Abstract
The transcription factor forkhead box P2 (FOXP2) is involved in the development of language and speech in humans. Two amino acid substitutions (T303N, N325S) occurred in the human FOXP2 after the divergence from the chimpanzee lineage. It has previously been shown that when they are introduced into the FOXP2 protein of mice they alter striatal synaptic plasticity by increasing long-term depression in medium spiny neurons. Here we introduce each of these amino acid substitutions individually into mice and analyze their effects in the striatum. We find that long-term depression in medium spiny neurons is increased in mice carrying only the T303N substitution to the same extent as in mice carrying both amino acid substitutions. In contrast, the N325S substitution has no discernable effects.
Similar content being viewed by others
Introduction
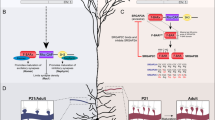
Humans heterozygous for pathogenetic variants of FOXP2 have difficulties in learning and performing complex orofacial movements, including those used for speech, as well as deficits in receptive and expressive language1,2,3. Although the transcription factor FOXP2 is thus involved in a trait unique to humans, only three out of the 715 amino acids in the FOXP2 protein differ between humans and mice, making it among the 5% most conserved proteins between the two species4. Remarkably, two of these amino acid substitutions (T303N and N325S) occurred on the human evolutionary lineage since its divergence from the chimpanzee lineage (Fig. 1). They have therefore been suggested to be of relevance for the evolution of speech and language4,5,6. Mice where these two human substitutions have been introduced into the endogenous Foxp2 gene (Foxp2hum mice) are generally healthy but show enhanced synaptic plasticity in the form of stronger long-term depression (LTD) in the medium spiny neurons (MSN) of the striatum, suggesting that cortico-basal ganglia circuits are affected7,8,9. However, it is unclear whether one or both of the two amino acid changes cause these effects.
To better understand the contributions of the two amino acid substitutions, we have generated one mouse line which carries only the threonine-to-asparagine substitution at position 302 (T302N; N303 in humans) and one mouse line which carries only the asparagine-to-serine at position 324 (N324S; S325 in humans) in their endogenous Foxp2 genes. Here, we analyze how these Foxp2 alleles (Foxp2T302N, Foxp2N324S) affect LTD in MSNs.
Results
Generation of Foxp2 mice
Mice carrying Foxp2 alleles encoding each of the two single amino acid substitutions (Foxp2N324S, Foxp2T302N) were generated (Fig. 1) as previously described for the Foxp2hum mice7. Targeted integration of the constructs and mutations was verified by Southern blot analysis and sequencing (Fig. S1). We have previously shown that the Foxp2 gene is expressed at the RNA and protein levels in in the identically constructed Foxp2hum mice (Figures S1 and S2 in7). We observed no loss-of-function phenotypes, such as postnatal death, weight loss or other developmental abnormalities that are characteristic for mice homozygous for null alleles of Foxp210,11. We compared animals homozygous for each of the two Foxp2 versions (Foxp2N324S, Foxp2T302N) with littermates or cousins homozygous for the wild type allele (Foxp2wt). Hence, phenotypic differences among the animals carrying the different Foxp2 versions are likely to be caused by the amino acid differences in Foxp2 (Fig. 1).
Synaptic plasticity in Foxp2 T302N and Foxp2 N324S mice
We have previously shown that LTD in neurons of the central7 and dorsolateral parts8,9 of the striatum of Foxp2hum mice is enhanced. Using identical protocols, we made dorsolateral recordings from Foxp2T302N (N = 8) and Foxp2N324S (N = 12) and their wildtype littermates (N = 3 and N = 8, respectively) and compared them to previously published data of Foxp2hum mice8,9. At 30–40 min after high-frequency stimulation, LTD of Foxp2T302N cells is increased compared to Foxp2wt cells (Mann Whitney U test (MWU): P = 0.011, Fig. 2A,C, Supplementary Table S1). When compared to the previously published data for Foxp2hum cells, no significant differences are seen (MWU: P = 0.48). In contrast, LTD in Foxp2N324S cells differs from Foxp2T302N cells (MWU: P = 0.028, Fig. 2C) and is not detectably different from Foxp2wt cells (MWU: P = 0.93; Fig. 2B,C, Supplementary Table S1).
Effects of human amino acid substitutions in FOXP2 on synaptic plasticity in striatal neurons in (A) Foxp2T302N mice and (B) Foxp2N324S mice. LTD was induced in MSN by high-frequency stimulation (100 Hz, black bars; see “Experimental procedures”). Amplitudes normalized to baseline levels (mean ± SEM, t = 10 to 0) is shown. Insets in A and B show representative EPSP traces averaged over 5 min at time point 1 (pre high-frequency stimulation) and 2 (post high-frequency stimulation, scale bars 2 mV, 20 ms). For comparison, mean amplitudes for wild-type (Foxp2wt, grey line) are given in A and B. Boxplots in (C) illustrate the LTD effect for each strain (average reduction of EPSP amplitude t = 30–40 min compared to baseline (t = − 10 to 0 min, *P = 0,028, **P = 0,011, MWU).
Discussion
A question regarding the two amino acid substitutions that affected the FOXP2 protein during human evolution is if they both have physiological consequences or if only one of them has effects. Here we show that mice homozygous for the T302N substitution exhibit increased LTD in the dorsolateral striatum to an extent similar to mice carrying both amino acid substitutions. In contrast, LTD in mice homozygous for N324S substitution does not differ from LTD in their wildtype littermates (Fig. 2). Thus, whereas the T302N substitution has clear effects in terms of synaptic plasticity, the N324S substitution does not have such effects. More work is needed to understand how these observations relate to phenotypic effects that the two amino acid substitutions have in the mouse, notably subtle differences in vocalization5 and enhanced transition from declarative to procedural performance during certain learning tasks9.
However, the variation in FOXP2 amino acid sequences among and within species can give indications about how important the two amino acid substitutions may be. Among vertebrates, the N324S substitution occurs also in carnivores and birds and is thus not unique to humans. Furthermore, 26% of western gorillas carry a substitution two amino acids away at position 326 (A326S)12. In contrast, the T302N substitution has not been observed in any other organism than humans. Among 125,642 humans for which exome sequences are available (gnomAD, v2.1.1), no individual carry the ancestral amino acid variants at either position. However, two individuals carry a serine to arginine substitution at the position corresponding to position 324 in the mouse (S325R). Thus, whereas the T303N substitution in humans affects a position that is extremely conserved and not known to vary among humans today, the N325S substitution has occurred also among other mammals and the positions varies among humans, albeit very rarely.
It should be noted that a signature of a recent selective sweep that was originally linked to the two changes5 is not supported when using sequence data from hundreds of globally distributed humans13. However, the two amino acid substitutions analyzed here are shared with Neandertals and Denisovans14—human forms that diverged from the ancestors of present-day humans 550,000–765,000 years ago15. Hence, signatures of recent selection, which are extremely unlikely to be detected when they occurred that long ago, are uninformative with respect to these two amino acid changes. Nevertheless, other functional changes may have affected the FOXP2 gene on the human evolutionary lineage more recently. For example, a substitution in a transcription factor-binding site in intron 8 of the FOXP2 gene is unique to modern humans and thus occurred during the past 500,000 years. The transcription factor that binds to this site, POU3F2 (also called BRN2), is expressed uniquely in the nervous system and is involved in neuronal differentiation and the substitution reduces the efficiency with which POU3F2 dimers bind to the binding site and drive transcription16. It is thus possible that multiple changes that affect both the function of the FOXP2 protein and the expression of the FOXP2 gene have occurred on the human evolutionary lineage. However, in terms of the effects of the protein on synaptic plasticity, the T303N rather than the N325S substitution is of importance.
Experimental procedures
Generation of Foxp2 N324S and Foxp2 T302N expressing mice
The vectors for generating the Foxp2N324S and Foxp2T302N allele were created by mutagenesis of the targeting vector used for the Foxp2hum allele (details see7). Linearized, sequence verified vectors were electroporated into Bruce4 C57BL/6 ES cells by Ozgene (Bentley, Australia) as described7. ES clones were screened and verified for targeted integration by Southern blot analysis (Fig. S1A,B; the original blots as provided by the company Ozgene are given in S1C) and point mutations in exon 7 were confirmed by sequencing before generation of chimeras. The resulting founder mice were bred, point mutations again confirmed by sequencing (Figure S1D) and then crossed to mice transgenic for the recombinase FLPe under the control of the human ACTB promoter (Jackson Laboratory, Stock Number 003800; C57BL/6J17) to generate Foxp2N324S or Foxp2T302N alleles in which the FRT-flanked neomycin resistance cassettes have been removed as described7. The recombinase transgenes were outcrossed using C57BL/6J mice in the next generation and further crossings were made in C57BL/6J mice (C57BL/6J@Rj; Janvier, St. Berthevin, France). Hence, all alleles are on the same genomic background and lack the neomycin resistance cassette of the targeting vector. Mice used for recordings were homozygous for the wildtype (Foxp2wt) or the FoxP2 locus (Foxp2N324S or Foxp2T302N). They were either derived directly from the first generation of crossings of heterozygous animals or from the following second generation where homozygous wildtype or FoxP2 siblings from the first generation were crossed. Thus, mice compared with each other were either matched littermates or second generation offsprings of such littermates (cousins). Genotyping was done as described7. All mouse experiments were overseen and approved by the Institutional Animal Welfare Officer of the Max Planck Institute for Evolutionary Anthropology (Dr. Gerd Möbius, Fac. of Veterinary Medicine, Univ. Leipzig). They were performed in accordance to the German Animal Welfare Legislation (“Tierschutzgesetz”), and approved and registered with the Federal State Authority Landesdirektion Sachsen (No. 24-9162.11 (T 38/12)).
We recently discovered that a wildtype deletion as described for the Harlan line C57BL/6JOlaHsd (365 kb between pos. 60.976 and 61.341 Mb of Chr. 6, including the Snca and Mmrn1 locus18,19) occurred in some of our lines (incl. the FLPe line). Hence, we tested all animals used in these experiments with PCR protocols as given in19. No animals carrying this deletion were found.
Slice electrophysiology
Brains of slightly anesthetized mice (P21–P53; isoflurane) were prepared into ice-cold sucrose-based cutting solution (in mM: 85 sucrose, 60 NaCl, 3.5 KCl, 6 MgCl2, 0.5 CaCl2, 38 NaHCO3, 1.25 NaH2PO4, 10 HEPES, 25 glucose). Coronal slices (250 µm) were cut (Vibroslice 7000smz, Campden Instruments, UK), incubated in artificial cerebrospinal fluid (aCSF; in mM: 120 NaCl, 3.5 KCl, 1 MgCl2, 2 CaCl2, 30 NaHCO3, 1.25 NaH2PO4, 15 glucose) supplemented with 5 mM HEPES, 1 MgCl2 for 30 min at 35 °C and allowed to recover at room temperature for at least 40 min.
MSN were identified as in20. They were recorded in the current clamp configuration with the bridge mode enabled (EPC-10 amplifier, Patch- and Fitmaster software; HEKA, Lambrecht, Germany). The internal solution contained (in mM): 150 K-gluconate, 10 NaCl, 3 Mg-ATP, 0.5 GTP, 10 HEPES and 0.05 EGTA adjusted to pH = 7.3 and 310 mOsm with the liquid junction potential (15 mV) corrected online. Slices were perfused (2–3 ml/min, aCSF, 21–24 °C) in presence of the GABAAR antagonist gabazine (SR-95531, 10 µM, Sigma). All solutions were continuously oxygenated with 95% O2, 5% CO2 gas.
Glutamatergic excitatory afferents where stimulated intrastriatally with aCSF-filled theta-glass electrodes typically ~ 100–150 µm away from the MSN soma (position of stimulation electrode between MSN and corpus callosum). A bipolar voltage pulse (0.1 ms, ± 5 to ± 30 V) at 0.2 Hz induced subthreshold excitatory postsynaptic potentials (EPSPs; 4–10 mV). Following 10–15 min baseline recording synaptic plasticity was induced by a high frequency protocol (four 100 Hz tetani, 3 s long, separated by 30 s; holding potential − 70 mV). Recordings were rejected if the membrane potential was more positive than − 80 mV or the input resistance changed by more than 30%. We verified that no background long-term potentiation was present as APV ((2R)-amino-5-phosphonovaleric acid), a specific blocker of a subtype of glutamate receptors, did not alter the effect in wildtype mice9.
Statistical analysis
EPSP amplitudes were normalized to a mean baseline level at t = − 10 to 0 min. LTD magnitude of individual cells was calculated by averaging amplitudes 30–40 min after induction with the high frequency protocol. For comparisons we used previously published data for Foxp2wt and Foxp2hum obtained under identical conditions8,9.
All analyzed cells, their associated information (animal, age, litter) and their LTD magnitude are listed in Supplementary Table S1. Genotypes were blinded for experimenters and initial evaluation. All methods are reported in accordance to the ARRIVE guidelines.
Data availability
All analyzed cells, their associated information (animal, age, litter) and their LTD magnitude are listed in Supplementary Table S1. Additional data, including the original reports by the company OzGene on generating the mice are available from the corresponding author on request.
References
Morison, L. D. et al. In-depth characterisation of a cohort of individuals with missense and loss-of-function variants disrupting FOXP2. J. Med. Genet. https://doi.org/10.1136/jmg-2022-108734 (2022).
Liegeois, F., Morgan, A. T., Connelly, A. & Vargha-Khadem, F. Endophenotypes of FOXP2: Dysfunction within the human articulatory network. Eur. J. Paediatr. Neurol. 15, 283–288. https://doi.org/10.1016/j.ejpn.2011.04.006 (2011).
Vargha-Khadem, F., Gadian, D. G., Copp, A. & Mishkin, M. FOXP2 and the neuroanatomy of speech and language. Nat. Rev. Neurosci. 6, 131–138. https://doi.org/10.1038/nrn1605 (2005).
Enard, W. FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Curr. Opin. Neurobiol. 21, 415–424. https://doi.org/10.1016/j.conb.2011.04.008 (2011).
Enard, W. et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418, 869–872. https://doi.org/10.1038/nature01025 (2002).
Zhang, J., Webb, D. M. & Podlaha, O. Accelerated protein evolution and origins of human-specific features: Foxp2 as an example. Genetics 162, 1825–1835. https://doi.org/10.1093/genetics/162.4.1825 (2002).
Enard, W. et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell 137, 961–971. https://doi.org/10.1016/j.cell.2009.03.041 (2009).
Reimers-Kipping, S., Hevers, W., Paabo, S. & Enard, W. Humanized Foxp2 specifically affects cortico-basal ganglia circuits. Neuroscience 175, 75–84. https://doi.org/10.1016/j.neuroscience.2010.11.042 (2011).
Schreiweis, C. et al. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc. Natl. Acad. Sci. USA 111, 14253–14258. https://doi.org/10.1073/pnas.1414542111 (2014).
Shu, W. et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc. Natl. Acad. Sci. USA 102, 9643–9648. https://doi.org/10.1073/pnas.0503739102 (2005).
French, C. A. et al. Generation of mice with a conditional Foxp2 null allele. Genesis 45, 440–446. https://doi.org/10.1002/dvg.20305 (2007).
Staes, N. et al. FOXP2 variation in great ape populations offers insight into the evolution of communication skills. Sci. Rep. 7, 16866. https://doi.org/10.1038/s41598-017-16844-x (2017).
Atkinson, E. G. et al. No Evidence for recent selection at FOXP2 among diverse human populations. Cell 174, 1424-1435.e1415. https://doi.org/10.1016/j.cell.2018.06.048 (2018).
Krause, J. et al. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr. Biol. 17, 1908–1912. https://doi.org/10.1016/j.cub.2007.10.008 (2007).
Prufer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49. https://doi.org/10.1038/nature12886 (2014).
Maricic, T. et al. A recent evolutionary change affects a regulatory element in the human FOXP2 gene. Mol. Biol. Evol. 30, 844–852. https://doi.org/10.1093/molbev/mss271 (2013).
Rodriguez, C. I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25, 139–140. https://doi.org/10.1038/75973 (2000).
Specht, C. G. & Schoepfer, R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2, 11. https://doi.org/10.1186/1471-2202-2-11 (2001).
Specht, C. G. & Schoepfer, R. Deletion of multimerin-1 in alpha-synuclein-deficient mice. Genomics 83, 1176–1178. https://doi.org/10.1016/j.ygeno.2003.12.014 (2004).
Pawlak, V. & Kerr, J. N. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 28, 2435–2446. https://doi.org/10.1523/JNEUROSCI.4402-07.2008 (2008).
Acknowledgements
We are grateful to K. Bruns and R. Voigtländer for animal care and to I. Bliesener and V. Wiebe for technical assistance. This work was supported by the Max Planck Society and the NOMIS Foundation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
U.B. and W.H. conducted all electrophysiological recordings; U.B., W.E. and W.H. designed the experiments, performed data analyses, and wrote the manuscript; W.E., W.H. and S.P. supervised experiments and H.Z. and S.P. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bornschein, U., Zeberg, H., Enard, W. et al. Functional dissection of two amino acid substitutions unique to the human FOXP2 protein. Sci Rep 13, 3747 (2023). https://doi.org/10.1038/s41598-023-30663-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30663-3
This article is cited by
-
From fossils to mind
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.