Abstract
Water care is an imperative duty in industries with effluents loaded with pollutants such as heavy metals, especially chromium (VI), extremely dangerous for humans and the environment. One way of treating water is possible through a continuous system with dry and crushed vegetable biomass of cellulose xanthogenate because it can adsorb heavy metals, especially due to its low production costs. Through continuous systems and with the waste of PET plastics, it is possible to develop a water treatment process adapting this system and biomass. The objective of this research is the development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Where a mass balance conducted to determine the adsorption capacity of this heavy metal, corroborating it through the Thomas model. The treatment process eliminated around 95% of Cr (VI) present in the water, in addition, biomass reuse cycles carried out, which maintained a considerable adsorption capacity in all the cycles conducted through EDTA reagent.
Similar content being viewed by others
Introduction
The world needs a new way of taking care of water due to the incessant growth of cities and industries that pollute water resources. One form of care is innovative treatments that are efficient and cheap. Cellulose biomass is ideal for treating water contaminated with heavy metals due to its ability to chemisorb these contaminants1.
Chemical precipitation is the most applied, for the removal of heavy metals. However, this method generates substantial amounts of highly contaminated sludge, and its implementation and operation are very expensive2.
Therefore, advanced methods with redefined approaches need in pursuit of sustainable development of industrial expansion. Chemical adsorption is a process that uses materials of biological origin as adsorbents to remove distinct types of contaminants, especially toxic metals3.
This technology stands out for being simple, ecological, economical and at the same time flexible to scale, that is, for the continuous treatment of substantial amounts of polluted effluents with low concentrations of metals1,4
In different investigations on alternative treatment systems, it was concluding that cellulose has a relevant capacity when it comes to removing heavy metals present in the water due to its power to exchange these contaminants for hydroxyl groups5,6,7. The dried and crushed cellulose residues of E. crassipes are an alternative because they have around 70% cellulose, and it is a somewhat expensive residue for final disposal1,4. The aquatic plant, E. crassipes, expands on the surface of water bodies, creating a layer that limits the transfer of oxygen into the water, affecting ecosystems8,9,10,11,12.
The transformation of cellulose with carbon disulfide (CS2) forms cellulose xanthogenate, which is an alkaline biomass charged with ions (OH−) that allows easy chemisorption of metal cations from industrial wastewater13,14. The used the xanthogenate of cellulose was implemented for eliminated Cr (VI)15.
The tests to determine the removal efficiencies of heavy metals through this type of biomass are generally carried out in batches, a condition under which the removal efficiencies, adsorption capacities and adsorption kinetics obtained16. It is generally carried out on a laboratory scale where their experiments carried out in small volumes (milliliters), from the work at the laboratory level the necessary data can obtained so that, through an adequate scaling technique, a process can defined at the pilot level and this, in turn, serves to define an industrial plant17.
Subsequently, column adsorption tests used to establish the efficiency of the biomass in the removal of the contaminant, in an arrangement like that used in the industry18. Obtaining rupture curves in specific operating conditions whose behavior depends on the adsorption kinetics, adsorption capacity and hydraulic factors19 and in general these tests carried out on a pilot scale, a pilot plant is a process to small scale. The purpose pursued when designing, building, and operating a pilot plant is to obtain information on a certain physical or chemical process, which allows determining if the process is technically and economically viable, as well as establishing the optimal operating parameters of said process. for later design20 The adjustment and calibration of continuous treatment systems could couple based on pollutant loads and flow, simulating performance to design processes, using mathematical models21,22. The mass balance has used successfully to predict rupture curves, calibrate the input load, and meet discharge standards, being the main parameter to model and define the designs of treatment systems23,24.
The complete treatment of system such as the one with fixed columns also consists of improving the treatment conditions by means of EDTA, since it is a substance used as a chelating agent that can form complexes with a metal that has an octahedral coordination structure25,26.
The objective of this research is the development of a treatment for water contaminated with Cr (VI) by means of E. crassipes xanthate cellulose on a pilot scale. Determining through FTIR and SEM images the characteristics of this biomass, where a mass balance modeled to determine the adsorption capacity of this heavy metal and subsequent elution with EDTA found to determine the ideal concentration for different reuses.
Until now, in the open literature, there were no continuous pilot-scale treatment systems with cellulose xanthate biomass with these characteristics. Although the E. crassipes plant has used to remove Cr (VI) in continuous processes27,28, these treatment system design parameters were not established. Therefore, the novelty of this study is the implementation of the mass balance, where an equation obtained to determine design variables in construction.
Methods and materials
Use the crassipes
The leaves and roots were separated from the rest of the plant, washed with abundant tap water, followed by distilled water, where a characteristic population of about 40 plants, already dead, was collected.
The experimental research in plants, including the collection of plant material, complied with the relevant institutional, national and international guidelines and legislation, as stipulated in Decree Law 2376 of 201329, for experimental projects on the environment.
The leaves and roots were cut into small pieces and dried in an oven at 60 °C for 36 h. The pulverized biomass is sieved through a blade mill to obtain different particle sizes of 0.212 mm. The taxonomic level is (Eichhornia crassipes). The collection point is the municipality of Mosquera, outside Bogotá DC, located at coordinates: 4.682995, − 74.256673.
Carried out on February 15, 2021, by the researcher Uriel Fernando Carreño Sayago. The plant samples were identified by the research staff (Universidad Los Libertadores) of the Faculty of Engineering, Bogotá, D.C.
Obtaining cellulose with carbon disulfide (xanthogenate)
The dried and crushed biomass of E. crassipes of 40 g, added to 500 ml of NaOH 10 mg/l, then 150 ml of carbon disulfide CS2 added. Two variables of NaOH-transformed cellulose (EC-Na) and complete xanthogenate cellulose (EC-X) used. This procedure is based in13.
Chromium measurement
Chromium (VI) laboratory measurement: 50, 100, 200 and 300 mg/L of Cr (VI). Samples were being taken in the flask at each time interval, analyzing the residual chromium concentration. 20 µm samples were taking and then placed in the centrifuge (KASAI MIKRO 200). In sampling, aliquots of the reaction mixture were being analyzed for residual chromium concentration using a UV84.
Chromium determination
Using the diphenylcarbazide method (the amount of chromium (VI) residue is estimated. For this purpose, the phosphate buffer solution was prepared by adjusting it to a pH equal to 2 with 90% of the grade of purity (H3PO4). In an eppendorf tube 200 µl of 0.5% diphenylcarbazide (with 97% the grade of purity) and (W/V acetone with 97 of grade of purity), 900 µl of phosphate buffer and 100 µl of the residual sample were added. A suitable portion is transferred to an absorption cell and the absorbance is measured at 540 nm.
Spectrophotometer (evolution 300 spectrophotometer) by monitoring changes in absorbance
All procedures for the determination of chromium, for water and substrates, were being carried out using the implementation of APHA (Procedure of the American Public Health Association), for standard tests (standard methods for the examination of water and wastewater).
Adsorption experiments
Adsorption experiments were being carried out in a 100 ml glass vessel with constant stirring (IKA Ks 4000 shaker) at 20 °C, 250 rpm. Data were being taken every 20 min until 180 min were completing. The sample taken is 20 um. All experiments were being carried out in triplicate, with an average of the final values.
The 1000 (mg/l) Cr (VI) stock solution was prepared with distilled water using potassium dichromate. This stock solution used to prepare the test solutions of 200; 300; 400 and 600 mg/l Cr (VI). The batch adsorption study performed at a temperature of 24 °C. The adsorption capacity was determined by suspending 0.3 g of biomass in 100 ml of Cr (VI) solution for 140 min at 200 rpm.
Continuous experimentation
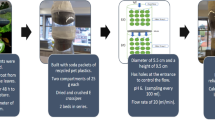
Development of treatment systems on a pilot scale using PET plastic containers. The treatment systems built using recycled PET containers (600 and 1000 ml). Capsules built where the dried and crushed biomass of ECx and EC-Na.
Each capsule connected to the next through a serial arrangement, with openings in the lids arranged at the bottom of each capsule to allow the flow of treated water to the next capsule.
The flow guaranteed by dripping on the upper capsule, keeping the system flow rate at 15 ml/min, also keeping the pH constant in the feed flow. The bed density was constant, and under temperature and pressure conditions of 20 °C and 1 bar, respectively, the system had manual flow control.
Evaluating initial concentrations of 600, 400, 300 and 200 mg/l of chromium (VI). All tests were performed in duplicate, calculating the average between the data obtained and with this the percentage of metal removal.
For this type of system, three compartments of 15 g each of the different types of biomasses used. In the process of designing and creating these systems, fundamental design parameters adjusted, creating a system that took advantage of the funnel-shaped effect of the containers.
The dry and crushed biomass passed through a 60 mesh, allowing the passage of particles of 0.212 mm in diameter.
The effluent water collected in containers with a capacity of 400 ml, once this capacity covered, the water sample to analyze take and a clean and dry container arranged to continue the collection. Preparations of Cr (VI). The 1000 (mg/l) Cr (VI) stock solution prepared with distilled water using potassium dichromate. This stock solution used to prepare the test solutions of 100 and 400 mg/l Cr (VI).
Desorption-adsorption
The experimental elution with EDTA carried out after each one of the treatments, when the biomass already presented saturation. The elution consisted of letting the biomass dry and later it passed through the treatment system under the concentration of:
-
1 g/l of reagent in 300 ml of water.
-
3 g/l of reagent in 300 ml of water.
-
5 g/l of reagent in 300 ml of water.
Once the optimal conditions determined, four sorption/desorption cycles performed in order to investigate the regeneration potential of the biomaterial. Between each cycle, the residue washed in a continuous flow of deionized water for 1 h.
Model evaluation
Mathematical modeling used to describe the behavior of the advance curves, which helps to understand and extend the system. Three different column adsorption models Yoon–Nelson, Thomas and Bohart fitted. To the data of the breakthrough curves to explain the biosorption process of Cr (VI) by EC-Na and ECx in fixed bed configuration.
The Thomas model used to estimate the maximum adsorption capacity and predict the rupture curves, assuming the kinetics of reversible second-order reactions and the Langmuir isotherm30,31.
This equation represented for each of the biomasses since it is one of the most representative in the literature to predict the rupture curves and estimate the adsorption capacity, it used to validate the equation.
The Yoon–Nelson model assumes that the adsorption rate decreases proportionally to the adsorbate removal and adsorbent breakdown curve, without considering information such as adsorbate properties, adsorbent type, and adsorption column specifications32.
The Bohart equation that describes the relationship between C/Co and t in a continuous system has been widely used to describe and quantify other types of systems. This model assumes that the sorption rate is proportional to the residual capacity of the solid and to the concentration of the retained species and used to describe the initial part of the rupture curve33 In the Table 1. Show the different model of the adsorptions.
TESCAN FE-MEB LYRA3
TESCAN FE-MEB LYRA3 scanning electron and focused ion beam microscope. The SEM has an integrated X-ray energy dispersion spectroscopy microanalysis system, EDS (Energy Dispersive X-ray Spectroscopy). (EDS) is an of the most efficient techniques for the qualitative and quantitative analysis of organic samples and through the SEM microphotographs the samples evaluated in the present investigation observed in detail, the diffraction of an X-ray beam by the atoms of the sample interacts with the X-ray beam, producing regions of diffraction intensity, or peaks, for the diagnosis of each of the elements.
Result
Analysis of FTIR
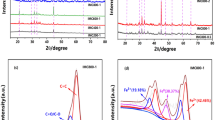
Understanding the functional groups involved in the biosorption of toxic metals is essential to elucidate the mechanism of this process. Groups such as carboxylic, hydroxyl and amine are among the main responsible for the absorption of metals by cellulose34 In the Fig. 1, show the FTIR of ECx.
According to13 the bandwidth at 3000–3600 cm−1 corresponds to bonds related to the -OH group. These hydrogen bonds are useful tools for cation exchange with heavy metals. This evidenced in the color spectrum (dark green) that represents an ECx sample with attached Cr (VI) after the adsorption process, where the stretching of the (OH) group lost part of its extension. The change observed in the peak from 3420 cm−1 of ECx to 3440 cm−1 in ECx-Cr indicates that these groups have a participation in the bond with the Cr (VI) ions. The variation of bands in the peak of the amines after adsorption confirms the participation of these groups in the adsorption process. This result confirmed by the ion exchange evaluation experiment discussed later in section SEM–EDX.
The change in peak 3280, after Cr (VI) adsorption, indicates that EC removed Cr (VI) based on interaction with (OH), part of (OH) lost due to formation of vibrations of ascension O–Cr. Also, after Cr (VI) biosorption on ECx, the peak of the EC-S group is shifted to 590. This can be explained by surface complexation or ion exchange35.
In general, comparable results reported in the literature for cellulose in the absorption of other toxic metals, as for other cellulose-derived biosorbentes in the removal of Cr (VI) ions36.
One way to corroborate the information presented in the FTIR measurements is through SEM images since with these images it is possible to observe the distribution of the reagents in the ECx biomass treatment and subsequently the Cr (VI) adsorption process.
SEM–EDX
Figure 2 shows the micrographs obtained for the biomass before (a) the adsorption of Cr (VI), in addition to showing the distribution of the different biomass chemical modifications in (b) and in (c) it shows the distribution of chromium around all biomasses.
From Fig. 2a, it can see that the biomass has a very irregular rough surface, with macropores and cracks. Many of these irregularities may associated with damage caused by the delignification process of E. crassipes cellulose with NaOH14. In Fig. 2b it is possible to visualize the components of the cellulose xanthogenate, coming from sodium, distributed throughout the biomass, a result like that reported in other studies35 The colored dots represent the elements in the samples, green dots represent carbon, red dots represent oxygen, and yellow dots represent the places where sodium lodged.
Table 2 shows that, in addition to carbon and oxygen, the element with the greatest presence in the composition of pure waste is sodium and sulfur from the xanthogenate cellulose transformation process. Table 2 shows the physicochemical characterization of the ECx sample, through EDS.
Cellulose xanthogenate, is one of the cellulose transformations to improve the adsorption performance of heavy metals, this compound produced from dry and ground biomass, mixing with sodium hydroxide (NaOH) to remove lignin, creating alkaline biomass, then disulfide (CS2) added13,14. (CS2) reacts with hydratable hydroxycellulose, forming C-SNa complexes; these are responsible for the cation exchange with heavy metals. Metal ions enter the interior of E. crassipes with (CS2), exchanging with Na36,37.
The SEM morphology of ECx and coupled with the high content of sulfides (7.3%) determined by the spectrum in Table 2, it further confirms that xanthate groups are successfully grafted onto the biomass of E. crassipes, and Fig. 3 represents this information based on13,36,37,38.
Exchange biochemistry is usually identified as the main mechanism for the adsorption of metals in cellulose and its derivatives35 and through the evaluation of EDS this process could verify. Similar observations were made by36 where the adhesion of Cr (VI) in this biomass was observed. Also, in xanthogenate cellulose processes, the adhesion of Pb (II) to this type of biomass verified, concluding that this cellulose is important in the removal of heavy metals from water13.
The SEM morphology of ECx with Cr (VI) coupled with the high content of sulfides determined by the spectrum in Table 3, was the determinate for the chemisorption’s of Cr (VI). The mechanism of Cr (VI) sorption by cellulose xanthate is:
where [4(C6H12O6)] *2CS2Na represents the xanthogenate biomass, and Cr2O7–2 represents the Cr (VI), that 4 parts of glucose xanthate react with the dichromate. In the Tables 3 and 4, the relationship between cellulose xanthogenate and Cr (VI), with related weights of 10.4 for Cr (VI).
Mass balance in treatment
Adsorption is the phenomenon through which the removal of Cr (VI) achieved in the treatment systems; this quantified by means of the general balance equation of the treatment system as shown in Fig. 3.
Adsorption is the phenomenon through which the removal of Cr (VI) achieved in treatment systems, this quantified by mass balance. Equation (1) shows the general balance of matter in the treatment system, together with the accumulation, inputs, and outputs of the system and the chemical process of adsorption.
Accumulation represents by Eq. (1), where ∂C(VI) is the contaminant input to the treatment system, (ε) is the porosity of the bed, which calculated as the ratio between the density of the bed of treatment and the density of the microparticle of this biomass. This parameter must be above 0.548 achieved using particle diameters less than 0.212 mm, which favors contact between the contaminant and the particle49. The contaminant input to the treatment system represents by the design speed and the amount of contaminant that the system could treat. The output in the treatment system represents by the same input speed and the amount of contaminant that comes out. With these equations, the general material balance will be complete, summarized in Eq. (2), where it can see that the accumulation is equal to the input to the system, minus the output, and minus the adsorption.
where V = System volume (ml), ε = Porosity, Co = Initial concentration of Cr (VI) (mg/ml), C = Final concentration Cr (VI) in the treated solution (mg/ml), Q = design flow (ml/min), Tb = Breaking time (Min), M = amount of biomass used (g), q = Adsorption capacity of the biomass used (mg/g).
Depending on the most important parameters when building a treatment system, Eq. (3) could use to model and validate the best form of treatment, for example, the necessary amount of biomass to use to treat a certain amount of contaminant, in the present investigation it used to establish the adsorption capacity in these initial treatment conditions. The remaining Eq. (4) determines the adsorption capacity.
Adsorption capacity is generally taken through Eq. (5) for both batch and continuous experiments20,21But unlike Eqs. (5), (4) takes into account design variables such as flow rate (Q), rupture time (Tb), particle bed porosity ε, and vessel design volume (v).
where m: Mass used in the treatment, V: Volume, Co: Initial concentration, C: Final Concentration, Q: adsorption capacity.
However, unlike Eqs. (5), (4) considers the design variables such as flow rate (Q), rupture time (Tb), particle bed porosity ε and vessel design volume (v).
When a desorption-elution process is involved for the reuse of biomass, Eq. (4) would be:
where Q = design flow (ml/min), Tbj = Break time of use number j (Min), Co = Initial concentration of Cr (VI) (mg/ml), C = Final concentration Cr (VI) in the treated solution (mg/ml), V = System volume (ml), ε = Porosity, M = amount of biomass used (g), q_T = Total adsorption capacity of the biomass used (mg/g).
This model (6) is design to determine the adsorption capacity when different elution processes have conducted, it will used to determine the new adsorption capacity and is one of the contributions of the present investigation.
Result process of adsorptions
In Fig. 4 shows the Cr (VI) adsorption process of the system.
Various researchers have extensively studied the influence of factors such as bed height, flow rate and metal inlet concentration on rupture (Tb) curves. For example, the influence and similarity of the initial contaminant concentrations should be reflected as in the case of a tannery, with initial concentrations of 600 mg/l. Figure 4 shows the progress curves obtained for the study of Cr (VI) removal by the studied biomasses, reflecting the percentage of Cr (VI) removal in contrast to the treated volume, which is a very important parameter to time to scale the process.
Regarding the effect of the input concentration, it can see in Fig. 5 that the breakpoint had a better performance in all the initial concentrations in the ECx biomass. comparing it with the EC-Na biomass (see Fig. 5), always obtaining breakpoints with more treated volume.
The difference between the rupture curves between ECx and EC-Na indicates that the cellulose xanthate modification scheme should completed, although it can also elucidate that the EC-Na biomass has high yields compared to other biomass studied. for example, in Ref.34 investigate the biomass of E. crassipes without modifying, having removals below this alkaline cellulose.
Adsorption capacities
Through Eq. (3), the adsorption capacity of ECx, using the initial concentration of 600 mg/l, since it was the maximum concentration used.
The break point was around 1200 ml according to Fig. 6 and together with the flow rate of 15 ml/min; the break time obtained in 80 min.
q: Adsorption capacity, Co: 0.6 mg/ml, C: 0.06 mg/ml, M: 40 g, Tb: rupture time 80 min, Q: 15 Flow rate ml/min, ε: 0.6649, V: Occupied volume: 70 ml.
A result of 16 mg/g obtained in this continuous study for the biomass ECx. With this same equation it gives the capacity of the biomass EC-Na, with 11 mg/g.
Desorption-Elution and reuse
Through Eq. (6), the sum of the Cr (VI) adsorption capacities established, after different biomass reuses due to EDTA elution. In the second treatment process, it yielded the following results under concentrations of 6 g/l of EDTA.
Co: 0.6 mg/ml, C: 0.06 mg/ml, M: 45 g Biomass eluted with EDTA, Tb: rupture time: 60 min, Q: 15 Flow ml/min, ε: 0.6649, V: Occupied volume: 68 ml, q: 10 mg/g.
Five Cr (VI) adsorption cycles performed using ECx and EC-Na cellulose in a continuous system to evaluate the regeneration and reuse potential. Between each biosorption cycle, a desorption cycle performed using three different concentrations of EDTA eluent.
According to Figs. 6 and 7, although the adsorption capacity gradually decreases from the first adsorption process, it could consider that it is a satisfactory biomass recycling process and a design parameter for later stages of this treatment system.
In the experiments with concentrations of 6 g/l, five reuse processes obtained, obtaining a final sum of 52 mg/g. In concentrations of 3 g/l of EDTA, final capacities of 51 mg/g obtained lower than concentrations of 6 g/l but with half of this reagent. With concentrations of 1 g/l, final capacities of 33 mg/g obtained.
The desorption processes of the EC-Na biomass with initial capacities of 11 mg/g were also evaluated and through desorption processes with EDTA of 3 g/l this biomass recycled on 5 occasions, reaching 32 mg/l in capacities of adsorption and like the EC-Na biomass, the ideal concentration in the process for desorption processes is 3 g/l, due to the considerable increase in reuse processes and low concentration compared to 6 g/l, which, although higher, does not this value is significant in the absorption capacity.
Through Eq. (6) and with different bibliographic references, representative data obtained to feed this equation, determining the capacities of each of these biomasses together with the new capacities determining the desorption power of the different eluents shown and summarized in Table 4.
For the EDTA eluent and with Eq. (6), satisfactory results evidenced by removing Al (II), reaching almost 150% of its adsorption capacity, corroborating what presented in the present investigation, also the EDTA reagent obtained interesting yields to recycle the cassava biomass increasing up to 40 mg/g. In Ref.39 used the biomass of Phanera vahlii to remove Cr (VI) obtaining results of 30 mg/g and with NaOH they reached capacities in the reuse process of this biomass up to 62 mg/g, reaching almost double of its total capacity41, also used NaOH for desorption processes with green synthesized nanocrystalline chlorapatite biomass, achieving results of 75% more. The eluent HCl is also a good chemical agent to use in desorption processes since it reached more than 100% in the reuse of biochar alginate for Cr (VI) but not so significant with biomass A. barbadensis Miller to remove Ni (II) and in40 significant results were also obtained to remove Pb (II) with pine cone Shell biomass. With the chemical agent HNO3, interesting contaminant recycling processes obtained, since more than 100% of the adsorption capacity of the biomasses used in this process used1,45.
Mathematical models of adsorption
In general, the models presented R2 greater than 0.95 for the adjustment of all the advance curves, which indicates a good adherence to the data, the model that best describes the behavior of the ECx system was the phenomenological model Thomas, which presented all the R2 values above 0.99.
This model could use for the extension of the Cr (VI) ion biosorption system using cellulose xanthogenate, in the literature it is possible to observe that this model often tends to better adapt to the experimental data of the adsorption systems that use cellulose for the absorption of toxic metals28,30,31.
With qt values remarkably close to the experimental values of Eq. (4) designed and presented in this investigation, indicating the validity of this equation where it reflects the maximum capacity obtained. Table 5 shows the adsorption constant of the Thomas model (Kt), which corresponds to the adsorption rate of Cr (VI) in the biomass49 This value was 0.048 (ml/mg*min) reflecting the speed with which Cr (VI) is chemisorbed in the biomass of ECx, in the EC-Na cellulose there was a Thomas model speed of 0.039 (ml/ mg*min) evidencing a lower adsorption rate than ECx. In the adsorption of Cr (VI) by rice biomass, the Thomas constant is 0.1 (ml/mg*min)47,50 also in the adsorption of Cr (VI) by biomass. Nanocrystalline chlorapatite biomass obtained at the Thomas constant 0.013 (ml/mg*min)49.
In the Table 6, it presents summary of the experiments obtained with material EC-Na.
The Cr (VI) adsorption process in the EC-Na biomass represented through the Bohart equation, since the sorption rate is proportional to the biomass capacity, obtaining an adsorption rate of 0.85(ml/mg*min). Having an alkalized biomass represents this model due to the homogeneity of this adsorbent.
Mathematical models in desorption processes
The continuous desorption process with its fit to the Thomas model for biomass ECx always shows the fit of this model with significance, because this type of model fits representatively to desorption processes with good performance32,51 It can also verify that with values of qt it is close to the experimental values of Eq. (6) designed and presented in this research, indicating the validity of this equation again, where it reflects the maximum capacity obtained.
In the Table 7. Show Summary of the experiments obtained with material ECx in process of desorption’s.
In the Table 8 the EC-Na biomass had a different behavior and in its second and third cycle it adjusted to the Yoon model and later to the Bohart model.
This behavior is due to the alkalinization of the biomass and this process makes the biomass a little more unstable. The values of qt, although a resemblance evidenced, were not so representative due to the little adjustment that there was with respect to the Thomas model.
Conclusions
In this work, a treatment system designed and evaluated to remove Cr (VI) through cellulose from E. crassipes xanthate. SEM/EDX analysis tests showed that ion exchange mechanisms involved in the removal of Cr (VI) by ECx and EC-Na. The regeneration and reuse studies of sorbents showed that the removal capacity remained satisfactory in the five cycles conducted, being the 3 g/l EDTA solution the best result for the reuse of the evaluated biomasses. The model developed in the present investigation through mass balance validated through the Thomas model since this model was the one that best described the adsorption process. Concluding that cellulose xanthate is a promising alternative biomass for the removal of Cr (VI) ions in a dynamic system. Future studies should carry out on larger scales with a view to industrial application, since this biomass presents, in addition to its good adsorption capacity, several advantages such as low cost, high availability, and promising regeneration/reuse.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
de Sá Costa, H. P., da Silva, M. G. C. & Vieira, M. G. A. Fixed bed biosorption and ionic exchange of aluminum by brown algae residual biomass. J. Water Process Eng. 42, 102117 (2021).
Carreño Sayago, U. F. Design, scaling, and development of biofilters with E. crassipes for treatment of water contaminated with Cr (VI). Water 13(9), 1317 (2021).
Saman, N. et al. High removal efficacy of Hg (II) and MeHg (II) ions from aqueous solution by organoalkoxysilane-grafted lignocellulosic waste biomass. Chemosphere 171, 19–30 (2017).
Lin, C. et al. A study on adsorption of Cr (VI) by modified rice straw: Characteristics, performances and mechanism. J. Clean. Prod. 196, 626–634 (2018).
Anirudhan, T. S., Deepa, J. R. & Christa, J. Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt (II) from nuclear industry wastewater samples. J. Colloid Interface Sci. 467, 307–320 (2016).
Martínez, C., Torres, L. M. & de la Cruz, R. F. G. Evaluación de la cinética de adsorción de Zn2 + y Cd2 + a partir de soluciones binarias Unitarias y por Eichhornia crassipes Raíces de Typha latifolia y. Avances en Ciencias e Ingeniería 4(2), 1–14 (2013).
Olivito, F. & Jagdale, P. New technologies to decontaminate pollutants in water: A report about the state of the art. Toxics 10(3), 128 (2022).
Olivito, F. et al. Cellulose citrate: A convenient and reusable bio-adsorbent for effective removal of methylene blue dye from artificially contaminated water. RSC Adv. 11(54), 34309–34318 (2021).
Adornado, A. P., Soriano, A. N., Orfiana, O. N., Pangon, M. B. J. & Nieva, A. D. Simulated Biosorption of Cd (II) and Cu (II) in single and binary metal systems by water hyacinth (Eichhornia crassipes) using aspen adsorption. ASEAN J. Chem. Eng. 2, 21–43 (2017).
Rani, N., Singh, B. & Shimrah, T. Chromium (VI) removal from aqueous solutions using Eichhornia as an adsorbent. J. Water Reuse Desalin. 7(4), 461–467 (2017).
Mahunon, S. E. R. et al. Optimization process of organic matter removal from wastewater by using Eichhornia crassipes. Environ. Sci. Pollut. Res. 25(29), 29219–29226 (2018).
Tare, V. & Chaudhari, S. Evaluation of soluble and insoluble xanthate process for the removal of heavy metals from wastewaters. Water Res. 21(9), 1109–1118 (1987).
Deng, L. et al. Effect of chemical and biological degumming on the adsorption of heavy metal by cellulose xanthogenate prepared from Eichhornia crassipes. Bioresour. Technol. 107, 41–45 (2012).
Zhou, Y., Fu, S., Zhang, L., Zhan, H. & Levit, M. V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb (II). Carbohyd. Polym. 101, 75–82 (2014).
Kumar, V., Singh, J. & Kumar, P. Regression models for removal of heavy metals by water hyacinth (Eichhornia crassipes) from wastewater of pulp and paper processing industry. Environ. Sustain. 3(1), 35–44 (2020).
Purnomo, R. O. Delignifikasi adsorben dari batang eceng gondok (Eichhornia crassipes) untuk adsorpsi nikel (II) menggunakan metode batch. SKRIPSI Mahasiswa UM (2021).
Gaballah, M. S. et al. Effect of design and operational parameters on nutrients and heavy metal removal in pilot floating treatment wetlands with Eichhornia Crassipes treating polluted lake water. Environ. Sci. Pollut. Res. 28(20), 25664–25678 (2021).
Mitra, T., Singha, B., Bar, N. & Das, S. K. Removal of Pb (II) ions from aqueous solution using water hyacinth root by fixed-bed column and ANN modeling. J. Hazard. Mater. 273, 94–103 (2014).
Nag, S., Mondal, A., Mishra, U., Bar, N. & Das, S. K. Removal of chromium (VI) from aqueous solutions using rubber leaf powder: Batch and column studies. Desalin. Water Treat. 57(36), 16927–16942 (2016).
Pavlidis, G. et al. Performance of pilot-scale constructed floating wetlands in the removal of nutrients and pesticides. Water Resour. Manag. 36(1), 399–416 (2021).
Choy, K. K., Ko, D. C., Cheung, C. W., Porter, J. F. & McKay, G. Film and intraparticle mass transfer during the adsorption of metal ions onto bone char. J. Colloid Interface Sci. 271(2), 284–295 (2004).
Javanbakht, V., Ghoreishi, S. M. & Javanbakht, M. Mathematical modeling of batch adsorption kinetics of lead ions on modified natural zeolite from aqueous media. Theor. Found. Chem. Eng. 53(6), 1057–1066 (2019).
Qiao, L., Li, S., Li, Y., Liu, Y. & Du, K. Fabrication of superporous cellulose beads via enhanced inner cross-linked linkages for high efficient adsorption of heavy metal ions. J. Clean. Prod. 253, 120017 (2020).
Xiao, Y. et al. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 140, 225–233 (2019).
Jiang, X., An, Q. D., Xiao, Z. Y., Zhai, S. R. & Shi, Z. Versatile core/shell-like alginate@ polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mater. Res. Bull. 118, 110526 (2019).
Jin, Y. et al. Batch and fixed-bed biosorption of Cd (II) from aqueous solution using immobilized Pleurotus ostreatus spent substrate. Chemosphere 191, 799–808 (2018).
Carreño-Sayago, U. F. Development of microspheres using water hyacinth (Eichhornia crassipes) for treatment of contaminated water with Cr (VI). Environ. Dev. Sustain. 23(3), 4735–4746 (2020).
Golie, W. M. & Upadhyayula, S. Continuous fixed-bed column study for the removal of nitrate from water using chitosan/alumina composite. J. Water Process Eng. 12, 58–65 (2016).
Decree 1376. Reglamenta el permiso de recolección de especímenes de especies silvestres de diversidad biológica con fines de investigación científica no comercial. Ministerio De Medio Ambiente y Desarrollo Sostenible De Colombia. (2013). Taken on January 20, 2020. https://www.minambiente.gov.co/images/normativa/decretos/2013/dec_1376_2013.pdf.
Tejada-Tovar, C. N., Villabona-Ortíz, A., Alvarez-Bajaire, G. & Granados-Conde, C. Influencia de la altura del lecho sobre el comportamiento dinámico de columna de lecho fijo en la biosorción de mercurio. TecnoLógicas 20(40), 71–81 (2017).
Chen, S. et al. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. Biores. Technol. 113, 114–120 (2012).
Arukula, D. et al. Treatment of tannery wastewater using aluminium formate: Influence of the formate over sulphate-based coagulant. Glob. NEST J 20, 458–464 (2018).
Yahya, M. D., Abubakar, H., Obayomi, K. S., Iyaka, Y. A. & Suleiman, B. Simultaneous and continuous biosorption of Cr and Cu (II) ions from industrial tannery effluent using almond shell in a fixed bed column. Results Eng. 6, 100113 (2020).
Sayago, U. F. C. Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI). Sci. Rep. 11(1), 1–16 (2021).
Wang, C., Wang, H. & Gu, G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb (II) sorption. Carbohyd. Polym. 182, 21–28 (2018).
Wang, N. et al. One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: Effect of plant species and roles of xanthation. Chemosphere 266, 129129 (2021).
Pillai, S. S. et al. Biosorption of Cd (II) from aqueous solution using xanthated nano banana cellulose: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 98, 352–360 (2013).
Sayago, U. F. C., Castro, Y. P., Rivera, L. R. C. & Mariaca, A. G. Estimation of equilibrium times and maximum capacity of adsorption of heavy metals by E. crassipes. Environ. Monit. Assess. 192(2), 1–16 (2020).
Ajmani, A., Shahnaz, T., Subbiah, S. & Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 26, 32137–32150 (2019).
Gupta, S. & Jain, A. K. Biosorption of Ni (II) from aqueous solutions and real industrial wastewater using modified A. barbadensis Miller leaves residue powder in a lab scale continuous fixed bed column. Clean. Eng. Technol. 2021(5), 100349 (2021).
Han, X. et al. Enhanced Cr (VI) removal from water using a green synthesized nanocrystalline chlorapatite: Physicochemical interpretations and fixed-bed column mathematical model study. Chemosphere 264, 128421 (2021).
Ghasemabadi, S. M., Baghdadi, M., Safari, E. & Ghazban, F. Investigation of continuous adsorption of Pb (II), As (III), Cd (II), and Cr (VI) using a mixture of magnetic graphite oxide and sand as a medium in a fixed-bed column. J. Environ. Chem. Eng. 6(4), 4840–4849 (2018).
Haroon, H. et al. Activated carbon from a specific plant precursor biomass for hazardous Cr (VI) adsorption and recovery studies in batch and column reactors: Isotherm and kinetic modeling. J. Water Process Eng. 38, 101577 (2020).
Martín-Lara, M. A., Blázquez, G., Calero, M., Almendros, A. I. & Ronda, A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fixed bed columns. Int. J. Miner. Process. 148, 72–82 (2016).
Barquilha, C. E., Cossich, E. S., Tavares, C. R. & da Silva, E. A. Biosorption of nickel and copper ions from synthetic solution and electroplating effluent using fixed bed column of immobilized brown algae. J. Water Process Eng. 32, 100904 (2019).
Simate, G. S. & Ndlovu, S. The removal of heavy metals in a packed bed column using immobilized cassava peel waste biomass. J. Ind. Eng. Chem. 21, 635–643 (2015).
Zhou, G., Luo, J., Liu, C., Chu, L. & Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 131, 246–254 (2018).
Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling (Walter de Gruyter, 2012).
Iváñez, M. Diseño de un sistema de adsorción para la remoción de fenol presente en disolución acuosa (Doctoral dissertation) (2017).
Saralegui, A. B., Willson, V., Caracciolo, N., Piol, M. N. & Boeykens, S. P. Macrophyte biomass productivity for heavy metal adsorption. J. Environ. Manage. 289, 112398 (2021).
Sayago, U. F. C., Castro, Y. P. & Rivera, L. R. C. Design of a fixed-bed column with vegetal biomass and its recycling for Cr (VI) treatment. Recycling 7, 71 (2022).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayago, U.F.C., Ballesteros Ballesteros, V. Development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Sci Rep 13, 1970 (2023). https://doi.org/10.1038/s41598-023-28292-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28292-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.