Abstract
A class of proteins, 1-aminocyclopropane-1-carboxylate oxidase (ACO), is required in the final step of production of ethylene from its immediate precursor 1-aminocyclopropane-1-carboxylic acid (ACC). Despite the crucial and regulatory role of ACO gene family in the fiber development, it has not been thoroughly analyzed and annotated in G. barbadense genome. In the present study, we have identified and characterized all isoforms of ACO gene family from genomes of Gossypium arboreum, G. barbadense, G. hirsutum and G. raimondii. Phylogenetic analysis classified all ACO proteins into six distinct groups on the basis of maximum likelihood. Gene locus analysis and circos plots indicated the distribution and relationship of these genes in cotton genomes. Transcriptional profiling of ACO isoforms in G. arboreum, G. barbadense and G. hirsutum fiber development exhibited the highest expression in G. barbadense during early fiber elongation. Moreover, the accumulation of ACC was found highest in developing fibers of G. barbadense in comparison with other cotton species. ACO expression and ACC accumulation correlated with the fiber length in cotton species. Addition of ACC to the ovule cultures of G. barbadense significantly increased fiber elongation while ethylene inhibitors hindered fiber elongation. These findings will be helpful in dissecting the role of ACOs in cotton fiber development and pave a way towards genetic manipulations for fiber quality improvement.
Similar content being viewed by others
Introduction
Cotton fiber produced by the Gossypium spp. is the global source of natural fiber used in the textile industry. Cotton fiber is a single-celled trichome produced on the seed testa and the elongation of this trichome determines the length of mature fiber i.e. the most desirable characteristic for ginning industry. Although, the complete mechanism of cotton fiber elongation has not been fully understood but ethylene production is known to directly affect cotton fiber cell elongation1 by modulating the expression of multiple classes of genes. Ethylene serves as a signaling molecule due to its solubility in lipids, making its diffusion easier across the membranes2,3. The presence of endogenous ethylene precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), is essential for ethylene synthesis in higher plants4,5. ACC is oxidized into ethylene6 by ACO proteins (Fig. 1). Ethylene synthesis is under the strict control of 1-aminocyclopropane-1-carboxylate oxidase (ACO) regulated by plant’s developmental and environmental state7,8. Thus the gene expression of ACO isoforms determines the rate of ethylene evolution and ACO can be the rate limiting factor for ethylene production9,10. Down regulation of ACO isoforms through gene silencing techniques resulted in a drastic reduction in ethylene biosynthesis11.
A transcription factor (MYB) has been extensively studied for its role in secondary cell wall biosynthesis12,13 and the presence of MYB binding sites in the promoters of ACOs shows the involvement of this ACO family in fiber length regulation14. Moreover, the upregulation of an ethylene responsive transcription factor WRI1 and its correlation with fiber length has been reported in the developing fibers of cotton15. Recently, upregulation of ACO in G. arboreum developing fiber supports its direct involvement in fiber elongation with a positive correlation between the fiber length and the amount of ethylene production16. All these findings provide strong bases for studying the mechanism of action of ACOs in cotton fiber development. Among higher plants, the main hindrance for the analyses of ACO multigene family is the incomplete information of all the gene family members. The present study focuses on the identification and characterization of ACO homologues in Egyptian cotton (G. barbadense) and their role in fiber elongation.
Results
Genome wide ACO gene family distribution
In the present study, we conducted genome wide survey of four cotton species i.e. G. arboreum (A2-genome), G. raimondii (D5-genome), G. hirsutum (AD1-genome) and G. barbadense (AD2-genome) and identified all the ACO homologues. For this purpose, nucleotide sequences of functional ACO1, ACO2, ACO3 and ACO410 were used as query sequences to retrieve all homologues in the above mentioned four cotton genomes. A total 34 unigenes were identified in which 5 belonged to G. arboreum, 6 to G. raimondii, 11 to G. barbadense and 12 to G. hirsutum genome (Table 1).
The genes of ACO isoforms in G. arboreum were located on chromosome 01, 03, 08 and 10. From these GaACO1, GaACO2 and GaACO3 were located on chromosome 10, 08 and 03 respectively. On the other hand, both GaACO4 and GaACO4-like were located on chromosome 01 (Table 1).
G. raimondii also harbored a uniform distribution of 6 GrACO gene members on 05 chromosomes (01, 04, 07, 09 and 10). Very similar to G. arboreum (A2), both GrACO4 and GrACO4-like were located on the same chromosome (01) while GrACO1, GrACO2, GrACO3 and GrACO3-like are located on chromosomes 9,10, 4 and 7 respectively (Table 1). All these uniform distribution patterns and variations provide us the clues for the evolutionary relations of the ACOs in different cotton genomes.
Similarly, among all the retrieved sequences, 11 were from G. barbadense tetraploid cotton (AD2). The GbACO genes were uniformly distributed on both A and D sub-genomes of five chromosomes (chromosome 5, 6, 7, 8 and 11). Both A and D homologues of chromosome 05 accommodate one copy of GbACO1 each. Similarly, GbACO2 isoforms are located on chromosome A06 and D06 homologues each, while GbACO3 homologues are located on A08 and D08. Both members of GbACO4 and one gene of GbACO4-like were located on homologues of chromosome 07 (Table 1). Both homologues of GbACO3-like were located on A11 and D11 sub genomes.
A total of 12 GhACOs were identified in the G. hirsutum tetraploid genome (AD1) which were uniformly distributed on both A and D genomes of this allopolyploid species on chromosome 05, 06, 07, 08 and 11 (Table 1). Distribution of ACO isoforms in G. hirsutum (AD1) genome matched largely with G. barbadense (AD2) except for GhACO4-like members. G. hirsutum contained 2 GhACO4-like members, one located on each A and D sub-genome of chromosome 07 while G. barbadense D genome harbored GbACO4-like but A genome missed that (Table 1).
Gene structure analysis for ACO isoforms
The intron–exon boundaries of all the ACOs were identified and gene structures were constructed (Fig. 2). All members of ACO1 gene subfamily contained three introns and four exons which differ widely in their lengths (Fig. 2). The six members of ACO2 subfamily showed much variation in coding region lengths as well as in the length of introns and exons. In G. hirsutum, the ACO2 isoform of D genome (GhACO2b) contains 5 exons and 4 introns, while the rest of the members of this gene subfamily contain three exons and two introns. Presence of 4 exons and 3 introns distinguishes ACO3 and ACO3-like subfamilies from ACO4 and ACO4-like subfamilies, although variations in gene lengths are there (Fig. 2). In ACO4 and ACO4-like subfamily, all gene members have two and three exons respectively, except GaACO4-like where the gene exists with four exons and three introns.
Conserved regions in ACO isoforms of cotton species
Iron (Fe II) is a cofactor required for the enzymatic activity of ACOs. Scanning of ACO proteins on Prosite database of protein domains (http://www.prosite.expasy.org) lead to the identification of Fe2OG dioxygenase domain at position 155–254 in ACO1 family of G. barbadense (GbACO1). This domain was found conserved among all isoforms of ACOs indicating its functional activity. In this domain triad His177 (H177), Asp 179 (D179) and His234 (H234) constitute an iron binding region17. We performed the residue analysis of ACOs from G. barbadense along with three other cotton species through multiple sequence alignment (Fig. 3) and confirmed the presence of highly conserved iron binding residues at position (H178, D180 and H235) in ACO1 and ACO2 with the exception of Gohir.D06G158400 and Gorai.010G184900 where iron binding triad is located at position H152, D154 and H201 and H187, D189 and H244 respectively. In all other ACO classes iron binding site is located at H177, D179 and H234 (Fig. 3).
Bicarbonate and ascorbate serve as activators for ACO to catalyze the oxidation of ACC to ethylene in plant species17,18,19. The residues involved in bicarbonate binding are Lysine159 (K159), Arg176 (R176) and Arg300 (R300). We found bicarbonate binding residues at position K159, R176 and R300 in G. barbadense. Ascorbate provides binding site for oxygen in the oxidation of ACC to ethylene19 and reported the specific sites for ascorbate binding in this family. In the aligned sequences of cotton, these conserved sites were identified at K159, K293 and F301.
Various other residues i.e. C29, T158, K159, R176, Q189, K200, K231, R245, S247, K293, E295, E298, R300, F301 and E302 have been described earlier in Malus domestica19 as essential residues for the activity of ACOs. Most of these residues (C29, T158, K159, R176, R245, S247, K293, E298, R300 and F301) were found conserved in all ACO isoforms. Although two residues i.e. Q189 and K200 were also found conserved but were absent in Gohir.D06G158400 and Cotton_A_18687 respectively. Interestingly, K231 was found absent in ACO3-like subfamily and has been replaced by R231, although conserved in all other ACO subfamilies. On the other hand, important activity residue E295 was found only in ACO2 subfamily while in all other subfamilies, it has been replaced by the residue Q. Similarly, E302 was found absent in ACO4-like subfamily and replaced by Q.
Another motif i.e. RXS motif (constituted by the residues R244 and S246 separated by X i.e. any amino acid) is involved in substrate binding of ACC to ACO19. We found conserved RMS at position 245–247 in G. barbadense and confirmed its conservation throughout the cotton ACO gene family. Glutathione (GSH), γ-glutamyl-cysteinyl-glycine, is a small cytosolic molecule which serves as a strong non-enzymatic antioxidant20 and modulates the mRNA stability of ACO by S-glutathionylation at Cys63 residue21. We found this residue conserved throughout the ACO family at position C61 (Fig. 3).
Classification and phylogenetic analyses of ACO isoforms from Gossypium species
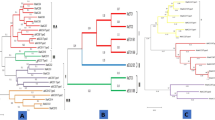
ACO1 (Gbar_A05G017390) and ACO2 (Gbar_A06G016410) coding regions were isolated from G. barbadense var Bar 14/5 cDNA library and sequenced. The amino acid sequences of G. barbadense ACO isoforms were obtained and aligned with the ACOs from other cotton species to find the evolutionary relationship. Phylogenetic tree was constructed through maximum likelihood method with 1000 bootstrap value (Fig. 4). The peptide sequences of GbACO1 and GbACO2 from G. barbadense were also included in this analysis to find their phylogenetic relevance with ACO homologues. The isolated GbACO1 and GbACO2 exhibited 99 and 99.5% homology with GbarA05G017390 and GbarA06G016410, respectively. Phylogenetic analysis distributed the ACO gene family into four ACO1, ACO2, ACO3 and ACO4 subfamilies. In addition to these, two ACO-like (ACO3-like and ACO4-like) subfamilies were also identified (Fig. 4).
Four ACO subfamilies (ACO1, ACO2, ACO3 and ACO4) were constituted by six gene members i.e. one from G. arboreum, 2 from G. barbadense, 2 from G. hirsutum and one from G. raimondii. ACO3-like subfamily comprised of five gene members having 2, 2 and one homologues from G. barbadense, G. hirsutum and G. raimondii, respectively (Fig. 4). ACO4-like subfamily also comprised of five homologues. This subfamily constituted one member from G. arboreum, one from G. barbadense, two from G. hirsutum and one from G. raimondii.
Genomic divergence of ACO families in Gossypium spp.
We constructed the circos figures to observe the trends and correlations among the members of ACO subclasses in A and D subgenomes (Fig. 5). Individually, all members of ACO1 subclass shared high homology (98.4–99.7%) with other members of ACO1 family, thus showing lesser diversity within this sub-class (Fig. 5A). Relative sequence complexity and diversity was observed while comparing the gene members of ACO2 sub-class as shown in Fig. 5B. In this subclass, G. arboreum (Cotton_A_30303), both A and D genome of G. barbadense (Gbar_A06G016410 and Gbar_D06G017100) and A genome of G. hirsutum (Gohir.A06G152100) showed maximum and approximately equal (more than 99%) homology with each other. Moreover, D genome of G. hirsutum (Gohir.D06G158400) showed approximately 24% homology with others except G. raimondii (Gorai.010G184900) where only 5.4% homology could be observed. Thus, ACO2 from G. raimondii (Gorai.010G184900) showed maximum diversity in comparison to other gene members (Fig. 5B).
Sub-genomic characterization of ACO (1-aminocyclopropane-1-carboxylate oxidase) subfamilies (A) ACO1, (B) ACO2, (C) ACO3, (D) ACO4, (E) ACO3-like, (F) ACO4-like. The thickness of the bands represents the percent homology while the colors of the band represent the cotton species from which they originate.
In case of sub-class ACO3, the homology among its members was 98.7–100% indicating common ancestors (Fig. 5C). While ACO4 subclass shows diverse homology patterns among its members i.e. 10.2–99% (Fig. 5D). ACO4 of G. raimondii shares more than 84% homology with the A and D sub-genomes of both allotetraploids i.e. G. hirsutum and G. barbadense (Fig. 5D). ACO4 of G. arboreum (Cotton_A_34794) shows only 10–20% homology with other members of this subclass. ACO3-like sub-class indicates less variability similar to ACO3 subclass (Fig. 5E). Within this sub-class, more than 97% homology was observed when all members were compared to each other. ACO4-like isoform from G. raimondii shows more than 98% homology with other members of this sub-class (Fig. 5F) indicating common ancestors while ACO4-like from G. arboreum (Cotton_A_18687) shows 61–62% homology with other members of this family.
Functional modulation of ACOs during cotton fiber development
Cotton fiber development is a very important process culminating in the formation of natural fiber required in the textile industry. Transcript abundance of ACO isoforms was measured through realtime RT-PCR in different cotton species at 5, 7, 10, 14, 17 and 21 DPA developing fibers. Temporal expression of ACO1 and ACO2 gene was found highest in G. barbadense in comparison with other cotton species during early fiber elongation stage (5 DPA). The ACO1 expression was 31 fold higher at 5 DPA in the fibers of G. barbadense and 8.4 fold in G. hirsutum in comparison with G. arboreum (Fig. 6). The ACO2 expression was found 63 fold and 8 fold higher in G. barbadense and G. hirsutum in comparison with G. arboreum respectively. In the later stages of fiber development, there was a drastic decrease in ACO1 and ACO2 expression in both species. In G. arboreum, the relative expression of ACO1 and ACO2 remained almost at bottom line throughout the fiber elongation process. Similar expression pattern of both genes indicate functional redundancy of these genes.
ACO3 showed higher transcriptional abundance at 5 DPA in G. arboreum in comparison with other species. This expression decreased at 7 DPA but showed a gradual rise and reached at its peak at 14 DPA and again reached at basal line at 17 DPA (Fig. 6C). Almost the same but low pattern of transcriptional expression was observed in G. hirsutum. On the other hand, in G. barbadense, the transcriptional expression remained highest at 7 DPA which decreased to basal line throughout its later fiber developmental stages. In case of ACO4, at 7 DPA the transcriptional abundance was found highest in G. arboreum while of the other two species remained almost at bottom line. At 10 DPA, it was again almost at the same level in the three cotton species. Interestingly, a rise in transcriptional expression was observed only in G. barbadense at 14 DPA. At 17 DPA, the transcriptional expression was almost at basal line in all species (Fig. 6D).
1-Aminocyclopropane-1-carboxylic acid (ACC) content during cotton fiber development
As ACC is the substrate of ACOs for ethylene production, so we wanted to quantify the endogenous ACC pool in cotton fibers of G. barbadense in comparison with other cotton varieties. We determined ACC content in cotton fibers at different developmental stages through high performance liquid chromatography (Fig. 7). The endogenous ACC level was found highest in G. barbadense throughout the fiber developmental stages as compared to G. arboreum (Fig. 8). At 0DPA, the endogenous ACC concentration was approximately 11, 13 and 10 pmol/g of fiber in G. arboreum, G. barbadense and G. hirsutum respectively. During fiber elongation stage (3 DPA), a sudden rise in ACC concentration was observed in the three cotton species. In G. barbadense, at 3 DPA it was approximately 126 pmol per gram of the tissue while at 21 DPA it reached to its peak i.e. 132 pmol/g of fiber with the slight fluctuations during intermediate fiber developmental phases (Fig. 8). G. arboreum showed a drastic decline in ACC concentration from 114 pmol to only 12 pmol/g fiber from 3 to 5 DPA. Again at 14 DPA it restored its endogenous concentration up to 112 pmol/g fiber which remained almost static during further fiber developmental stages. Contrary to G. barbadense which had highest ACC concentration at 21 DPA fibers, G. hirsutum showed highest ACC concentration at initial fiber developmental phase. At 3 DPA it reached up to 124 pmol/g of fiber. After a small decline during further fiber developmental stages it again reached up to 119 pmol/g fiber at 17 DPA. Moreover, the endogenous ACC concentration was measured in the vegetative leaves of cotton species. It was 108.137, 116.344 and 111.95 pmol/g of leaf tissue in G. arboreum, G. barbadense and G. hirsutum respectively (Fig. 9).
Effect of ACC, AgNO3 and CoCl2 on fiber length
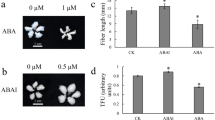
Ovules of G. barbadense at 0DPA were cultured in BT medium22 and fiber growth was documented at 21 DPA. When cultures were supplemented with ACO substrate (ACC) for the production of ethylene, relatively longer fibers (23 mm) were observed as compared to un-supplemented control where fiber length was 18 mm (Fig. 10A,B). When cultures were supplemented with previously reported23 ethylene inhibitors (AgNO3 and CoCl2), the fiber growth was retarded (Fig. 10).
Ovule cultures of G. barbadense in modified BT medium22 at 21 DPA (A) control BT without any supplement (B) medium supplemented with 10 µM ACC (C) 40 µM of CoCl2 (D) 30 µM of AgNO3.
Discussion
Ethylene is an important growth factor participating in various physiological and developmental processes in higher plants under normal as well as stress conditions24,25,26. In higher plants, 1-aminocyclopropane 1-carboxylic acid (ACC) is the immediate ethylene precursor converting to ethylene and this bioconversion is under the control of ACC-oxidase enzymes. Molecular oxygen and ascorbate serve as the co-substrates during this biochemical conversion11. In G. hirsutum, four ACO isoforms namely ACO1, ACO2, ACO3 and ACO4 have been identified10. In a recent report, 20 ACOs have been identified in G. hirsutum1. In other plants, numerous members of this family have been described and their classification is thus a complex phenomenon.
ACOs and ethylene production play vital role in the fiber elongation (Fig. 10) and G. barbadense is the longest and finest fiber producing species as shown in our published data15. So it’s very important to thoroughly investigate ACOs family in G. barbadense and compare it with the other tetraploid and diploid species of cotton. In the present work, two tetraploid cotton species, i.e., G. barbadense and G. hirsutum and two diploid species belonging to ancestral genome A (G. arboreum) and D (G. raimondii) were used to study the ACO gene families. ACO isoforms were identified in the genomes of tetraploid and diploid cotton species and molecular characterization was performed. Phylogenetic analysis grouped all these isoforms in four classes of ACOs and two ACO-like classes (Fig. 4). The gene structure analyses also revealed the structural variations and gene architecture among these classes (Fig. 2).
Gene expression of ACOs determine ethylene production and silencing of ACO resulted in a drastic reduction in ethylene biosynthesis11. In order to access the amount of ethylene production at different stages of fiber development in diploid and tetraploid varieties of cotton, we determined the expression of ACOs as well as concentration of ACC in the developing fibers. Among the cotton species under study, G. barbadense revealed the highest level of ACO1, and ACO2 transcript accumulation during early fiber elongation phase and upregulation of ACO4 at later developmental stages (Fig. 6). Upregulation of ACO genes has been described to promote cell wall loosening and changes in cytoskeletal arrangement to facilitate fiber elongation27. ACO1 and ACO2 transcription levels exhibited here correlate with the lengths of mature fibers. G. barbadense produces longest cotton fibers followed by G. hirsutum and G. arboreum respectively15. G. hirsutum which is the second longest fiber producing species, reveals the activation of ACO3 along with ACO2 and ACO1 during early fiber elongation stages while G. arboreum displays activation of ACO3 and ACO4 during fiber elongation (Fig. 6).
Endogenous ACC pool which serves as a substrate for ethylene production has been described as limiting factor in ethylene production4,5. We investigated this important component and found that ACC pool was biggest in the vegetative leaf tissue as well as in the developing fibers of G. barbadense in comparison with G. hirsutum and G. arboreum (Figs. 8, 9). We also studied the effect of ethylene production on fiber length in G. barbadense by growing ovules in medium supplemented with ethylene precursor or ethylene inhibitors. We found that the ethylene precursor which is the substrate of ACOs enhanced while ethylene inhibitors reduced fiber length (Fig. 10). In our previous studies, the expression of wrinked-1, an ethylene responsive transcription factor showed highest expression in fibers of G. barbadense as compared to G. hirsutum and G. arboreum15. Transformation studies of Gbwri1 (EREB) in Arabidopsis thaliana have also demonstrated its role in fiber development28. The highest expression of this ethylene responsive transcription factor might also play a supportive tool in exploring the role of ethylene in fiber development. From our findings, in this study, it is quite obvious that ethylene biosynthesis is a complex phenomenon and can be affected by various factors like the presence of its precursor, the fiber developmental phase, etc. Furthermore, the promoter analyses and further investigations of ACO classes might be helpful in exploring and understanding the role of ethylene in cotton fiber development.
Conclusion
The relative expression of ACOs in cotton fibers at different stages of fiber elongation in G. arboreum, G. barbadense and G. hirsutum will further support the role of ACC oxidases in fiber cell elongation. The correlation between the endogenous ACC pool in the leaves and at different fiber developmental stages of the three Gossypium species also support the possible role of ethylene in cotton fiber elongation. Moreover, addition of ACO substrate enhances fiber length of G. barbadense in ovule cultures while ethylene inhibitors hinder fiber growth.
Materials and methods
Plant growth conditions and sampling
The seeds of G. arboreum (cv. FDH786), G. barbadense (cv. bar14/5) and G. hirsutum (cv. MNH886) were procured from Central Cotton Research Institute (CCRI), Multan, Pakistan, as a gift. FDH786 is a Pakistani origin cultivated variety of G. arboreum15,29,30 developed in University of Agriculture Faisalabad and can be searched on cottongen too. Bar 14/5 is Egypt origin germplasm present in the germplasm pool of CCRI, Multan while MNH886 of G. hirsutum is the commonly cultivated variety of Pakistan developed in 2012 (https://aari.punjab.gov.pk/cri_multan_achievements). Mature fibers of all the above three varieties were tested for fiber quality traits and published in a separated study15. During the cotton growing season (March–September 2019), seeds were sown in the field of School of Biological Sciences, University of the Punjab, Lahore under natural conditions following the standard protocols and good agricultural practices. Cotton flowers were tagged on the first day of opening of the flower and bolls at different developmental stages were harvested on ice and dissected to isolate the developing fibers which were immediately immersed in liquid nitrogen and stored in − 80 °C freezer15. Bolls were collected at multiple developmental stages i.e. 5, 7, 10, 14, 17 and 21 days’ post anthesis (DPA) from all cotton species under study. All methods and procedures were carried out in accordance with the relevant guidelines. G. raimondii was not included in the fiber related experiments due to its inability to produce spin-able fibers.
Identification and in-silico analysis of ACOs
Previously reported nucleotide sequences of ACO1 (DQ116442), ACO2 (DQ116443), ACO3 (DQ116444) and ACO4 (DQ122175)10 were used to search for the homologues in G. arboreum (A-genome), G. raimondii (D5-genome), G. barbadense (AD2-genome) and G. hirsutum (AD1-genome) sequences (CottonGen database) through nucleotide basic local alignment search tool (BLASTN). Splice/Sequence variants were combined to form unigenes. Amino acid sequences of the homologues were retrieved and aligned through ClustalW algorithm while phylogenetic tree was constructed using Maximum Likelihood method with 100 bootstrap values using pairwise deletion option with Poisson substitution model on MEGA version X31. An online resource, DNA Pattern Find was used to predict the molecular weights and Isoelectric points (pI). Moreover, Gene Structure Display Server (GSDS) was used to generate gene structure for all ACO homologues32. The chromosomal locations of all the ACO isoforms were analyzed in the Jbrowser portal of Cottogen database. Circos online tool was used to find the evolutionary relationship between diploid and tetraploid species of cotton (http://circos.ca).
Transcript profiling
From the developing fiber samples, RNA was isolated through modified hot borate method33. Complimentary DNA (cDNA) was synthesized from the isolated RNA samples using Invitrogen SuperScript™ II RT First Strand cDNA Synthesis kit using random hexamer primers. Forward and reverse primers (18–30 bp) were designed (Table 2) to study gene expression in the Gossypium species through online PRIMER3 software34. ACO1 and ACO2 primers were designed with the objective to amplify only family specific products. While ACO3 primers possessed the ability to amplify ACO3 and ACO3-like products and similarly ACO4 primers amplified both ACO4 and ACO4-like products. After primer optimization, quantitative expression was measured using Sybr green master mix in PikoReal thermocycler (Thermo Scientific, Waltham, MA, USA). Elongation Factor 1 (EF1) was used as the endogenous control to normalize ACOs expression during development. At least three biological and three technical replicates were used for every sample15,35.
Isolation and sequencing of ACO1 and ACO2 from G. barbadense
For isolating ACO genes, cDNA was synthesized from RNA isolated from the developing fibers (10 DPA) of G. barbadense using Oligo (dT) primers. From the cDNA library, full length sequences of ACO1 (GbACO1) and ACO2 (GbACO2) were amplified using gene specific primers (Table 2). The Amplified GbACO1 and GbACO2 were then cloned in pJET cloning vector using CloneJET PCR Cloning Kit (Thermo Scientific, MA, USA). The constructs were sequenced using insert flanking universal primers36.
ACC content estimation through high performance liquid chromatography (HPLC)
ACC endogenous pool was measured in developing fibers (5, 7, 10, 14, 17 and 21 DPA) and the whole gynoecium at 0 and 3 DPA, of the three cotton species under study. For this quantification, we used the modified method37 by preparing ACC derivative with o-phthaldialdehyde (OPT). Briefly, for ACC extraction, fibers were ground to fine powder and 5 ml of ice-chilled 70% ethanol were added to it. The mixture was homogenized thoroughly and was then centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was filtered through the 0.2 µm micro-filter. The working samples were prepared by dissolving 20 µl of extracted ACC, 80 µl of OPT and 900 µl of 70% ethanol. The mixture was mixed well and was allowed to stand for 30 min to complete the reaction and 20 µl of the prepared working samples were injected through a micro-injector. ACC-OPA derivative was detected through an attached fluorescence spectrophotometer at 1.0 ml/min flow rate using methanol as solvent A and acetonitrile as solvent B over 20 min. The calibration curve was prepared using standard ACC (Sigma Aldrich) to find the endogenous content in samples.
In-vitro ovule culturing
The ovules of G. barbadense were cultured on BT medium with some modifications. BT medium was prepared according to22 with pH 5. The medium was supplemented with 0.75µM Gibberellic acid (GA3) and 5 µM and Indole acetic acid (IAA). BT media were either supplemented with 10 µM ACC or ethylene inhibitors i.e. 30 µM silver nitrate (AgNO3) or 40 µM cobalt chloride (CoCl2)23,38.
The flowers along with their anthocaulus (approximately 1 cm) were collected at the day of anthesis. Bracts, petals and stamens were removed carefully without hurting the pistil. The whole tissue was immediately immersed in 0.1% (w/v) of mercuric chloride (HgCl2) for 15 min with continuous shaking. Afterwards, the tissue was rinsed 4–5 times with autoclaved distilled water under standard aseptic conditions. The shuck of the ovary was removed carefully to avoid damage to the ovules. Ovules were then placed gently on the surface of the medium within each culture plate having 5 ml of nutrient medium. The floating ovules were incubated at 30 ± 1 °C in dark without shaking.
The cultured ovules (21 DPA) were immersed in 75% (v/v) ethanol for 20 min. They were then placed on a clean glass slide and kept under running water to make their one side flow. The lengths of the fibers were then measured with the help of a ruler.
Data availability
All data generated or analysed during this study are included in this published article.
References
Yu, D. et al. Dynamic roles and intricate mechanisms of ethylene in epidermal hair development in Arabidopsis and cotton. New Phytol. 234(2), 375–391 (2022).
Chang, C. Q&A: How do plants respond to ethylene and what is its importance?. BMC Biol. 14(1), 7 (2016).
Vandenbussche, F. et al. Ethylene in vegetative development: A tale with a riddle. New Phytol. 194(4), 895–909 (2012).
Yoon, G. M. & Kieber, J. J. 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants 5, plt017 (2013).
Van de Poel, B. & Van Der Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene!. Front. Plant Sci. 5, 640 (2014).
Vanderstraeten, L. & Van Der Straeten, D. Accumulation and transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: Current status, considerations for future research and agronomic applications. Front. Plant Sci. 8, 38 (2017).
Ruduś, I., Sasiak, M. & Kępczyński, J. Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol. Plant. 35(2), 295–307 (2013).
Li, F. et al. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 33(5), 524–530 (2015).
Yang, S. F. & Hoffman, N. E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35(1), 155–189 (1984).
Shi, Y. H. et al. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell 18(3), 651–664 (2006).
Lin, Z., Zhong, S. & Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 60(12), 3311–3336 (2009).
Zhong, R. et al. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 20(10), 2763–2782 (2008).
Machado, A. et al. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 59(1), 52–62 (2009).
Li, F. et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 46(6), 567–572 (2014).
Qaisar, U. et al. Studies on involvement of Wrinkled1 transcription factor in the development of extra-long staple in cotton. Indian J. Genet. Plant Breed. 77(02), 298–303 (2017).
Verma, P. et al. Ethylene mediated regulation of fiber development in Asiatic cotton (Gossypium arboreum L.). South Afr. J. Bot. 135, 349–354 (2020).
Zhang, Z. et al. Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase—The ethylene-forming enzyme. Chem. Biol. 11(10), 1383–1394 (2004).
Brisson, L. et al. 1-Aminocyclopropane-1-carboxylic acid oxidase: Insight into cofactor binding from experimental and theoretical studies. J. Biol. Inorg. Chem. 17(6), 939–949 (2012).
Dilley, D. R. et al. 1-Aminocyclopropane-1-carboxylic acid oxidase reaction mechanism and putative post-translational activities of the ACCO protein. AoB Plants 5, plt031 (2013).
Hasanuzzaman, M. et al. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 23(2), 249–268 (2017).
Datta, R. et al. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 169(4), 2963–2981 (2015).
Beasley, C. & Ting, I. P. The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. Am. J. Bot. 60(2), 130–139 (1973).
Kumar, V., Parvatam, G. & Ravishankar, G. A. AgNO3: A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 12(2), 8–9 (2009).
Chandler, J. W. Class VIIIb APETALA2 ethylene response factors in plant development. Trends Plant Sci. 23(2), 151–162 (2018).
Wang, Y. et al. Ethylene increases the cold tolerance of apple via the MdERF1B–MdCIbHLH1 regulatory module. Plant J. 106(2), 379–393 (2021).
Tian, Y. & Lu, X. Y. The molecular mechanism of ethylene signal transduction. South Afr. J. Bot. 72(4), 487–491 (2006).
Wei, X. et al. Fiber-specific overexpression of GhACO1 driven by E6 promoter improves cotton fiber quality and yield. Ind. Crops Prod. 185, 115134 (2022).
Imran, M. et al. Ectopic expression of fiber related Gbwri1 complements seed phenotype in Arabidopsis thaliana. Int. J. Agric. Biol. 25(1), 123–130 (2021).
Jamal, A. et al. Water stress mediated changes in morphology and physiology of Gossypium arboreum (var FDH-786). J. Plant Sci. 2(5), 179–186 (2014).
Hassan, S. et al. Growth, physiological and molecular responses of cotton (Gossypium arboreum L.) under NaCl stress. Am. J. Plant Sci. 2014, 605–614 (2014).
Kumar, S. et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35(6), 1547–1549 (2018).
Hu, B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 31(8), 1296–1297 (2015).
Wan, C. Y. & Wilkins, T. A. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 223(1), 7–12 (1994).
Untergasser, A. et al. Primer3—New capabilities and interfaces. Nucleic Acids Res. 40(15), e115 (2012).
Qaisar, U. et al. Identification, sequencing and characterization of a stress induced homologue of fructose bisphosphate aldolase from cotton. Can. J. Plant Sci. 90(1), 41–48 (2010).
Maqbool, A. et al. Identification, characterization and expression of drought related alpha-crystalline heat shock protein gene (GHSP26) from desi cotton. Crop Sci. 47(6), 2437–2444 (2007).
Bushey, D. F., Law, D. M. & Davies, P. J. High-performance liquid chromatography analysis of 1-aminocyclopropane-1-carboxylic acid using o-phthaldialdehyde precolumn derivatization. Anal. Biochem. 167(1), 31–36 (1987).
Lau, O.-L. & Yang, S. F. Inhibition of ethylene production by cobaltous ion. Plant Physiol. 58(1), 114–117 (1976).
Acknowledgements
We acknowledge the School of Biological Sciences, University of the Punjab, Lahore, Pakistan for providing the infrastructure and funding for research. We are thankful to Dr. Waheed-uz-Zaman, School of Chemistry, University of the Punjab, Lahore, Pakistan, for his guidance in HPLC.
Funding
This research was financially supported by the School of Biological Sciences, University of the Punjab, Lahore, Pakistan.
Author information
Authors and Affiliations
Contributions
S.Y. and U.Q. planned the experiments, analysed data, and drafted the manuscript. S.Y. performed the experiments. T.R. and B.T. analysed the data. F.A. revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yousaf, S., Rehman, T., Tabassum, B. et al. Genome scale analysis of 1-aminocyclopropane-1-carboxylate oxidase gene family in G. barbadense and its functions in cotton fiber development. Sci Rep 13, 4004 (2023). https://doi.org/10.1038/s41598-023-30071-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30071-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.