hepatic lipase is mostly expressed in hepatocytes and endothelial cells in the liver. There are two possibilities for hepatic lipase: it can either stay attached to the liver or it can separate from liver endothelial cells and be free to enter the bloodstream19. Cholesteryl ester transfer protein gene (CETP), a protein that reduces high density lipoprotein (HDL) levels by transferring cholesteryl esters from HDL to particles that contain apolipoprotein B in exchange for triglycerides. Triglycerides and cholesterol esters are transported between VLDL, LDL, and HDL via the enzyme CETP. Lower CETP levels promote the synthesis of HDL. Since higher HDL levels are associated with a decreased risk of atherosclerosis20. The genomic DNA for the CETP gene has about 25 Kbp and 16 exons21. The upregulation of CETP is brought on by either an increase in dietary cholesterol or endogenous hypercholesterolemia22.
In numerous studies, probiotic treatment has been found to decrease the symptoms of NAFLD in animal models23,24,25. Probiotics improve liver function, restore the gut flora, and improve the lipid profile by assessing circulating total cholesterol, HDL, LDL, and triglycerides25. One of the most prevalent genera of probiotic bacteria, Lactobacillus, has a long history of safe use and is recognized by the US Food and Drug Administration as safe for human consumption26. The researchers found that the strain most successful at reducing total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) was Lactobacillus acidophilus27,28. The most popular probiotics, Lactobacillus species, are given for a lengthy period29. It was discovered to prevent NAFLD brought on by a high-fat diet (HFD) and to enhance gut permeability, inflammation, and gut flora modulation in a diet-induced obesity model30,31,32. The interaction of probiotic bacteria with bile acids via deconjugation events catalyzed by bile salt hydrolase enzymes (BSH) is assumed to be the basic mechanism of their cholesterol-lowering properties33. Probiotic strains containing BSH help deconjugate bile salts, which is the first stage in the colon's biotransformation of bile salts. Deconjugation is the term for the enzymatic dissolution of the C-24 N-acyl amide link connecting bile acids to their amino acid conjugates34.
So, this study aimed to determine the effect of a high cholesterol diet (HCD) inducing NAFLD on lipolytic gene expression, liver function, lipid profile, and antioxidant enzymes in rabbits and the modulatory effects of probiotic Lactobacillus acidophilus on it.
Material and methods
The current study was approved by the Ethical Committee for live birds sampling at the Faculty of Veterinary Medicine, Benha University (BUFVTM 01-09-22).
Rabbits
The current study was carried out in the Faculty of Veterinary Medicine, Benha University, Department of Animal Wealth Development, using 45 male New Zealand white rabbits (eight weeks old, about 1200 g body weight). They were obtained from SAN El-HAGAR, ASH SHARQIYAH, EGYPT. All animal handling procedures are in agreement with the ARRIVE guidelines from the National Center for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) 19 throughout the experimental period (eight weeks).
The experimental design
The rabbits were weighed individually and marked. The rabbits were divided into three groups at random; each group had three replicates of five rabbits. The rabbits were housed in wire mesh cages with identical housing and care practices; feed was applied twice a day, and water was applied constantly by the nipple system. The home was clean, disinfected, and well-ventilated, with the right environmental temperature. Lighting was provided for 16 h: 8 h of darkness throughout the experimental period. All methods were carried out in accordance with relevant guidelines and regulations.
The three groups were as follows:
-
Group I received the basal diet.
-
Group II received a high cholesterol diet (HCD), a 2% cholesterol diet35.
-
Group III received HCD with probiotic (1 g/L water) in water. The probiotic Lactobacillus acidophilus (Lacto biotech®) is produced and exported by Mycrofeed Srl, Italy, and obtained by Cairomed Pharmaceuticals Company.
The experimental food was introduced to the rabbits gradually over a two-week adaptation period, the trial lasted for eight weeks. The ingredients and nutritional composition of the basal diet and HCD are represented in Tables 1 and 2.
Histopathological examination of the liver
Liver samples from various areas of the liver were obtained for histological evaluation and investigation for NAFLD. Samples were stored in 10% buffered neutral formalin, and then exposed to microscopic examination in accordance with the method described by Davis36.
Scoring of hepatic steatosis was done according Nassir et al.37 in which grading was done on the basis of the percentage of fat within the hepatocytes: grade 0 (healthy, < 5%), grade 1 (mild, 5%-33%), grade 2 (moderate, 34%-66%), and grade 3 (severe, > 66%).
Determination of lipolytic genes expression
Samples collection
On day 56 following the commencement of the trial (at the end of eight weeks), 12 representative rabbits (selected at random as three rabbits per replicate) had been sacrificed by overdose injection of pentobarbital sodium at 60 to 70 mg/kg live weight for sampling. Liver samples had been taken and kept at − 80 °C for subsequent study.
Quantitative real-time PCR analysis
Following the manufacturer's instructions, 50 mg of tissue was homogenized in a sterile collection tube with 750 µl of Trizol solution using a rotor Tissue Ruptor to extract the total RNA (Qiagen, GmbH, Germany). By measuring the absorbance in a nanodrop spectrophotometer (BMG Lab Tec. GmbH, Germany), the concentration and purity of RNA were assessed. The A260/A280 ratio of undiluted RNA is (1.8–2.0). The primers were created using NCBI Primer-BLAST Software, and their sequences are displayed in Table 3. Two µg of total RNA was reverse transcribed into cDNA using 2X Reverse Transcriptase Master Mix (Applied Biosystem, USA) following the manufacturer's instructions. The Applied Biosystems 7500 Fast Real-time PCR, USA, was used to quantify the mRNA. The quantitative PCR was conducted using the SYBER Green Master Mix in a 20 µL reaction mixture (TOPreal TM qPCR 2X PreMIX). The initial activation (3 min/95 °C), denaturation (3 s/95 °C), and annealing/extension (30 s/60 °C) were used to justify the cycling condition, and 40 cycles were used in total, according to38. The GAPDH gene served as the standard for all gene expression levels. Utilizing the 2-ΔΔCt technique, gene expression has been compared and quantified39.
Biochemical analysis of blood
At the completion of the study period, blood samples were taken from each animal. After an overnight fast, blood samples were taken from the ear vein of the rabbits by means of a serum-separating blood collection tube and a vacuum gel tube with a clot activator. Samples were drawn into dry, clean test tubes and allowed to clot for half an hour at room temperature to separate the serum. Clear sera were separated by centrifugation at 3500 rpm for 15 min and then collected using automatic micropipettes in Eppendorf’s tubes. The serum was maintained at − 20 °C in a deep freezer to determine the following parameters: alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), cholesterol, triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total protein, albumin, glucose, and total bilirubin.
Determination of liver antioxidants
Liver antioxidants were determined in liver tissue homogenate according to methods adopted by Weissman40 for glutathione peroxidase (GPx), Aebi41 for catalase (CAT), Beutler et al.42 for reduced glutathione (GSH), and Nishikimi et al.43 for superoxide dismutase (SOD).
Statistical analysis
The statistical program SPSS was used to analyze the data (version 21; SPSS Inc., Chicago, IL, USA). The results were mean ± SE, according to the independent sample T-test analysis, Significant statistically (P ≤ 0.05).
Ethical approval
The current study was approved by the Ethical Committee for live birds sampling at the Faculty of Veterinary Medicine, Benha University (BUFVTM 01-09-22).
Guidelines
All methods were carried out in accordance with relevant guidelines and regulations.
ARRIVE guidelines
The authors confirm that the study was carried out in compliance with the ARRIVE guidelines.
Result
Concerning histopathological changes in liver sections from control rabbits, they showed the liver's typical histological structure, which was made up of hepatic lobules with radiating hepatocytes surrounding a central vein and irregular blood sinusoids separating them (Fig. 1). HCD supplemented group exhibited pronounced hepatic vacuolation coupled with fat cytoplasmic vacuoles, (Fig. 2). Hepatic fatty changes in the group receiving probiotic supplements and HCD were significantly reduced, and there was very slight glycogen infiltration, as shown in Fig. 3. Quantitative scoring of histological sections involving the criteria of the percentage of fat within the hepatocytes showed that the control group scored at grade 0 (2 ± 0.58), HCD group at grade 2 (50 ± 1.58), and the HCD + probiotic group at grade 1 (20 ± 2.89).
The impact of probiotic supplementation and HCD-induced fatty liver on lipolytic gene expression in the liver was depicted in Figs. 4 and 5. The HCD group showed a significant (P ≤ 0.05) increase in LPL, HL, and CETP gene expression as well as a significant decrease in LDLr gene expression when compared to the control group and probiotic-supplemented group. Supplementation of probiotics reversed the effects of HCD on gene expression, as no significant differences were found between the control group and probiotic-supplemented group for all genes studied.
Figures 6 and 7 indicated the impact of a high-cholesterol diet (HCD) and probiotic supplementation on the liver enzymes ALT, AST, ALP, and LDH. These results showed that the ALT, AST, ALP, and LDH enzymes were significantly higher in the HCD group than in the other groups. There were no significant differences between the control group and the HCD with a probiotic-supplemented group.
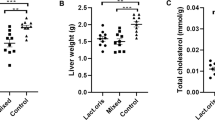
Figures 8 and 9 illustrate how supplementing with probiotics and HCD affects the lipid profile [cholesterol, triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL)]. These results showed that the HCD group showed a significant increase in cholesterol, TG, and LDL with a significant decrease in HDL compared to the control and probiotic-supplemented groups. The results indicated that probiotic supplementation reversed the effect of HCD.
Figures 10, 11, and 12 showed the impact of HCD and probiotic supplementation on liver function (total protein, albumin, glucose, and total bilirubin). These results demonstrated a marked increase in glucose and total bilirubin, as well as a marked decrease in total protein and albumin in the HCD-supplemented group when compared to the other groups, while the HCD & probiotic-supplemented group exhibited a marked decrease in glucose and total bilirubin, as well as a significant increase in total protein when compared to the HCD group.
Figures 13 and 14 showed how probiotic supplementation and HCD-induced fatty liver affect antioxidant levels [glutathione peroxidase (GPx), catalase (CAT), reduced glutation (GSH), and superoxide dismutase (SOD)]. These findings indicate that the liver's concentrations of GPx, CAT, GSH, and SOD were significantly (P ≤ 0.05) lower in the HCD-supplemented group than they were in the control group and the probiotic-supplemented group. There were no significant differences in enzyme levels between the probiotic-supplemented group and the control group.
Discussion
The HCD-supplemented group's liver sections showed significant hepatic vacuolation along with fat cytoplasmic vacuoles. This is similar to Helal et al.44, who revealed hepatocytes that appeared to be enlarging and ballooning. In rats with induced fatty liver, cells throughout the hepatic lobule have macrovacuoles scattered throughout the cytoplasm. Also, Wang et al.45 demonstrated that there were numerous vacuoles in the hepatocytes of the rabbits fed the HCD, according to an investigation of the liver's histology. Probiotic supplements dramatically minimized hepatic fatty alterations, and there was very little glycogen infiltration. These results are consistent with Rishi et al.46, who observed that probiotic administration improved the liver's morphology.
The result showed that there was up-regulation in lipoprotein lipase (LPL) gene expression in the HCD group. This finding is similar to that of Zhang et al.47, who discovered that a high-cholesterol diet increases the expression of the LPL gene. Also, similar to Teratani et al.48, who reported that NAFLD and nonalcoholic steatohepatitis (NASH) patients' livers had significantly greater levels of LPL messenger RNA (mRNA) expression than did healthy subjects' livers. The development of NAFLD in their mice model is accompanied by a considerable increase in hepatic LPL mRNA levels. Pardina et al.49 compared morbidly obese humans with steatosis to control subjects, and LPL mRNA activity was significantly higher (nearly double). Perla et al.50 said that increased LPL is linked to NAFLD, while in the HCD plus probiotic-supplemented group, the LPL gene is down regulated. This result is consistent with the findings of Wang et al.51, who found that the gene expression of LPL was considerably lower in the Lactobacillus johnsonii group than in the control group. Furthermore, Karimi et al.52 discovered that probiotic administration, whether single or multiple species, significantly reduced LPL gene expression. LPL expression is increased in both humans and mice by serum obesity-related substances such as leptin, IL-6, and free fatty acids (FFA)48. According to reports, the putative signal transducer and activator of transcription 3 (STAT3)-binding site is located in the LPL promoter, and STAT3 signalling elevates LPL expression53. It can be claimed that taking a probiotic supplement boosted the expression of (PPARG), which in turn up-regulates the expression of angiopoietin-like 4. (ANGPTL-4). Lower TG levels result from the downregulation of LPL caused by the upregulation of ANGPTL-452.
The LDLr gene expression was lower in the HCD group than in the other groups. This result is similar to that reported by Chen et al.54, who reported that, compared to the normal diet group, the high-fat diet group's levels of LDLr mRNA expression were considerably lower. Also, similar to Zhang et al.47, who reported that, compared to the control group, the mRNA expression of LDLr was significantly lower in the high-fat, high-cholesterol groups. This is also similar to Xin et al.55, who found that evidently, a high-fat, high-sucrose diet reduced the expression of LDLr mRNA and protein in the liver. Also, similar to Song et al.56, who reported that, compared to mice on a regular diet, animals on a high-fat diet had significantly fewer LDLr genes in the liver. The expression was higher in the HCD supplemented with probiotics than in the HCD supplemented group. This outcome is comparable to that of Palaniyandi et al.57, who discovered that the high-cholesterol diet group supplemented with probiotics had higher levels of LDLr gene expression in the liver than the high-cholesterol diet control group. Tamtaji et al.58 discovered that selenium and probiotic-supplemented patients had significantly higher levels of LDLr gene expression than those who just received selenium supplements. Similarly, Song et al.56 found that administering L. acidophilus NS1 increases LDLr expression in the liver, which was previously suppressed by a high-fat diet.
The expression of the hepatic lipase (HL) gene was increased in the HCD group more than in other groups. This result is similar to that reported by Miksztowicz et al.59, who reported that the patients with hepatic steatosis showed considerably higher hepatic lipase activity than controls, and this activity was higher in the most severe state of hepatic steatosis. This result is incompatible with Yang et al.60, who reported that hepatic lipase expression levels dramatically dropped in the high-fat diet groups. When compared to the HCD group, the expression of HL in the probiotic-supplemented group was significantly lower. It might be caused by probiotics like Lactobacillus acidophilus, which increased HDL and decreased total and LDL cholesterol in experimental animals61. Increased hepatic lipase has a role in promoting a more atherogenic profile, as evidenced by the direct correlation between LDL cholesterol and hepatic lipase and the inverse associations with HDL cholesterol59.
The expression of cholesteryl ester transfer protein (CETP) was increased in the HCD group. These results are similar to those of Lucero et al.62, who reported that patients with hepatic steatosis exhibit elevated CETP activity; they are also similar to those of Lottenberg et al.63, who said that a high level of CETP activity is frequently seen in hypercholesterolemic people. Blauw et al.64 recorded that it is conceivable that metabolic liver inflammation won't significantly decrease CETP expression and production. The expression of CETP decreased in HCD with the probiotic-supplemented group, as probiotics could have caused it. In test animals, Lactobacillus acidophilus boosted HDL and decreased total and LDL cholesterol61. It's thought that increased activity of CETP is associated with low HDL65.
The obtained data revealed that HCD supplementation in normal rabbits exhibited a significant increase in serum ALT, AST, and ALP activities when compared with the control. These findings are remarkably identical to those of previous investigations45,66,67. On the other hand, these results disagree with those of Kainuma et al.68, who reported that there is no significant difference between high cholesterol diets and control diets rabbits. The considerable increase in ALT, AST, ALP, and LDH following high-cholesterol diet (HCD)-induced nonalcoholic fatty liver disease (NAFLD) was attributed to an increase in the hepatic cell membrane’s fragility, which caused enzyme release into the bloodstream. Due to the liver's compromised structural integrity, these cytoplasmic enzymes are released into the circulation following an autolytic breakdown or cellular necrosis69. The level of these enzymes decreased in HCD with the probiotic-supplemented group. These findings are similar to those of Adesiji et al.70 and Li et al.71, who reported that Lactobacillus acidophilus decreases liver enzyme levels. This might be viewed as a positive side effect of taking Lactobacillus acidophilus, which is effective in preserving the health and activity of the epithelial cells lining the biliary duct, showing how probiotics directly affect liver function72.
The levels of serum triglycerides (TG) and cholesterol were rising. These outcomes resemble those reported by Sigrist-Flores et al.73; Xing et al.74; and Lee et al.75. TG and cholesterol levels were significantly lower in the HCD probiotic supplemented group compared to the HCD supplemented group. These resemble the reports of Mazloom et al.76 and Lee et al.77, who reported that Lactobacillus acidophilus has a hypocholesterolemic impact and lowers blood triglycerides. Also, Song et al.56 reported that hepatic cholesterol and TG levels may be decreased by L. acidophilus. Also, Kullisaar et al.78 reported that probiotic supplementation dramatically decreased total cholesterol and triglycerides. These results may be attributed to a decrease in the host's intestinal absorption of fatty acids as a result of L. acidophilus79. Moreover, Park et al.80 reported that L. acidophilus improves lipid metabolism.
The result showed that there was a decrease in high-density lipoprotein (HDL) and an increase in low-density lipoprotein (LDL) in serum. These findings are similar to those of Paul et al.81, who reported that lower blood HDL and greater LDL particle content were independently linked to NAFLD. Also, Briseño-Bass et al.82 reported that a rise in LDL and a reduction in HDL were both substantially correlated with hepatic steatosis, and Sigrist-Flores et al.73 reported that chronic intake of the fat-enriched diet inducing fatty liver caused a high level of LDL and a low level of HDL. Moreover, Kainuma et al.68 reported that many physiopathological characteristics of NAFLD are shared by cholesterol-fed rabbits, where this model may be useful for elucidating the mechanism of NAFLD related primarily to hyperlipidemia because it did not exhibit insulin resistance or obesity. When compared to the HCD-supplemented group, the HCD with probiotic supplementation significantly increased HDL and decreased LDL. These findings are similar to those of Song et al.56, who reported that high LDL cholesterol levels may be reduced as a result of L. acidophilus-induced liver low-density lipoprotein receptor (LDLr) recovery, which may make it easier for the liver to absorb plasma LDL. Also, Jouybari et al.61 found that ingestion of yoghurt containing Lactobacillus acidophilus in their experimental animals resulted in a rise in HDL and a decrease in total and LDL cholesterol. Kullisaar et al.78 found that due to probiotic use, LDL cholesterol levels and total cholesterol all reduced dramatically, while HDL cholesterol showed a trend to improve. Probiotics are thought to decrease cholesterol by blocking the reabsorption and subsequent excretion of bile salts through their action of deconjugating bile salt, which prevents its recycling83.
The findings showed that there was a significant decrease in total protein and albumin in the HCD group compared to other groups. These findings are consistent with those of Helal et al.44, who found that fatty liver had a significant decrease in total protein and albumin levels. In addition, Mikolasevic et al.84; Grgurevic et al.85; and Kawaguchi et al.86 reported that patients who had NAFLD showed a low level of serum albumin. The results disagreed with Cho et al.87, who reported that patients with fatty liver showed a higher level of total protein. Serum total protein and albumin significantly increased in the HCD group with probiotic supplementation compared to the HCD group. These findings are similar to those of Ayyat et al.88, who claimed that taking a probiotic supplement increased serum total protein and albumin levels significantly. Moreover, Adriani et al.89 stated that broiler chicken treated with dry probiotics had the greatest levels of blood protein and albumin. These findings didn't agree with Alkhalf et al.90, who declared that their study's probiotic supplementation had no effect on the serum concentrations of total protein or albumin. The inclusion of probiotics in the diet increases the amount of aminoethyl cysteine and lysine analogues in the digestive system, which are then converted to lysine and cysteine amino acids to enhance the retention of proteins important for the development of meat91.
The research showed that the HCD group's serum glucose rose in comparison to the other groups. This is analogous to Helal et al.44, who reported that fatty liver-induced lab animals had higher serum glucose levels, and likewise in line with Cho et al.87 and Paul et al.81, who reported that individuals with fatty livers have high serum glucose levels. The results showed that the HCD in the probiotic-supplemented group had a significantly lower serum glucose level. These results concur with those of Adesiji et al.70, who noted that the lactobacillus acidophilus-treated rat groups showed a considerable reduction in blood glucose levels. This observation can be the result of appropriate insulin release, which helps to control blood glucose levels92. Endogenous insulin production may be enhanced by promoting glucose storage in the liver, increasing the body's usage of glucose, or giving probiotics that may have improved the beta cells' declining activity93.
The study revealed that the HCD group's serum total bilirubin was significantly higher than that of the other groups. This is similar to Jain and Singhai94, who reported that the affected liver's serum bilirubin levels have significantly increased. Nevertheless, HCD with probiotic supplementation significantly reduced serum total bilirubin levels. This agrees with Mutlu et al.95, who reported that the probiotic supplementation group in their study had reduced levels of bilirubin. In this case, glucuronidase activity may be inhibited.
The results showed that there was a decrease in liver antioxidants (GPx, CAT, GSH, and SOD) in the HCD-supplemented group. These data are analogues to Videla et al.96, who reported that GPx, GSH, CAT, and SOD activity were decreased in NAFLD patients. Compared to the HCD group, these antioxidants were significantly higher in the group receiving probiotic supplements with HCD. These results are consistent with those of Amdekar and Singh97, who found that L. acidophilus maintained oxidative stress markers in collagen-induced arthritic rats. Moreover, Dowarah et al.98 discovered that certain lactic acid bacteria strains might boost the production of antioxidant enzymes or control and alleviate circulatory oxidative stress to shield cells from oxidative stress-related harm. Although the Food and Drug Administration has not approved any medications to treat NAFLD, current treatment options depend on lifestyle modification and dietary restrictions. In addition, treatment is based on the use of antioxidants as probiotics or the treatment of associated metabolic diseases like obesity, type 2 diabetes, and dyslipidemia, which are all directly related to NAFLD. Recently, there are many drugs in the pipeline that are reckoned as good candidates to cure NAFLD/NASH99.
Conclusion
Many of the physiopathological characteristics of NAFLD were shared by cholesterol-fed rabbits. This model may be useful for elucidating the mechanism of NAFLD related primarily to hyperlipidemia because it did not exhibit insulin resistance or obesity. However, the current study showed that the enzymatic activity of the serum liver profile, liver function tests, liver tissue antioxidants and peroxide, and lipid profile were all improved when a probiotic was administered throughout the rabbit's rearing period. Also, the supplements improved the pathological organ damage brought on by HCD.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904. https://doi.org/10.1016/j.jhep.2018.05.036 (2018).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153. https://doi.org/10.1136/gutjnl-2017-313884 (2017).
Tarantino, G. et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: a cross-sectional retrospective study. J. Clin. Med. 10(2), 346 (2021).
Pais, R. et al. NAFLD and liver transplantation: Current burden and expected challenges. J. Hepatol. 65, 1245–1257. https://doi.org/10.1016/j.jhep.2016.07.033 (2016).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922. https://doi.org/10.1038/s41591-018-0104-9 (2018).
Huang, Y., Cohen, J. C. & Hobbs, H. H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 286, 37085–37093. https://doi.org/10.1074/jbc.M111.290114 (2011).
Sookoian, S. & Pirola, C. J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatol. 53, 1883–1894. https://doi.org/10.1002/hep.24283 (2011).
Singal, A. G. et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 109, 325–334. https://doi.org/10.1038/ajg.2013.476 (2014).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356. https://doi.org/10.1038/ng.2901 (2014).
Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 13, 643-654.e9. https://doi.org/10.1016/j.cgh.2014.04.014 (2015).
Mead, J., Irvine, S. & Ramji, D. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 80, 753–769. https://doi.org/10.1007/s00109-002-0384-9 (2002).
Uhlén, M. et al. Tissue-based map of the human proteome. Science (80-.) https://doi.org/10.1126/science.1260419 (2015).
Marais, A. D., Firth, J. C. & Blom, D. J. Homozygous familial hypercholesterolemia and its management. Semin. Vasc. Med. 4, 43–50. https://doi.org/10.1055/s-2004-822985 (2004).
Jeon, H. & Blacklow, S. C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 74, 535–562. https://doi.org/10.1146/annurev.biochem.74.082803.133354 (2005).
Kong, W. J., Liu, J. & Jiang, J. D. Human low-density lipoprotein receptor gene and its regulation. J. Mol. Med. 84, 29–36. https://doi.org/10.1007/s00109-005-0717-6 (2006).
Nykjaer, A. & Willnow, T. E. The low-density lipoprotein receptor gene family: A cellular Swiss army knife?. Trends Cell Biol. 12, 273–280. https://doi.org/10.1016/S0962-8924(02)02282-1 (2002).
Vázquez, J. Human physiology. Am. Biol. Teach. 68, 169–169. https://doi.org/10.2307/4451958 (2006).
Fox, S. I. Human Physiology (McGraw-Hill, 2016).
Karackattu, S. L., Trigatti, B. & Krieger, M. Hepatic lipase deficiency delays atherosclerosis, myocardial infarction, and cardiac dysfunction and extends lifespan in SR-BI/apolipoprotein E double knockout mice. Arterioscler. Thromb. Vasc. Biol. 26, 548–554. https://doi.org/10.1161/01.ATV.0000202662.63876.02 (2006).
Seidman, M. A. & Stone, J. R. Cholesterol Ester Transfer Protein Pathophysiology of Atherosclerosis Pharmacogenetics CETP Deficiency and Concerns in CETP Inhibitor Development (2014).
Agellon, L. B. et al. Organization of the human cholesteryl ester transfer protein gene. Biochemistry 29, 1372–1376. https://doi.org/10.1021/bi00458a004 (1990).
Tall, A. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64, 235–257. https://doi.org/10.1146/annurev.biochem.64.1.235 (1995).
Esposito, E. et al. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J. Nutr. 139, 905–911. https://doi.org/10.3945/jn.108.101808 (2009).
Nardone, G. et al. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am. J. Physiol. Liver Physiol. 299, G669–G676. https://doi.org/10.1152/ajpgi.00188.2010 (2010).
Meroni, M., Longo, M. & Dongiovanni, P. The role of probiotics in nonalcoholic fatty liver disease: A new insight into therapeutic strategies. Nutrients 11, 1–24. https://doi.org/10.3390/nu11112642 (2019).
Khalesi, S. et al. A review of probiotic supplementation in healthy adults: helpful or hype?. Eur. J. Clin. Nutr. 73, 24–37. https://doi.org/10.1038/s41430-018-0135-9 (2019).
Shimizu, M., Hashiguchi, M., Shiga, T. & Tamura, H. Meta-analysis: Effects of Probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals 1–16 (2015). https://doi.org/10.1371/journal.pone.0139795
Sun, J. & Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 47, 430–440. https://doi.org/10.3109/07853890.2015.1071872 (2015).
Segers, M. E. & Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb. Cell Fact. 13, S7. https://doi.org/10.1186/1475-2859-13-S1-S7 (2014).
Ritze, Y. et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 9, e80169. https://doi.org/10.1371/journal.pone.0080169 (2014).
Xin, J. et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 98, 6817–6829. https://doi.org/10.1007/s00253-014-5752-1 (2014).
Kawano, M., Miyoshi, M., Ogawa, A., Sakai, F. & Kadooka, Y. Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high- fat diet. J. Nutr. Sci. 5, e23. https://doi.org/10.1017/jns.2016.12 (2016).
Pavlović, N., Stankov, K. & Mikov, M. Probiotics—interactions with bile acids and impact on cholesterol metabolism. Appl. Biochem. Biotechnol. 168, 1880–1895. https://doi.org/10.1007/s12010-012-9904-4 (2012).
Ridlon, J. M., Kang, D. & Hylemon, P. B. review Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259. https://doi.org/10.1194/jlr.R500013-JLR200 (2006).
Dornas, W. C., De Oliveira, T. T., Augusto, L. E. F. & Nagem, T. J. Aterosclerose experimental em coelhos. Arq. Bras. Cardiol. 95, 272–278. https://doi.org/10.1590/S0066-782X2010001200020 (2010).
Davis, M. M. Paediatric surgical pathology: An illustrated handbook of the paediatric biopsy. J. Pediatr. Surg. 31, 1603. https://doi.org/10.1016/S0022-3468(96)90228-6 (1996).
Nassir, F., Rector, R. S., Hammoud, G. M. & Ibdah, J. A. Pathogenesis and prevention of hepatic steatosis. Gastroenterol. Hepatol. 11(3), 167 (2015).
Zhao, F. J., Ma, J. F., Meharg, A. A. & McGrath, S. P. Arsenic uptake and metabolism in plants. New Phytol. 181, 777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real- time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Weissman, S. M. Red cell metabolism. A manual of biochemical methods. Yale J. Biol. Med. 49(3), 310 (1976).
Aebi, H. Catalase in vitro, in: Methods in Enzymology 121–126 https://doi.org/10.1016/S0076-6879(84)05016-3 (1984).
Nishikimi, M., Appaji Rao, N. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. https://doi.org/10.1016/S0006-291X(72)80218-3 (1972).
Beutler, E., Duron, O. & Kelly, B. M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888 (1963).
Helal, E. G. E., Abd El-Wahab, S. M., Zedan, G. A. & Sharaf, A. M. M. Effect of Zingiber officinale on fatty liver induced by oxytetracycline in albino rats. Egypt. J. Hosp. Med. 46, 26–42. https://doi.org/10.21608/ejhm.2012.16354 (2012).
Wang, Y. et al. Establishment of a novel non-alcoholic fatty liver disease model using cholesterol-fed rabbits with reference to the potential role of endoplasmic reticulum stress. Mol. Med. Rep. 18, 2898–2904. https://doi.org/10.3892/mmr.2018.9258 (2018).
Rishi, P., Arora, S., Kaur, U. J., Chopra, K. & Kaur, I. P. Better management of alcohol liver disease using a ‘microstructured synbox’ system comprising L. plantarum and EGCG. PLoS ONE 12, e0168459. https://doi.org/10.1371/journal.pone.0168459 (2017).
Zhang, L. et al. Cholesterol induces lipoprotein lipase expression in a tree shrew (Tupaia belangeri chinensis) model of non- alcoholic fatty liver disease. Sci. Rep. 5, 1–12. https://doi.org/10.1038/srep15970 (2015).
Teratani, T. et al. Lipoprotein lipase up- regulation in hepatic stellate cells exacerbates liver fibrosis in nonalcoholic steatohepatitis in mice. Hepatol. Commun. 3, 1098–1112. https://doi.org/10.1002/hep4.1383 (2019).
Pardina, E. et al. Lipoprotein lipase expression in livers of morbidly obese patients could be responsible for liver steatosis. Obes. Surg. 19, 608–616. https://doi.org/10.1007/s11695-009-9827-5 (2009).
Perla, F., Prelati, M., Lavorato, M., Visicchio, D. & Anania, C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children 4, 46. https://doi.org/10.3390/children4060046 (2017).
Wang, H. et al. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol. 8, 1–14. https://doi.org/10.3389/fmicb.2017.01073 (2017).
Karimi, G. et al. Single-species versus dual-species probiotic supplementation as an emerging therapeutic for obesity. Nutr. Metab. Cardiovasc. Dis. 27, 910–918. https://doi.org/10.1016/j.numecd.2017.06.020 (2017).
Rozovski, U. et al. Aberrant LPL expression, driven by STAT3, mediates free fatty acid metabolism in CLL cells. Mol. Cancer Res. 13, 944–953. https://doi.org/10.1158/1541-7786.MCR-14-0412 (2015).
Chen, P., Li, Y. & Xiao, L. Berberine ameliorates nonalcoholic fatty liver disease by decreasing the liver lipid content via reversing the abnormal expression of MTTP and LDLR. Exp. Ther. Med. 22, 1–8. https://doi.org/10.3892/etm.2021.10543 (2021).
Xin, P. et al. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem. Toxicol. 52, 12–18. https://doi.org/10.1016/j.fct.2012.10.026 (2013).
Song, M. et al. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J. Dairy Sci. 98, 1492–1501. https://doi.org/10.3168/jds.2014-8586 (2015).
Palaniyandi, S. A., Damodharan, K., Suh, J. W. & Yang, S. H. Probiotic characterization of cholesterol-lowering lactobacillus fermentum MJM60397. Probiotics Antimicrob. Proteins 12, 1161–1172. https://doi.org/10.1007/s12602-019-09585-y (2020).
Tamtaji, O. R. et al. Probiotic and selenium co- supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 38, 2569–2575. https://doi.org/10.1016/j.clnu.2018.11.034 (2019).
Miksztowicz, V. et al. Hepatic lipase activity is increased in non- alcoholic fatty liver disease beyond insulin resistance. Diabetes. Metab. Res. Rev. 28, 535–541. https://doi.org/10.1002/dmrr.2312 (2012).
Yang, Y. et al. Fatty liver and alteration of the gut microbiome induced by diallyl disulfide. Int. J. Mol. Med. 44, 1908–1920. https://doi.org/10.3892/ijmm.2019.4350 (2019).
Jouybari, M. G., Pour, V. R., Mohammad, M. & Nagharchi, Z. The effect of novel probiotic on blood parameters and performance in broiler chickens. J. Cell Anim. Biol. 3, 141–144. https://doi.org/10.5897/JCAB.9000151 (2009).
Lucero, D. et al. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome?. Clin. Chim. Acta 412, 587–592. https://doi.org/10.1016/j.cca.2010.12.012 (2011).
Lottenberg, A. M. P. et al. Plasma cholesteryl ester synthesis, cholesteryl ester transfer protein concentration and activity in hypercholesterolemic women: Effects of the degree of saturation of dietary fatty acids in the fasting and postprandial states. Atherosclerosis 126, 265–275. https://doi.org/10.1016/0021-9150(96)05914-X (1996).
Blauw, L. L. et al. Metabolic liver inflammation in obesity does not robustly decrease hepatic and circulating CETP. Atherosclerosis 275, 149–155. https://doi.org/10.1016/j.atherosclerosis.2018.06.004 (2018).
Barter, P. J. The causes and consequences of low levels of high density lipoproteins in patients with diabetes. Diabetes Metab. J. 35, 101. https://doi.org/10.4093/dmj.2011.35.2.101 (2011).
Sanyal, D. et al. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J. Endocrinol. Metab. 19, 597. https://doi.org/10.4103/2230-8210.163172 (2015).
Taylor, E. et al. MRI of atherosclerosis and fatty liver disease in cholesterol fed rabbits. J. Transl. Med. 16, 1–11. https://doi.org/10.1186/s12967-018-1587-3 (2018).
Kainuma, M. et al. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. J. Gastroenterol. 41, 971–980. https://doi.org/10.1007/s00535-006-1883-1 (2006).
Motawi, T. K., Hamed, M. A., Shabana, M. H., Hashem, R. M. & Aboul Naser, A. F. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutr. Metab. 8, 40. https://doi.org/10.1186/1743-7075-8-40 (2011).
Adesiji, Y., Owolabi, S., Ayelagbe, O. G. & Olowe, A. Effects of lactobacillus acidophilus on biochemical indices and liver histology in streptozotocin-induced diabetic rats. J. Clin. Diagnostic Res. https://doi.org/10.7860/jcdr/2019/40762.13001 (2019).
Li, H. et al. Lactobacillus plantarum KLDS1.0344 and lactobacillus acidophilus KLDS1.0901 Mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways. J. Agric. Food Chem. 69, 183–197. https://doi.org/10.1021/acs.jafc.0c06346 (2021).
Al-Qayim, M. A. J. Effects of probiotics (Lactobacillus acidophilus) on liver functions in experimental colitis in rats. Iraqi J. Vet. Med. 38, 48–54. https://doi.org/10.30539/iraqijvm.v38i2.223 (2014).
Sigrist-Flores, S. C. et al. Chronic intake of moderate fat-enriched diet induces fatty liver and low-grade inflammation without obesity in rabbits. Chem. Biol. Interact. 300, 56–62. https://doi.org/10.1016/j.cbi.2019.01.004 (2019).
Xing, Y. W., Wu, Q. H., Jiang, Y., Huang, M. X. & Lei, G. T. Procyanidin B2 protects against diet-induced obesity and nonalcoholic fatty liver disease via the modulation of the gut microbiota in rabbits. World J. Gastroenterol. 25, 955–966. https://doi.org/10.3748/wjg.v25.i8.955 (2019).
Lee, M. et al. Recombinant Lactococcus lactis expressing Ling Zhi 8 protein ameliorates nonalcoholic fatty liver and early atherogenesis in cholesterol-fed rabbits. Biomed Res. Int. https://doi.org/10.1155/2020/3495682 (2020).
Mazloom, Z., Yousefinejad, A. & Dabbaghmanesh, M. H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 38, 38–43 (2013).
Lee, N. Y. et al. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin. Mol. Hepatol. 27(1), 110 (2021).
Kullisaar, T., Zilmer, K., Salum, T., Rehema, A. & Zilmer, M. The use of probiotic L. fermentum ME-3 containing Reg’Activ Cholesterol supplement for 4 weeks has a positive influence on blood lipoprotein profiles and inflammatory cytokines: An open-label preliminary study. Nutr. J. 15, 1–6. https://doi.org/10.1186/s12937-016-0213-6 (2016).
Jang, H. R. et al. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 51(8), 1–14 (2019).
Park, S. S. et al. Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J. Endocrinol. 237(2), 87–100 (2018).
Paul, A. et al. Nonalcoholic fatty liver disease and serum lipoproteins : The multi-ethnic study of atherosclerosis. Atherosclerosis 227, 429–436. https://doi.org/10.1016/j.atherosclerosis.2013.01.022 (2013).
Briseño-Bass, P., Chávez-Pérez, R. & López-Zendejas, M. Prevalence of hepatic steatosis and its relation to liver function tests and lipid profile in patients at medical check-up. Rev. Gastroenterol. México 84, 290–295. https://doi.org/10.1016/j.rgmxen.2018.05.024 (2019).
Begley, M., Hill, C. & Gahan, C. G. M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72, 1729–1738. https://doi.org/10.1128/AEM.72.3.1729-1738.2006 (2006).
Mikolasevic, I. et al. Nonalcoholic fatty liver disease (NAFLD) – a new factor that interplays between inflammation, malnutrition, and atherosclerosis in elderly hemodialysis patients. Clin. Interv. Aging 9, 1295. https://doi.org/10.2147/CIA.S65382 (2014).
Grgurevic, I. et al. Natural history of nonalcoholic fatty liver disease: implications for clinical practice and an individualized approach. Can. J. Gastroenterol. Hepatol. 2020, 5–7. https://doi.org/10.1155/2020/9181368 (2020).
Kawaguchi, K. et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 100, e26835. https://doi.org/10.1097/MD.0000000000026835 (2021).
Cho, J.-H., Namgung, J.-S., Lee, J., Moon, D.-H. & Lee, H.-K. Analysis of biochemical markers related to fatty liver patients. J. Phys. Ther. Sci. 26, 1865–1868. https://doi.org/10.1589/jpts.26.1865 (2014).
Ayyat, M. S., Al-Sagheer, A. A., Abd El-Latif, K. M. & Khalil, B. A. Organic selenium, probiotics, and prebiotics effects on growth, blood biochemistry, and carcass traits of growing rabbits during summer and winter seasons. Biol. Trace Elem. Res. 186, 162–173. https://doi.org/10.1007/s12011-018-1293-2 (2018).
Adriani, L. et al. Improving blood protein and albumin level using dried probiotic yogurt in broiler chicken. Jordan J. Biol. Sci. 14, 1021–1024. https://doi.org/10.54319/jjbs/140521 (2021).
Alkhalf, A., Alhaj, M. & Al-Homidan, I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 17, 219–225. https://doi.org/10.1016/j.sjbs.2010.04.005 (2010).
Diba, F. S., Hossain, K. M., Azim, M. A. & Moinul Hoque, M. D. Isolation, characterization and determination of antimicrobial properties of lactic acid bacteria from human milk. Jordan J. Biol. Sci. 6, 111–116. https://doi.org/10.12816/0000268 (2013).
Andreasen, A. S. et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 104, 1831–1838. https://doi.org/10.1017/S0007114510002874 (2010).
Duan, F. F., Liu, J. H. & March, J. C. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 64, 1794–1803. https://doi.org/10.2337/db14-0635 (2015).
Jain, N. K. & Singhai, A. K. Protective effects of Phyllanthus acidus (L.) Skeels leaf extracts on acetaminophen and thioacetamide induced hepatic injuries in Wistar rats. Asian Pac. J. Trop. Med. 4, 470–474. https://doi.org/10.1016/S1995-7645(11)60128-4 (2011).
Mutlu, M., Irmak, E., Aslan, Y. & Kader, Ş. Effects of lactobacillus rhamnosus gg as a probiotic on neonatal hyperbilirubinemia. Turk. J. Pediatr. 60, 482. https://doi.org/10.24953/turkjped.2018.05.003 (2018).
Videla, L. A. et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 106, 261–268. https://doi.org/10.1042/CS20030285 (2004).
Amdekar, S. & Singh, V. Lactobacillus acidophilus maintained oxidative stress from reproductive organs in collagen-induced arthritic rats. J. Hum. Reprod. Sci. 9, 41–46. https://doi.org/10.4103/0974-1208.178638 (2016).
Dowarah, R., Verma, A. K., Agarwal, N., Singh, P. & Singh, B. R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 13, e0192978. https://doi.org/10.1371/journal.pone.0192978 (2018).
Negi, C. K., Babica, P., Bajard, L., Bienertova-Vasku, J. & Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metab. 126, 154925 (2022).
Acknowledgements
The authors would like to extend their sincere appreciation to The Science, Technology and Innovation Funding Authority (STDF) and the Egyptian Knowledge Bank (EKB).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Investigation, Methodology, and Writing – original draft, F.E. and M.A.; Supervision, S.H.; revised and edited manuscript, G.M.A.; Writing – review and editing, S.E.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aziz, M., Hemeda, S.A., Albadrani, G.M. et al. Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits. Sci Rep 13, 6312 (2023). https://doi.org/10.1038/s41598-023-32584-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32584-7
This article is cited by
-
Effects of probiotics in patients with morbid obesity undergoing bariatric surgery: a systematic review and meta-analysis
International Journal of Obesity (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.