Abstract
The main objective of this study was to evaluate the effect of left anodal transcranial direct current stimulation (tDCS) on hypothalamic–pituitary–adrenal axis (HPAA) activity in individuals with depression. We conducted a 3-week, randomized, triple-blind pilot trial with 47 participants (dropout rate: 14.89%) randomly assigned to either the tDCS or control group (sham stimulation). Salivary cortisol was used as an HPAA activity marker since cortisol is the effector hormone of the HPAA. The primary outcome was the effect of tDCS on the diurnal cortisol pattern (DCP and area under the curve with respect to ground -AUCg-). Secondary outcomes included tDCS effects on cortisol awakening response (CAR) and cortisol decline (CD), as well as the variation of cortisol concentrations between the initiation of tDCS and 2 weeks later. Intention-to-treat and per-protocol analyses were conducted. Our primary outcome showed an absent effect of tDCS on DCP and AUCg. Additionally, tDCS had an absent effect on CAR, CD, and cortisol concentration variation before-after stimulation. Our pilot study suggests that anodal tDCS showed an absent effect on HPAA activity in individuals with depression. More studies are needed to confirm these findings.
Similar content being viewed by others
Introduction
Depression is a mood disorder characterized by permanent sadness, anhedonia, loss of perspective, and suicidal behavior1,2. The World Health Organization (WHO) estimates that approximately 300 million people worldwide suffer from depression, resulting in a global prevalence of 4.4%, with substantial costs to regional health systems and high disability-adjusted life years3.
Although numerous hypotheses have been proposed regarding the causes and pathophysiology of depression, they remain uncertain. However, recent studies have revealed a correlation between depressive episodes and inflammation4 or high hypothalamic–pituitary–adrenal axis (HPAA) activity, which is manifested by altered diurnal cortisol patterns5,6,7, elevated morning salivary cortisol8,9, and increased cortisol awakening response10 during depression. Additionally, research has shown that pharmacological treatment leads to a reduction in HPAA activity11, with the modulatory effect on cortisol metabolism being a critical component for the treatment of depression.
International guidelines for treating depressive episodes typically recommend antidepressant agents combined with psychotherapeutic treatment12,13. However, about 15–30% of patients with depression do not respond to guideline-recommended therapies14. Novel augmentation treatments, such as non-invasive procedures like transcranial direct current stimulation (tDCS)15,16,17,18, have been developed in the last three decades to address non-response19,20.
The evidence linking tDCS and depression is growing, but there is a lack of research on the effect of tDCS on HPAA activity in depression, as depression itself can alter HPAA activity21,22,23. Additionally, no studies have evaluated the effect of tDCS on cortisol levels in depressed patients, despite evidence that other stimulation procedures (e.g., electroconvulsive therapy)24,25 and pharmacological agents (e.g., mirtazapine and tricyclic antidepressants)11,26 have been linked to reductions in cortisol levels and improved clinical outcomes in depressive episodes. Given the critical role of the prefrontal cortex (PFC) in regulating the HPAA stress response27, the hypoactivity of the PFC and the dysregulation of the HPAA observed in mood disorders, stimulation of the PFC through tDCS may increase its activity and regulate HPAA and cortisol metabolism28.
Therefore, the main objective of our randomized, triple-blind pilot trial was to assess the effect of tDCS on HPAA activity in depressed participants by evaluating salivary cortisol levels. We hypothesized that the tDCS would be an effective treatment for depression and reduce salivary cortisol levels in participants with depression.
Materials and methods
Study design
This randomized, triple-blind pilot trial was conducted at the Central Institute of Mental Health (Mannheim, Germany). The pilot trial aimed to compare the effect of tDCS on HPAA activity in patients with depressive episodes, and included an experimental group (tDCS) and a control group (sham stimulation). Diurnal cortisol pattern (DCP), area under the curve with respect to the ground (AUCg) of the diurnal cortisol pattern, cortisol awakening response (CAR), cortisol decline (CD), and variation of cortisol before and after stimulation were calculated in both groups. Pharmacological and psychotherapeutic treatments were continued throughout the trial. The trial schedule and measurements are presented in Table 1. This study is registered in the German Clinical Trials Register (DRKS; register Number ID DRKS00029994; registration date: 16/08/2022).
Randomization and blinding
Study participants, who were patients of an inpatient affective disorders’ unit, were randomly assigned in a 1:1 ratio to either an experimental group receiving tDCS or a control group receiving sham stimulation. To mitigate potential bias in the results, especially experimental bias, each patient was assigned to one of the four stimulation devices (A, B, C, and D) based on the order of their inclusion in the study. Two of the four devices were tDCS (A and C), and the remaining two were sham stimulation devices (B and D). To ensure compliance with the quality criterion of reliability, all stimulation treatments were carried out by the same practitioner (H.S.) using the same devices, in the same rooms, and always on weekdays (Monday–Friday) between 2:00 and 4:00 PM. The same trial physician always collected the assessment questionnaires. During the trial, neither the patients, the practitioner, nor the trial physician were informed about the devices’ mode (sham stimulation or tDCS) or whether the treatment was real or simulated. This blinding status was maintained throughout the evaluation of the raw data, and disclosure only took place at the beginning of the final data analysis to further reduce possible experimental bias.
Participants
Initially, between August 15th, 2016 and September 20th, 2017, 47 patients (20 female and 27 male participants; mean age: 45.30 ± 14.20) with depressive disorders were recruited from the inpatient unit “affective disorders” at the Central Institute of Mental Health, Mannheim for this pilot trial. The characteristics of the participants who completed the trial are presented in Table 2. To be included in the study, participants had to meet the DSM-5 criteria for single or recurrent major depressive episode, as evaluated through SCID-I interviews, and sign the informed consent form. The interviews were conducted by clinical experts at the Central Institute of Mental Health who were not involved in the study. Patients were included if they had a Hamilton Depression Rating Scale (HDRS) score of at least 18 points with baseline stability (i.e., less than 25% improvement 1 week before the beginning of stimulation). Before starting the study, we ensured that participants had an ECG sinus rhythm. Additionally, this pilot trial did not require a therapy-naïve status, so participants could continue their prescribed treatments at the inpatient unit. We chose a three-week trial interval (21 days) to avoid the occurrence of essential changes in the pharmacological treatment. Finally, participants taking benzodiazepines as a pro re nata (PRN) treatment could participate with doses no greater than 1.5 mg/days of Lorazepam or equivalent to minimize additional pharmacological effects. Participants taking hypnotics, such as zopiclone and zolpidem, as PRN treatments were not excluded from this pilot trial.
Patients with psychotic disorders, post-traumatic stress disorders, panic disorders, and borderline personality disorders were excluded from the study. We also excluded pregnant female participants, participants with a conservatorship or legal guardianship, and patients who were unable to provide consent due to severe mental illness. Furthermore, patients who met the following criteria were excluded: those with metal implants or medical devices (e.g., cardiac pacemakers), those with illegal drug and alcohol dependency at the time of the study, those experiencing acute suicidal crisis, those with medical conditions that alter adrenal functions, those taking glucocorticoids or medications that alter heart rate and variability (such as beta-blockers), those with any type of arrhythmia, those meeting DSM-5 criteria for dementia syndrome, those with a past medical history of severe cranioencephalic trauma, those with any neurological disease, those with severe decompensated medical conditions (such as therapy-resistant arterial hypertension, heart insufficiency, or respiratory insufficiency), and those with neoplastic or infectious diseases of any kind.
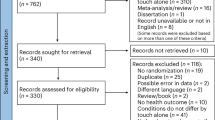
Of the 47 patients initially included in the study, 40 completed the three-week treatment, resulting in a dropout rate of 14.89%. One 20-year-old participant was interrupted on the third day of stimulation due to a newly identified skin rash on the scalp, and a 78-year-old participant was discharged from the inpatient ward, interrupting the trial on the seventh day. Three participants (ages 34, 48, and 53) were terminated from the trial before the first stimulation appointment due to new diagnostic assessments, resulting finally in exclusion. Additionally, one participant (age 38) left the study before the first stimulation appointment due to the onset of a manic phase. Lastly, one participant (age 27) withdrew from the pilot study due to post-stimulation headaches.
Regarding medication, all 40 participants (35 participants with unipolar depression and 5 participants with bipolar depression) who completed the stimulation trial received direct current stimulation as an add-on therapy to guideline-compliant pharmacotherapy for depression. Among them, seven received monotherapy with an antidepressant agent (SSRI, SNRI, bupropion, and trazodone), 14 were treated with two antidepressants (SSRI + mirtazapine, SSRI + trazodone, bupropion + trazodone), and one with a combination of three antidepressants (bupropion + mirtazapine + trazodone). Six patients were prescribed an antidepressant plus augmentation therapy (such as aripiprazole, quetiapine, or lithium), and five received a combination of two antidepressants and augmentation therapy. One patient received a quadruple combination of three antidepressants and quetiapine, and two received a triple combination of lithium, quetiapine, and an antidepressant. Two patients received quetiapine monotherapy, and two received dual therapy with lithium and quetiapine. There were no significant differences between the two groups concerning pharmacotherapy (Cramer’s V = 0.70, p = 0.42).
Transcranial direct current stimulation (tDCS)
Stimulation was administered using a CE-certified microprocessor-controlled device (Sooma tDCS, Sooma Medical, Helsinki, Finland) that emitted a direct current (DC) with a maximum output of 2 mA. A pair of conductive rubber square electrodes (anode and cathode) with dimensions of 5 × 7 cm (35 cm2; ELM2 Electrodes, Sooma Medical, Sooma Oy, Helsinki, Finland) were used to apply DC. Prior to electrode placement, tDCS electrode sponges (50 × 70 mm; SPM200, Sooma Medical, Sooma Oy, Helsinki, Finland) were soaked in a 144 mol/L NaCl solution to lower the physiological skin resistance. The electrodes were placed based on the 10–20 international system, with the anode positioned at EEG point F3 (left dorsolateral PFC) and the cathode at EEG point Fp2 (right dorsolateral PFC). Correct placement was facilitated by a hood (Large/Medium/Small Flex cap, Sooma Medical, Sooma Oy, Helsinki, Finland) and chin strap (HCHI, Sooma Medical, Sooma Oy, Helsinki, Finland), adapted to the head of the participant. The cap was pulled taut with the edge ending approximately 1.5–2 cm above the eyebrows and positioned correctly. The chin strap was then secured in place.
The four stimulation devices (A, B, C, and D) were preprogrammed to provide either real stimulation (devices A and C, with a DC of 2.00 mA) or sham stimulation (devices B and D, with a DC of 0.30 mA). In the sham stimulation mode, the devices initially provided DC of 2.00 mA, immediately ramping down to a DC of 0.30 mA and maintaining this current for 30 min (treatment duration), thereby producing sensations similar to real stimulation.
Stimulation was carried out according to the manufacturer’s protocol, which entailed three weeks of treatment with daily 30-min stimulation sessions for 15 sessions. This protocol was used irrespective of the device mode (real or sham stimulation). In our trial, participants received tDCS or sham stimulation until the end of week 3, from Monday to Friday, between 2:00 and 4:00 PM, with stimulation pauses at weekends.
Cortisol assessment
General procedure
The activity of the HPAA was assessed by measuring cortisol in saliva samples. Salivary cortisol was preferred due to its practicability in sample collection, which is less invasive and requires less effort from participant compared to other methods9,29. Studies have demonstrated a good correlation between salivary and plasma cortisol30,31, verifying its effectiveness for assessing plasma cortisol concentration. Salivary cortisol measurements were acquired as reliable indicators of total free plasma cortisol, albeit with marginally lower concentrations due to the presence of 11β-HSD2 in saliva32.
Diurnal cortisol data were obtained using Salivette® tubes (Sarstedt™, Leicester) containing an untreated cotton swab. Four saliva samples were collected from participants on a typical ward routine day. The participants were all inpatients on the same ward with identical routines. Participants were instructed to chew on the saliva collectors after awakening (Cor I), 30 min after getting up (Cor II), 8 h after awakening (Cor III), and 14 h after awakening (Cor IV), while still in bed. Additional instructions were provided by ward health workers (i.e., nurses, postgraduate trainees or interns) regarding precautions related to meals, drinks, teeth brushing, and smoking. The ward health workers also recorded the date and time of sampling and placed the tubes with saliva samples in the refrigerator. All samples were stored at – 25 °C, and after thawing, they were centrifuged for 5 min at 3000 rpm, resulting in a clear supernatant of low viscosity. Salivary cortisol levels were measured using a time-resolved immunoassay with fluorescence detection33,34. The inter- and intra-assay coefficients of variation were less than 10% across the expected range of cortisol levels, and the lower limit of detection was 0.43 nmol/L. The concentrations were calculated in nmol/L33,34.
Saliva samples for DCP, AUCg, cortisol awakening response, cortisol decline, and stimulation-related cortisol levels
Two sets of DCP samples were collected during the study. Participants were instructed to collect saliva four times during the day before the first stimulation (baseline) and at the end of the third week (W3)35. Additionally, the area under the curve with respect to the ground (AUCg) was calculated based on the DCP data using the formulae of Pruessner et al.36. AUCg reflects the total cortisol concentration over the course of a day, as measured by the area under the diurnal cortisol curve using a trapezoidal formula.
Based on samples Cor I and Cor II, the cortisol awakening response (CAR) was calculated from the difference between the two samples (i.e., CAR = Cor II−Cor I). Additionally, the cortisol decline (CD) was calculated from the difference between the first morning cortisol value (i.e., sample Cor I) and the last value (i.e., sample Cor IV). Finally, salivary cortisol was collected "immediately before stimulation" (pre), "immediately after stimulation" (post), and "2 h after stimulation" (2 h-post) in W0 and W2 (Table 1) to record the short-term effects of tDCS on HPAA activity.
It is important to note that the examination of diurnal cortisol parameters (i.e., CAR, CD and DCP) has been established as an important aspect in both clinical and epidemiological research37. In addition, recent studies highlight the significance of diurnal salivary cortisol profiles in understanding adrenocortical functioning and regulation of the HPAA. The relevance of diurnal cortisol parameters, including CAR, CD, and DCP, is also due to the fact that cortisol is a hormone that displays a circadian rhythm and varies throughout the day, and the level of salivary cortisol changes depending on the time of day of the analysis37.
Implausible morning cortisol values (i.e., values of samples Cor I or Cor II ≤ 3 nmol/L) were replaced with half of the minimum morning cortisol value (in this case, 1.50 nmol/L), as recommended elsewhere38. In our case, five replacement procedures for four participants were required, with four participants having a Cor I value less than or equal to 3 nmol/L and being replaced with a value of 1.50 nmol/L. One participant had both Cor I and Cor II values below 3 nmol/L, and their data were also replaced with a value of 1.50 nmol/L.
Outcomes
As the main objective of this pilot trial was to assess the effect of tDCS on HPAA activity in depressed participants by evaluating salivary cortisol levels, we defined our primary outcome as the detection of any changes in the DCP from the baseline to W3 in the tDCS group compared to the control group, including also changes in AUCg for salivary cortisol. Moreover, our secondary outcomes comprised changes in CAR and CD from baseline to W3, as well as changes in cortisol levels associated to stimulation between W0 and W2.
We also examined any variations in the Montgomery-Asberg Depression Rating Scale (MADRS) scores throughout the trial period for both groups. Following the recommendation of previous studies39, we defined clinical response as a reduction of ≥ 50% in the MADRS total values compared to the initial value, and remission as a final score of ≤ 9 points. Finally, also as described elsewhere40, we defined partial response if the MADRS total values showed a reduction of ≥ 25% of the initial value.
Statistical analysis
Numeric variables that followed a gaussian distribution are presented as mean (standard deviation), while those with a non-gaussian distribution (i.e., median ≠ mean) are shown as median (interquartile range, 3rd quantile–1st quantile). Category variables and count data are specified as numbers or fractions, with values rounded to two decimals. Values smaller than 0.005 are presented as < 0.005, and values greater than 1 million are expressed in scientific notation4. Descriptive data are presented in tables. For significance testing, t-tests were used for continuous gaussian distributed data, while the U-test for continuous non-gaussian distributed data. The χ2 or the Cramer’s V for the category and/or count data. Statistical significance was defined as a two-tail-p-value of less than or equal to 0.05.
Primary and secondary outcomes, as well as MADRS scores, were analyzed blindly by one of the authors (B.P.P) who did not know the group assignments. Both intention-to-treat (ITT) and per-protocol analyses (PP) were performed using linear mixed models (LMM) for the primary and secondary outcomes. We calculated the interaction time * daytime * group (DCP) and time * group (AUCg) for the primary outcome, and time * group (CAR and CD) and time * daytime * group (stimulation-related cortisol levels) for the secondary outcome.
Interactions were corrected for gender, age, and weight as confounding factors, with multiple imputations with linear interactions and a maximum of 1000 iterations in case of missing values. For both trial groups, CAR, DCP, AUCg, CD, and stimulation-related cortisol levels were estimated in the multiple imputations using the variables gender and age of the participants. In the LMM, fixed effect omnibus tests were carried out to define the main effects of the variables in the model and to evaluate the variable in the model against the null model. The ITT results were reported graphically, and both ITT and PP analyses’ results were described in the text using 95% confidence intervals (95CI). Cohen’s d was calculated for ITT and PP analyses to estimate the sample size effect. Post-hoc tests were performed for the LMM when differences between the time * group (CD, CAR, AUCg) and the time * daytime * group (DCP and stimulation-related cortisol levels) were significant.
Statistical analyses and descriptive data were conducted using IBM SPSS Statistics software, version 26.0 (International Business Machines Corporation, New York, United States of America). LMM analyses were performed using the R-based software jamovi 2.0.041 and the GAMLj toolbox42. Graphs were generated using Prism 8 GraphPad software (GraphPad Software Inc., California, United States of America).
Ethics approval and consent to participate
Each participant was fully informed about the objectives and procedures of the study, as well as the potential adverse effects, and gave their written consent to participate. The study protocol and all study procedures were reviewed and approved by the ethics committee of the Medical Faculty Mannheim of the University of Heidelberg. Additionally, this pilot trial was carried out according to the Helsinki Declaration.
Results
MADRS scores during the trial
The ITT analysis for MADRS scores revealed a significant decrease in the total MADRS score of 10.68 points (34.26% reduction from baseline scores) after 3 weeks, regardless of the trial group (95CI [− 12.92; − 8.44]; Cohen’s d = − 1.65). Although reductions in MADRS scores occurred in both groups, the ITT analysis showed absent effects for the interaction time * group (percentage of responders in sham stimulation group: 34.78%, percentage of responders in the tDCS group: 29.17%; Fig. 1). Interestingly, all four remitters in the study belonged to the placebo group. Finally, the PP analysis also showed absent effects for the interaction time * group but significant changes in the MADRS values during the trial time.
Adverse effects
Adverse effects resulting from tDCS or sham stimulation were recorded using a self-assessment questionnaire at W0. These adverse effects included itching, tingling, burning, cutaneous rash, pain (χ21,40 = 0.83, p = 0.362), concentration deficit (absent in all participants), acute mood changes (χ21,40 = 0.17, p = 0.679), and visual perception disorders (χ21,40 = 0.17, p = 0.679). A statistically significant difference between the two stimulation groups was only observed for the side effect of tingling (χ21,40 = 4.96, p = 0.026). While only 6 of 13 participants in the sham group reported tingling as a side effect, this side effect occurred in 15 of the 21 participants in the tDCS group. The side effects of itching and burning were also reported more frequently by the tDCS group; however, the differences between the two groups concerning itching (χ21,40 = 0.47, p = 0.492) and burning (χ21,40 = 0.59, p = 0.444) were not significant. Four patients treated with tDCS and one with sham stimulation reported a cutaneous rash after stimulation, but differences between the groups were not significant (χ21,40 = 1.43, p = 0.233). Finally, no occurrence of a manic clinical condition was observed in any test person, either under sham stimulation or under tDCS.
Primary outcome
The ITT analysis showed a decrease in salivary cortisol concentration during the day, regardless of the trial group (tDCS and sham stimulation) or stimulation protocol time (baseline and W3), with a decrease of 7.92 nmol/L cortisol during the course of the day (95CI [− 10.73; − 5.12]; Cohen’s d = − 0.64). Cortisol concentrations regarding diurnal cortisol patterns are represented in Table 3. However, we found an absence of effects for group (tDCS and sham stimulation), the interaction daytime * time (baseline and W3), or the interaction group * daytime * time (estimate = − 5.57, 95CI [− 16.50; 5.67]; Cohen’s d = − 0.11; Fig. 2). Additionally, for AUCg, the ITT analysis showed an absence of effect for group * time (estimate = 24.06, 95CI [− 34.23; 82.65]; Cohen’s d = 0.24).
In the PP analysis, we observed a decrease in salivary cortisol concentration during the day, with a decrease of 8.85 nmol/L cortisol during the course of the day (95CI [− 11.72; − 5.98]; Cohen’s d = − 0.72). Similar to the ITT analysis, we found an absence of effects for the interactions group * daytime * time and daytime * time. Furthermore, for AUCg, the PP analysis showed an absence of effect for group * time.
Secondary outcomes
In the ITT analysis of secondary outcomes, there were absent effects for the interaction group * time and CAR (estimate = 0.14, 95CI [− 5.16; 5.44]; Cohen’s d = 0.01; Fig. 3B). Similarly, CD values also showed absent effects in the ITT analysis for the interaction group * time (estimate = 1.63, 95CI [− 4.86; 8.11]; Cohen’s d = 0.15; Fig. 3C). Finally, we performed an ITT analysis for the stimulation-related cortisol levels, taking into account the trial time (i.e., for W0 and W2) and the stimulation time (pre, post, and 2 h-post). However, the ITT analysis showed absent effects for the interaction group * stimulation time * trial time (estimate = − 2.33, 95CI [− 5.83; 1.18]; Cohen’s d = − 0.17 Fig. 3A). Table 3 represents cortisol concentrations related to CAR, CD, and stimulation.
Secondary outcomes; estimated marginal means (cortisol concentration in nmol/L) were calculated in each model. (A) Presents the stimulation-related cortisol concentrations for tDCS and sham stimulation groups. Here, we present cortisol concentrations immediately before stimulation (A), immediately after stimulation (B), and two hours after stimulation (C) for W0 and W2 (Table 3). (B) Presents the cortisol awakening response (CAR) of both tDCS and sham stimulation groups for baseline and W3. Finally, (C) presents cortisol decline for both tDCS and sham stimulation groups for baseline and W3.
In the PP analysis for CAR and CD, there were also absent effects for the interaction group * time. Additionally, the PP analysis of stimulation-related cortisol concentrations showed absent effects for the interaction group * stimulation time * trial time.
Discussion
To our knowledge, this pilot study is the first to report results regarding the effects of tDCS on HPAA activity in patients with depression43. Regarding our primary outcome, our main findings indicate that tDCS has an absent effect on DCP in depression and absent effects in diurnal cortisol patterns (AUCg). In particular, the interaction group * daytime * trial time showed absent effects for the tDCS group.
Currently, there are no clinical studies evaluating the effects of tDCS on HPAA activity in participants with depression, and the current evidence regarding tDCS and HPAA activity is inconclusive. Previous studies with healthy humans have reported negative results regarding tDCS and HPAA activity44,45. In addition, two studies with participants with Sjogren’s disease46 and with osteoarthritis of the knee47 found no significant effects of tDCS on HPAA activity. However, previous literature, such as the review by Castelo-Branco and Fregni, based on two studies with left anodal neurostimulation in healthy subjects28,48, suggested that the neurostimulation of the left dorsolateral PFC (DLPFC) can regulate cortisol concentrations and cause changes in HPAA activity49. Finally, a systematic review by Vignaud and colleagues reported that a single stimulation of the DLPFC, independent of the stimulation procedure (i.e., repetitive transcranial magnetic stimulation -rTMS- or tDCS), is the most appropriate method to reduce cortisol concentrations in acute stress situations in healthy subjects50.
Despite anodal stimulation of the left DLPFC being part of our study protocol, we observed absent effects of tDCS on HPAA activity in participants with depression, in contrast to other stimulation procedures like repetitive transcranial magnetic stimulation (rTMS), which showed effects in cortisol concentration28,51. This suggests that tDCS may lack the necessary effects to modify HPAA activity in depression, unlike rTMS. However, given the small sample size and the pilot nature of this study, further research is needed to confirm these findings.
Regarding the secondary outcomes of CAR and CD, we found absent effects of tDCS on awakening response and decline in participants with depression. To the best our knowledge, this pilot study is the first to report on the effects of tDCS on CAR and CD in depression43,52. Our results suggest that tDCS, similar to the DCP, has an absent effect for inducing changes in these variables. Other evidence has also shown negative results for the effect of tDCS on CAR in healthy subjects27. In contrast, other stimulation procedures, such as rTMS53, have demonstrated significant changes in the CAR of depressive patients, which suggests that, like DCP, tDCS lacks effects to change the awaking response in people with depression. Nevertheless, larger studies are needed to confirm this finding. Although CAR is dependent on other variables, such as age, gender, and BMI27,54,55, our results were adjusted for these potential confounding factors in both analyses, reducing the likelihood of their interaction in the analysis. Lastly, as tDCS showed absent effects in the DCP, it is expected that the tDCS did not modify the CD, which is obtained by subtracting the last value from the second value of the diurnal cortisol pattern.
In terms of the stimulation-related cortisol levels, tDCS showed absent effects for the interaction group * trial time * stimulation time in patients with depression. Comparable negative results were also reported in a study involving healthy subjects, which revealed that tDCS did not change the cortisol concentrations associated with stimulation27. However, the tDCS group in our pilot trial exhibited increased cortisol concentrations (Fig. 3A). One possible explanation for the increase in cortisol levels could be the differences in adverse effects (i.e., tingling) between tDCS and sham stimulation, as well as participants’ perceived excitement levels before and during the stimulation.
As for therapy response (MADRS total scores), we noted a reduction in the MADRS score during the trial, regardless of the group assignment. This implies the effectiveness of the pharmacological treatment, while the add-on stimulation treatment showed absent effects on the MADRS scores. One possible interpretation for the lack of differences between the two stimulation procedures is that the sham stimulation could be viewed as an “active placebo”. Some studies have suggested that even lower doses may have therapeutic efficacy, as seen in the case of 0.3 mA dose applied in the sham tDCS56. Therefore, it cannot be entirely ruled out that the sham tDCS had therapeutic effects on patients with depressive episodes. Similar findings are reported elsewhere in the literature57. Finally, compared to other neurostimulation methods like rTMS, tDCS is considered a weak stimulation procedure at 1–2 mA. This may explain the lack of effects found between the study groups in this pilot trial.
This pilot trial is the first to report data on the effects of tDCS and HPAA in participants with depression. While the obtained data are negative results58, it is important to consider the limitations of this pilot trial. First, the initial sample size was small, with only 47 participants, and only 40 completed the trial. Larger studies would be necessary to make reliable conclusions concerning the effects of tDCS on HPAA activity in depression. Nonetheless, the scope of our study was consistent with that of other tDCS studies. Secondly, there was heterogeneity in the participants included in this pilot study. However, the two groups did not differ in demographic and clinical characteristics. Third, our study included patients with bipolar depression, as in similar studies where patients with unipolar and bipolar depression as the main diagnosis were included59,60. In our case, bipolar patients (n = 5) included in the study did not differ from the unipolar patients in terms of demographic or clinical characteristics. Fourth, we included participants receiving other therapy in parallel during the pilot trial. Concomitant antidepressant medication has been shown to potentially influence tDCS61,62 and HPAA activity11,63,64,65. However, in our study, the inclusion of the “psychopharmacological treatment” variable had an absent effect on the ITT and PP analysis results. Fifth, the timing of cortisol sampling is crucial to achieving a reliable reproduction of diurnal cortisol66. Another concern is the computation of cortisol indices (decline and AUCg), which were estimated using rough mathematical approximations due to the low number of daily measurements. Finally, the tDCS stimulation positioning was restricted in our study to the left anodal stimulation of the DLPFC, a common standardized protocol, but showed absent effects on the HPAA activity in depression.
In conclusion, this pilot trial showed an absent effect of a 3-week tDCS regime on the HPAA activity in depression. Future studies should consider a larger sample of possibly therapy-naïve participants and compare with other neurostimulation methods, like rTMS68. Moreover, exploring stimulation of other brain areas, such as the ventromedial PFC, which are linked to stress, may provide insights into the effect on HPAA activity in depression, as suggested by Carnevalli et al.27. Additionally, to address intraindividual variation in rhythmicity, future studies may consider collecting multiple daily measurements at various time points for more accurate reproduction of the diurnal cortisol profile and computed indices67. Finally, including a follow-up period in future studies could provide insight into any potential post-treatment effects (including adverse effects from repeated tDCS sessions) of tDCS on HPAA activity in depression, especially in larger studies.
Data availability
The data sets generated and analyzed during the study are not publicly accessible due to the applicable data protection law of the State of Baden-Württemberg but they are available from the corresponding author on justified request.
References
Friedlander, A. H. & Mahler, M. E. Major depressive disorder. J. Am. Dent. Assoc. 132, 629–638 (2001).
Fuchs, T. Psychopathology of Depression and Mania: Symptoms, Phenomena And Syndromes 10 (2022).
Geneva. World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates (2017).
Pedraz-Petrozzi, B., Neumann, E. & Sammer, G. Pro-inflammatory markers and fatigue in patients with depression: A case-control study. Sci. Rep. 10, 9494 (2020).
Adam, E. K. et al. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41 (2017).
Deuschle, M. et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J. Clin. Endocrinol. Metab. 82, 234–238 (1997).
Joseph, J. J. & Golden, S. H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus: Role of cortisol in stress, depression, and diabetes. Ann. N. Y. Acad. Sci. 1391, 20–34 (2017).
Bhagwagar, Z., Hafizi, S. & Cowen, P. J. Increased salivary cortisol after waking in depression. Psychopharmacology 182, 54–57 (2005).
Knorr, U., Vinberg, M., Kessing, L. V. & Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 35, 1275–1286 (2010).
Bhagwagar, Z., Hafizi, S. & Cowen, P. J. Increase in concentration of waking salivary cortisol in recovered patients with depression. Am. J. Psychiatry 160, 1890–1891 (2003).
Deuschle, M. et al. Antidepressive treatment with amitriptyline and paroxetine: Effects on saliva cortisol concentrations. J. Clin. Psychopharmacol. 23, 5 (2003).
Guideline Development Panel for the Treatment of Depressive Disorders. APA Clinical Practice Guideline for the Treatment of Depression Across Three Age Cohorts (2019). https://doi.org/10.1037/e505892019-001.
Bennabi, D. et al. Clinical guidelines for the management of depression with specific comorbid psychiatric conditions French recommendations from experts (the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental). BMC Psychiatry 19, 50 (2019).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry 2006, 13 (2006).
Meron, D., Hedger, N., Garner, M. & Baldwin, D. S. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci. Biobehav. Rev. 57, 46–62 (2015).
Carvalho, S. et al. Transcranial direct current stimulation as an add-on treatment to cognitive-behavior therapy in first episode drug-naïve major depression patients: The ESAP study protocol. Front. Psychiatry 11, 563058 (2020).
Bennabi, D. & Haffen, E. Transcranial direct current stimulation (tDCS): A promising treatment for major depressive disorder?. Brain Sci. 8, 81 (2018).
Herrera-Melendez, A.-L., Bajbouj, M. & Aust, S. Application of transcranial direct current stimulation in psychiatry. Neuropsychobiology 79, 372–383 (2020).
Lolas, F. Brain polarization: Behavioral and therapeutic effects. Biol. Psychiatry 12, 37–47 (1977).
Fregni, F., Boggio, P. S., Nitsche, M. & Pascual-Leone, A. Transcranial direct current stimulation. Br. J. Psychiatry 186, 446–447 (2005).
Varghese, B. The hypothalamic-pituitary-adrenal axis in major depressive disorder: A brief primer for primary care physicians. Prim. Care Compan. J. Clin. Psychiatry 3, 5 (2001).
Menke, A. Is the HPA axis as target for depression outdated, or is there a new hope?. Front. Psychiatry 10, 101 (2019).
Mikulska, J., Juszczyk, G., Gawrońska-Grzywacz, M. & Herbet, M. HPA Axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 11, 1298 (2021).
Burgese, D. F. & Bassitt, D. P. Variation of plasma cortisol levels in patients with depression after treatment with bilateral electroconvulsive therapy. Trends Psychiatry Psychother. 37, 27–36 (2015).
Yrondi, A. et al. Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain Stimulat. 11, 29–51 (2018).
Laakmann, G., Hennig, J., Baghai, T. & Schüle, C. Mirtazapine acutely inhibits salivary cortisol concentrations in depressed patients. Ann. N. Y. Acad. Sci. 1032, 279–282 (2004).
Carnevali, L., Pattini, E., Sgoifo, A. & Ottaviani, C. Effects of prefrontal transcranial direct current stimulation on autonomic and neuroendocrine responses to psychosocial stress in healthy humans. Stress 23, 26–36 (2020).
Brunoni, A. R. et al. Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology 38, 58–66 (2013).
Wüst, S., Federenko, I., Hellhammer, D. H. & Kirschbaum, C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 25, 707–720 (2000).
Kerlik, J. et al. Comparison of salivary cortisol and calculated free plasma cortisol during low-dose ACTH test in healthy subjects. Clin. Biochem. 43, 764–767 (2010).
Perogamvros, I., Keevil, B. G., Ray, D. W. & Trainer, P. J. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocrinol. Metab. 95, 4951–4958 (2010).
Kirschbaum, C. & Hellhammer, D. H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 19, 313–333 (1994).
Gilles, M. et al. Maternal hypothalamus-pituitary-adrenal (HPA) system activity and stress during pregnancy: Effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology 94, 152–161 (2018).
Deuschle, M. & Gilles, M. Hypercortisolemic depressed women: Lean but viscerally obese?. Neuroendocrinology 103, 263–268 (2016).
Miller, G. E., Chen, E. & Zhou, E. S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 133, 25–45 (2007).
Pruessner, J. C., Kirschbaum, C., Meinlschmid, G. & Hellhammer, D. H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28, 916–931 (2003).
Miller, R. et al. The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology 73, 16–23 (2016).
Vexler, A., Tao, G. & Chen, X. A toolkit for clinical statisticians to fix problems based on biomarker measurements subject to instrumental limitations: From repeated measurement techniques to a hybrid pooled-unpooled design. Methods Mol. Biol. Clifton NJ 1208, 439–460 (2015).
Trivedi, M. H. et al. Remission, response without remission, and nonresponse in major depressive disorder: Impact on functioning. Int. Clin. Psychopharmacol. 24, 133–138 (2009).
Rush, A. J. et al. Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology 31, 1841–1853 (2006).
Love, J. et al. The jamovi project. https://www.jamovi.org (2020).
Gallucci, M. GAMLJ—General Analyses for Linear Models https://www.jamovi.org/library.html (2019).
Perrin, A. J. & Pariante, C. M. Endocrine and immune effects of non-convulsive neurostimulation in depression: A systematic review. Brain. Behav. Immun. 87, 910–920 (2020).
Raimundo, R. J. S., Uribe, C. E. & Brasil-Neto, J. P. Lack of clinically detectable acute changes on autonomic or thermoregulatory functions in healthy subjects after transcranial direct current stimulation (tDCS). Brain Stimul. 5, 196–200 (2012).
Kistenmacher, A. et al. Persistent blood glucose reduction upon repeated transcranial electric stimulation in men. Brain Stimulat. 10, 780–786 (2017).
Pinto, A. C. P. N. et al. Transcranial direct current stimulation for fatigue in patients with Sjogren’s syndrome: A randomized, double-blind pilot study. Brain Stimul. 14, 141–151 (2021).
Suchting, R., Colpo, G. D., Rocha, N. P. & Ahn, H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: A Bayesian residual change analysis. Biol. Res. Nurs. 22, 57–63 (2020).
Sarkar, A., Dowker, A. & Cohen-Kadosh, R. Cognitive enhancement or cognitive cost: Trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J. Neurosci. 34, 16605–16610 (2014).
Castelo-Branco, L. & Fregni, F. Home-based transcranial direct current stimulation (tDCS) to prevent and treat symptoms related to stress: A potential tool to remediate the behavioral consequences of the COVID-19 isolation measures?. Front. Integr. Neurosci. 14, 46 (2020).
Vignaud, P. et al. Can a single session of noninvasive brain stimulation applied over the prefrontal cortex prevent stress-induced cortisol release?. Prog. Neuropsychopharmacol. Biol. Psychiatry 121, 110667 (2023).
Baeken, C. et al. The impact of one HF-rTMS session on mood and salivary cortisol in treatment resistant unipolar melancholic depressed patients. J. Affect. Disord. 113, 100–108 (2009).
Sampaio, L. A. N. P. C., Fraguas, R., Lotufo, P. A., Benseñor, I. M. & Brunoni, A. R. A systematic review of non-invasive brain stimulation therapies and cardiovascular risk: Implications for the treatment of major depressive disorder. Front. Psychiatry 3, 87 (2012).
Clow, A. et al. Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress 17, 219–223 (2014).
Carnegie, R. et al. Cortisol awakening response and subsequent depression: Prospective longitudinal study. Br. J. Psychiatry 204, 137–143 (2014).
Steptoe, A. & Serwinski, B. Cortisol awakening response. In Stress: Concepts, Cognition, Emotion, and Behavior 277–283 (Elsevier, 2016). https://doi.org/10.1016/B978-0-12-800951-2.00034-0.
Loo, C. K. et al. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul. 11, 125–133 (2018).
Moirand, R. et al. Ten sessions of 30 Min tDCS over 5 days to achieve remission in depression: A randomized pilot study. J. Clin. Med. 11, 782 (2022).
Weintraub, P. G. The importance of publishing negative results. J. Insect Sci. 16, 109 (2016).
Dondé, C. et al. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 78, 123–131 (2017).
Brunoni, A. R. et al. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 96–101 (2011).
Brunoni, A. R. et al. The sertraline vs electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiat. 70, 383 (2013).
Nitsche, M. A., Müller-Dahlhaus, F., Paulus, W. & Ziemann, U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: Building models for the clinical use of CNS active drugs: Pharmacology of non-invasive brain stimulation. J. Physiol. 590, 4641–4662 (2012).
Heuser, I. J. et al. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am. J. Psychiatry 153, 93–99 (1996).
Schmid, D. A. et al. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 31, 832–844 (2006).
Schüle, C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J. Neuroendocrinol. 19, 213–226 (2007).
Kudielka, B. M., Broderick, J. E. & Kirschbaum, C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom. Med. 65, 313 (2003).
Hellhammer, J. et al. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology 32, 80–86 (2007).
Paulus, W. International conference on transcranial magnetic and direct current stimulation. Dtsch. Ärztebl. Int. (2009). https://doi.org/10.3238/arztebl.2009.0143.
Acknowledgements
The authors of this study would like to thank Ms. Anna Ziller and Laura Helger, who helped with the examination of participants and with use of the tDCS hood. Moreover, the study's authors would like to thank Ms. Svenja Bardtke, M. Psych., who assisted with the planning of the trial schedule. In addition, we would like to thank Ms. Susanne Laubender, who helped with the saliva sampling process. Moreover, we would like to thank Sooma Oy (Helsinki, Finland), who helped with the distribution of the tDCS and sham stimulation devices, as well as with financial support for the cortisol measurements in saliva. Finally, we would like to thank Mr. Martin Goldsworthy, MA, M.Sc., for his suggestions and improvements on English language.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported financially by Sooma Oy, Helsinki, Finland. Funding did not influence the study design, data collection, analysis, interpretation of the results, or any other aspect of the study. Employment: The authors of this study were not hired by any organization that may gain or lose financially through this publication. Personal financial interests: For this study, the authors did not receive any compensation (monetary, shares, etc.) from companies, consultation fees, or other forms of remuneration from organizations.
Author information
Authors and Affiliations
Contributions
B. P. P. Information: MD, Ph.D., Central Institute of Mental Health, Mannheim, Germany. E-Mail: Bruno.PedrazPetrozzi@zi-mannheim.de Contributions: B. P. P. wrote the introduction, performed the data analysis, contributed to the methods section, wrote the discussion section, and worked on all other parts of the manuscript preparation, including proofreading. H.S. Information: MD, Central Institute of Mental Health, Mannheim, Germany. E-Mail: Helena.sardinha@zi-mannheim.de Contributions: HS performed in the data collection, supervised the methods section of the manuscript, and was involved in preparing the discussion section and in proofreading of the manuscript. M. G. Information: MD, Medical Faculty Mannheim and Central Institute of Mental Health, Mannheim, Germany; research Co-group leader of the working group “Stress”. E-Mail: Maria.gilles@zi-mannheim.de Contributions: M. G. contributed to the organization of the study, with the participants’ recruitment and the methods, particularly with questions about the biological properties of salivary cortisol and an adequate statistical analysis. She participated in the preparation of the discussion section and in proofreading of the manuscript. M. D. Information: apl. Professor, MD, Medical Faculty Mannheim; Vice-chair of the Central Institute of Mental Health - Mannheim. Research group leader of the working group “Stress”. E-Mail: Michael.deuschle@zi-mannheim.de Contribution: M. D. developed the idea for the study, supervised the data collection, the methods and the analyses, and worked on proofreading of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pedraz-Petrozzi, B., Sardinha, H., Gilles, M. et al. Effects of left anodal transcranial direct current stimulation on hypothalamic–pituitary–adrenal axis activity in depression: a randomized controlled pilot trial. Sci Rep 13, 5619 (2023). https://doi.org/10.1038/s41598-023-32531-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32531-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.