Abstract
Flexible endoscopic evaluation of swallowing (FEES) is considered the gold standard in diagnosing oropharyngeal dysphagia. Recent advances in deep learning have led to a resurgence of artificial intelligence-assisted computer-aided diagnosis (AI-assisted CAD) for a variety of applications. AI-assisted CAD would be a remarkable benefit in providing medical services to populations with inadequate access to dysphagia experts, especially in aging societies. This paper presents an AI-assisted CAD named FEES-CAD for aspiration and penetration detection on video recording during FEES. FEES-CAD segments the input FEES video and classifies penetration, aspiration, residue in the vallecula, and residue in the hypopharynx based on the segmented FEES video. We collected and annotated FEES videos from 199 patients to train the network and tested the performance of FEES-CAD using FEES videos from other 40 patients. These patients consecutively underwent FEES between December 2016 and August 2019 at Fukushima Medical University Hospital. FEES videos were deidentified, randomized, and rated by FEES-CAD and laryngologists with over 15 years of experience in performing FEES. FEES-CAD achieved an average Dice similarity coefficient of 98.6\(\%\). FEES-CAD achieved expert-level accuracy performance on penetration (92.5\(\%\)), aspiration (92.5\(\%\)), residue in the vallecula (100\(\%\)), and residue in the hypopharynx (87.5\(\%\)) classification tasks. To the best of our knowledge, FEES-CAD is the first CNN-based system that achieves expert-level performance in detecting aspiration and penetration.
Similar content being viewed by others
Introduction
Dysphagia is a disorder of swallowing commonly occurring in patients with strokes and neurological diseases1. Oropharyngeal dysphagia is typically caused by abnormalities of muscles, nerves, or structures of the oral cavity, pharynx, or the sphincter at the top of the esophagus, and about 30–50\(\%\) of stroke patients may suffer from longstanding oropharyngeal dysphagia2,3. Oropharyngeal dysphagia after stroke is the root cause of health complications like aspiration pneumonia, dehydration, and malnutrition. Aspiration and penetration are the most severe complications caused by oropharyngeal dysphagia. Aspiration is defined as the passage of material below the vocal fold (into the subglottis), and penetration is the passage of material into the laryngeal vestibule but not into the subglottis. Jaderberg et al.4 suggest that mild aspiration in healthy adults may not bring about medical complications. However, Crausaz et al.5 record that a degree of aspiration in stroke patients can result in serious medical consequences, including bacterial pneumonia, chemical pneumonitis, and even death. As a tertiary prevention after stroke, the prevention and treatment of aspiration are critical in increasing stroke survivors’ survival rates6.
Flexible endoscopic evaluation of swallowing (FEES) and videofluoroscopic swallow study (VFSS) represent the gold standards in studying oropharyngeal dysphagia7. Considering the lower detection performance of FEES without video recording, FEES with video recording or videofluoroscopy is mainly used to detect aspiration and penetration accurately. Videofluoroscopy can detect aspiration and penetration more accurately but requires radiation exposure. Onofri et al.8,9,10,11 reported the comparison between the diagnostic values of FEES and VFSS regarding the quality of aspiration and penetration detection. Their experiments show that FEES has many advantages over VFSS: (1) uses test bolus with no radiation exposure or barium; (2) does not limit the exam length; (3) assesses patient endurance during a meal; (4) views the larynx and pharynx directly; and (5) can yield significant performance gain over VFSS in identifying aspiration and penetration. However, accurate detection of aspiration and penetration by FEES after swallowing remains a clinical challenge for inexperienced doctors because white-out and/or motion of swallowing is so fast, usually only lasting 0.3 s12. Furthermore, the interpretation results of FEES may vary among examiners with different levels of experience although they are required to evaluate swallowing impairment appropriately. As a result, the interdifference in diagnosis would significantly affect the clinical course of patients with swallowing impairment such as the incidence of aspiration pneumonia after FEES. One potential solution is to detect aspiration and penetration with artificial intelligence (AI)-assisted FEES.
To date, studies of AI-assisted methods have been performed using traditional machine learning methods13 and traditional image-based AI-assisted medical equipment14. However, traditional supervised learning models like support vector machines have not reached a satisfactory level of performance and were developed slowly in the past few decades. The breakthroughs of AI-assisted medical equipment took place in the last decade15 when researchers utilized a convolutional neural network (CNN) to analyze medical images and achieved the practical standard. Ahsan et al.16 developed a method based on CNNs to predict and differentiate between COVID-19 and non-COVID-19 patients. Islam et al.17 used a pretrained deep learning model’s weight to detect potato diseases from leaf images. Danala et al.18 proposed a deep transfer learning-based CAD to classify breast lesions with a relatively large and diverse retrospective dataset. Attention modules19 have arguably become a valuable component in deep learning. Attention mechanism are based in a common-sense intuition that people focus to a certain part when processing a large amount of information. CNN-based systems lack long-range pixel-pixel dependencies that are present in an image20. Many efforts have been made on exploiting long-range pixel-pixel dependencies for CNN to model global contextual representations in biomedical images, and achieved significantly improved biomedical image segmentation performance21,22,23. Islam et al.24 improved the performance of breast lesion classification with attention mechanisms. Rajpurkar et al.25 evaluated CNN-based computer-aided diagnosis (CAD) through competing with four radiologists with 4, 7, 25, and 28 years’ experiences in detecting pneumonia from frontal-view chest X-ray images. The CAD achieved expert-level performance on the F1 metric. Moreover, Tschandl et al.26 conducted a web-based comparison between their CNN based CAD and 95 dermatologists in diagnosing skin cancer, and the CAD achieved better performance than that of beginners and intermediate dermatologists but slightly lower performance than that of expert dermatologists with over 10 years’ experiences.
A massive number of annotated data are of prime importance to improve CNN’s performance. Many works27 were focused on leveraging images from large-scale nonmedical datasets such as ImageNet28. For medicine-related implementation, existing methods can only be retrained on small-scale medical datasets29. As a result, CNN has to initialize many hidden layers previously trained on large-scale nonmedical image segmentation to ensure fine-tuned models have promising results30. Transfer across acquisition protocols or imaging modalities (domain adaptation) has better performance than that of transfer from nonmedical images. Karimi et al.31 improved target task segmentation performance with images acquired by different acquisition protocols or imaging modalities. Ghafoorian et al.32 reported a CNN, that was transferred from images by different scanners and fine-tuned on two target samples, achieved an average Dice score of 48 \(\%\) over a unpretrained model. Zhu et al.33 used generative adversarial networks to transfer knowledge to medical images in a target modality from images in another.
However, each aforementioned domain-adapted CNN requires a feasible large-scale labeled medical dataset. This problem has been partially addressed by recent self-supervised and semisupervised learning methods and applications in medical image analysis. Self-supervised and semisupervised learning approaches aim to learn from carefully chosen unlabeled medical images and then transfer knowledge by finetuning CNN on labeled target medical images. Self-supervised methods34 pretrain CNN with unlabeled images. Surrogate task-based self-supervised methods transfer knowledge from discriminating between a set of classes on surrogate tasks, e.g., image inpainting35, image colorization36, relative pixel matching37, jigsaw puzzles solving38, and rotation prediction39. Contrastive learning40 is a popular form of surrogate task-based self-supervised learning. It reduces the distance of sampled pairs from the same surrogate class and enlarges the distance of sampled pairs from different classes.
The generalizability of transferred knowledge by surrogate task-based self-supervised learning relies on building a target-related and easy pretrained task41. However, collecting a suitable unlabeled dataset is difficult and may be impossible for a specific target task. The segmentation performance of recent AI-assisted CAD is lower in clinical practice than that in development stages because of data heterogeneity and interobserver variability42. The reliability of practical clinical applications at a hospital with a specific acquisition protocol and imaging modality may partially be improved through training by their own annotated datasets even though annotation is expensive and time-consuming.
Despite the prevalence of CNN, existing models43,44,45 and training strategies46,47 have rarely been used in detecting aspiration and penetration with FEES. Expert-level automated detection of aspiration and penetration from FEES would be clinically significant for beginner and intermediate clinicians because outward signs or symptoms of dysphagia have inter- and intraindividual differences. Moreover, AI-assisted CAD would be a remarkable benefit in providing healthcare to populations with inadequate access to dysphagia experts, especially in aging societies48. This paper presents an AI-assisted CAD that can automatically detect aspiration and penetration by segmenting the vocal fold, subglottis, laryngeal vestibule, test bolus, and white-out on video recording during FEES at an expert-level performance. For the experiment, the proposed FEES-CAD was trained using 25,630 expert-annotated images from 199 complete videos recorded during FEES and tested on 40 FEES videos. To the best of our knowledge, FEES-CAD is the first CNN-based system that achieves expert-level performance in detecting aspiration and penetration49,50.
Background
The proposed AI-assisted CAD named FEES-CAD addresses the need for aspiration and penetration detection on FEES by monitoring the movement of test bolus in oropharyngeal regions. Its main task is to segment objects of interest in FEES video frames, i.e., the vocal fold, subglottis, laryngeal vestibule, test bolus, and white-out. This section reviews recent approaches to medical image segmentation and their potential limitations.
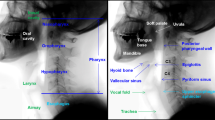
Many techniques have been proposed to realize more accurate segmentation, e.g., attention mechanism51, domain knowledge52, and uncertainty estimation53. In the past decade, CNN plus attention mechanism has been introduced in medical image segmentation. An attention mechanism that simulates the human brain’s capability to sort out information was first introduced by Bahdanau et al.54 for machine translation. An expansive path was designed to dynamically select useful source language words or subsequences for translation into a target language. Recently, its variant, self-attention55, is widely implemented in medical image segmentation. However, self-attention has a fundamental issue in supervised medical image segmentation, where extra parameters have to be introduced to CNN. This makes CNN easy to overfit with the training data. However, medical image segmentation tasks usually require higher accuracy than natural image segmentation tasks. Figure 1a shows an example of the original video frames, and Fig. 1b shows an example of the segmented video frames. The objects of interest include the aspiration area (red) created by the two anatomic landmark structures of the vocal fold and subglottis, the penetration area (purple) created by the laryngeal vestibule, and the annotation of jelly as test bolus (green). Changing the angle and lighting in a video recording during FEES makes some strong outliers in the training data in the test bolus. Classification of test bolus would locate more in the domain of the other classes, and then overfitting causes shifted boundaries of these outliers in some directions. Such shifted boundaries result in high false positives as partial domains belonging to other classes are occupied by the test bolus. Aspiration and penetration detection aims to analyze if the test bolus enters the larynx or even below the true vocal. While a high false positive may be uncritical in some medical tasks, it may cause unreliable aspiration and penetration detection results.
Methods
Flexible endoscopic evaluation of swallowing
As shown in Fig. 2a, FEES was performed using a laryngeal flexible endoscope with a diameter of 2.6 mm (ENF-V3, O, OLYMPUS, Corporation Tokyo, Japan), and the corresponding videos were recorded. Figure 3 illustrates the flowchart of this study. The study subjects were consecutive patients with suspected oropharyngeal dysphagia who underwent FEES between December 2016 and August 2019 at Fukushima Medical University Hospital56. The research protocol was prepared in accordance with the Declaration of Helsinki, and this research was approved by the Institutional Review Board and Ethics Committee of Fukushima Medical University (2019-154). Each participant signed informed consent for FEES before this examination was conducted. FEES, which was video-recorded, was performed by transnasally passing a laryngeal flexible endoscope. Aspiration and penetration are the most severe complications caused by oropharyngeal dysphagia, of which aspiration is when the passage of food below the vocal fold (into the subglottis), and penetration means the passage of food into the laryngeal vestibule but not into the subglottis. However, it is relatively hard to detect subglottis regions in swallowing endoscopy compared with the vocal fold. In this experiment, the aspiration area is composed of two anatomic landmark structures of the vocal fold and subglottis as it indicates whether food is too close to the windpipe (trachea). As shown in Fig. 1, the original video recording during FEES allows clinicians to investigate the condition of the hypopharynx, arytenoid, epiglottis, vestibule, vocal fold, subglottis, vallecula, and base of the tongue. By contrast, FEES video segmentation highlights the outline of the aspiration area (red), the penetration area (purple), and food,i.e.,test bolus (green). In addition to the mentioned classes, FEES-CAD also recognizes the“none”and “white-out”images Fig. 2b).“White-out”is the overexposed image caused by the reducing distance between the posterior wall of the pharynx and the light from the endoscope, indicating the motion of swallowing. The motion of swallowing is very fast and takes about 0.3 s. Therefore,“white-out”detection is a critical FEES-specific swallowing task. When FEES-CAD segments the “white-out”, it records the starting point for“white-out”and reminds the laryngologists. Therefore, FEES-CAD is designed for a five-class segmentation problem.
(a) Example of the original video frames. Outline of the eight different anatomic landmark structures in a FEES video: the hypopharynx (1), arytenoid (2), epiglottis (3), vestibule (4), vocal fold (5), subglottis (6), vallecula (7), and the base of the tongue (8). (b) Example of the segmentation video frames. Annotation of aspiration area (red) created by the two anatomic landmark structures of the vocal fold and subglottis, penetration area (purple) created by the laryngeal vestibule, and annotation of jelly as test bolus (green).
Artificial intelligence-assisted computer-aided diagnosis
Figure 4 shows the workflow of the proposed FEES-CAD. FEES-CAD captures video frames from a FEES video and then inputs video frames into a CNN. Implemented CNN is extended from the commonly used architecture named UNet. The network architecture of customized UNet is depicted in Fig. 4(right). It consists of four contracting paths, four expansive paths, and a bottleneck connecting the last contracting path and the first expansive path. Every contracting path, expansive path, and bottleneck has two \(3\times 3\) convolutions with strides \(1\times 1\), both of which are activated by a Gaussian error linear unit. Each contracting path follows a \(2\times 2\) max-pooling with stride \(2\times 2\) to extract high-level semantic representations for image recognition. Each contracting path starts with an unpooling with stride \(2\times 2\), and the upsampled feature maps concatenate the feature maps derived from the corresponding contracting path. In the contracting path, expansive path, and bottleneck, every convolution follows a batch normalization57 before activation. At the end of the customized UNet, a softmax activated \(1\times 1\) convolution with strides \(1\times 1\) predicts the segmentation outcome. Finally, segmented images convert to a segmented video. The number of channels in the customized UNet architecture starts with 32, doubles after the max-pooling layers, and halves after the unpooling layers.
Experiments
The network of FEES-CAD was developed using Python 3.9 and the Tensorflow 2.6.0 framework. It was trained on a workstation with an Intel core i7-10700F processor CPU, 32.0 GB RAM, and NVIDIA GeForce RTX 3090.
Datasets
The network of FEES-CAD was developed on 25,630 expert-annotated images from 199 FEES videos and tested on 40 FEES videos shown in Table 1. The training and validation set includes 159 and 40 videos randomly selected from the 199 FEES videos for model development. The frame per second (fps) and size of videos recordings are 60 fps and 1920–1080, respectively. The average duration of training and validation videos recordings is 381.1 s, and that of the test videos is 384.8 s. There was no significant difference in diseases, age, height, and weight among the training, validation and test datasets. Videos were deidentified, randomized, and rated by an expert panel of laryngologists as well as dysphagia experts with over 15 years of experience performing FEES. The diagnosis of the underlying disease (primary disease) was confirmed before the test by the expert panel and CAD. The ground truth was determined through repeated video observation and discussion between 2016 and 2019 by laryngologists and dysphagia experts with 20 years’experience in performing FEES and also certificated as swallowing consultation doctors by The Society of Swallowing and Dysphagia of Japan. The expert panel was blinded to all identifying information about examinees and examined the test videos in real time combined with frame-by-frame analysis.
Training methodology
The input image size was resized as \(256\times 256\). Data augmentations were applied to training imaging through zooming and color jitter to randomly change the value of brightness, contrast, saturation, and hue. The network of FEES-CAD is trained from scratch and initialized with weights by a He-normal initializer58. All networks in these experiments use nondifferentiable activation. Hence, it is better to choose the He-normal initializer that provides a more efficient gradient descent than that using a nondifferentiable activation function.
Loss function directly affects training convergence and generalization performance of the network. Most AI-assisted CAD in medical image segmentation was designed for binary segmentation tasks. However, aspiration and penetration detection is a multiclass segmentation problem. FEES video segmentation is also an imbalance problem. To choose a suitable loss function, we performed comprehensive experiments on our dataset using various loss functions. Let \(y_{ci}\) and \(p_{ci}\) correspond to the ground truth and the output prediction, where c and i denote class and pixel, respectively. Generalized Dice loss (GDL) (\(\mathscr {L}_{\mathrm {GD}}\))59 originates from an overlap index named Dice similarity coefficient. GDL has the class re-balancing properties of the generalized Dice overlap and thus achieved good performance on different multiclass segmentation tasks. It can be formally written as
where \(w_{c}\) provides invariance to different class properties. However, the training stability when using GDL is dependent on the inherent uncertainty in a task60. Generalized Tversky loss (GTL)(\(\mathscr {L}_{\mathrm {GT}}\)) addresses this problem by adjusting weights of false positive (\(\alpha\)) and false negative (\(\beta\)). GTL is defined as
where \(p_{c i}\) denotes the probability of pixel i belonging to the class c and \(p_{\bar{c} i}\) denotes the probability of pixel belonging to other classes. \(y_{\bar{c} i}\) is 1 for class c and 0 for other classes, and conversely, \(y_{c i}\) takes values of 1 for other classes and 0 for class c. \(\mathscr {L}_{\mathrm {GT}}\) balances the weights for training. By adjusting the hyperparameters \(\alpha\) and \(\beta\), \(\mathscr {L}_{\mathrm {GT}}\) balances the cost of false positives and false negatives. \(\mathscr {L}_{\mathrm {GT}}\) is equal to \(\mathscr {L}_{\mathrm {GD}}\) in the case of \(\alpha = \beta = 0.5\).
Another popular loss function for training multiclass segmentation networks is categorical cross-entropy loss (\(\mathscr {L}_{\mathrm {CCE}}\)). It computes how distinguishable two discrete probability distributions are from each other, and its gradient is nicer than \(\mathscr {L}_{\mathrm {GD}}\) and \(\mathscr {L}_{\mathrm {GT}}\). \(\mathscr {L}_{\mathrm {CCE}}\) is computed as
The loss function of the customized UNet is a combination of \(\mathscr {L}_{\mathrm {GT}}\) and \(\mathscr {L}_{\mathrm {CCE}}\) to allow for some diversity in the loss while benefiting from the stability of CCE61. \(\mathscr {L}_{\mathrm {CCE+GT}}\) denotes a weighted sum of \(\mathscr {L}_{\mathrm {GT}}\) and \(\mathscr {L}_{\mathrm {CCE}}\):
The comparison of the loss function is presented in the Supplementary Material. The network of FEES-CAD minimizes loss with an adaptive moment estimation (ADAM) optimizer62. ADAM is derived from two stochastic gradient descent approaches, adaptive gradients63 and root mean square propagation64. It adaptively computes different learning rates for individual parameters from estimates of the first and second moments of the gradients. ADAM optimizer is often the most efficient stochastic optimization in deep learning applications. Figure 5 illustrates the progress of the training (dotted line) and validation loss (dotted line) with the number of epochs during training with DC (Green), FT (Blue), CCE (orange), DC plus CCE (pink), and FT plus CCE (red) loss function. All networks were trained for 100 epochs using an ADAM optimizer with a batch size of 8 because neither of the networks improved after 100 epochs. The initial learning rate is dependent on the networks and decays 1e−4 every five epochs when validation loss is not improved. For a given class C, \(true \ positive\) is pixel classified correctly as C, \(false \ positive\) is pixel classified incorrectly as C, \(true \ negative\) is pixel classified correctly as not C, and \(false \ negative\) is pixel classified incorrectly as not C. The quantitative performance is evaluated by Accuracy (\(\%\)), Sensitivity (\(\%\)), Specificity (\(\%\)), \(Dice \ similarity \ coefficient\) (DSC, \(\%\)), and 95\(\%\) \(Hausdorff \ distance (HD95)\):
Aspiration and penetration are historically the only indicators of interest in FEES because they are associated with potentially severe medical consequences65. Residue recently has been used as another clinical sign of swallowing dysfunction. Residue in the vallecula and the hypopharynx increases the risk of aspiration and can be aspirated after finishing FEES. Residue stuck in throats can also cause serious symptoms to people with dysphagia66 such as weight loss and malnutrition67. Our experiments use four indicators for assessing swallowing impairment severity, penetration, aspiration, residue in the vallecula, and residue in the hypopharynx. Test dataset consists of 40 videos, and thus the number of all classifications is 160. The expert panel blindly analyzed the original FEES videos of the test dataset to judge the presence or absence of penetration, aspiration, residue in the vallecula, and residue in the hypopharynx, while FEES-CAD classified the segmented FEES videos. The corresponding quantitative classification performance is also evaluated by accuracy(%), sensitivity(%), and specificity(%).
Ethical approval
The research protocol was prepared in accordance with the Declaration of Helsinki, and this research was approved by the Institutional Review Board and Ethics Committee of Fukushima Medical University (2019-154). Each participant signed informed consent for flexible endoscopic evaluation of swallowing before this examination was conducted.
Results
FEES-CAD is designed for analyzing FEES videos after patients have undergone FEES. It can be noted that aspiration and penetration detection is not time-bound. FEES-CAD processes images at 3.76 frames per second. The segmentation performance of the customized UNet is shown in Table 2, and the comparison against other networks is presented in the Supplementary Material. Tables 3, 4 and 5 show the comparison of quantitative classification performance between the FEES-CAD and expert panel.
Results of test FEES videos
As shown in Table 2, customized UNet manages semantic segmentation with a high level of performance. For test FEES videos, we observed that the FT and CCE loss are optimum for obtaining accurate pixel-level segmentation. When the customized UNet was trained on a combination of FT and CCE loss, it achieved the highest segmentation performance based on DSC (98.63\(\%\)), sensitivity (98.56\(\%\)), specificity (99.68\(\%\)), and HD95 (0.00). This high-quality segmentation guarantees satisfactory classification performance.
Table 3 lists the classification results of the expert panel and FEES-CAD. We have three major observations from Table 3. First, FEES-CAD achieved an average of 95.45\(\%\) sensitivity for overall classification (penetration, aspiration, residue in the vallecula, and residue in the hypopharynx), outperforming the expert panel. FEES-CAD provides outlines of objects of interest as it makes the detection and following of test bolus easier and is very useful in aspiration and penetration detection. Second, FEES-CAD reached a satisfactory level of performance in accuracy and specificity. Particularly, the expert panel outperformed FEES-CAD in specificity because dysphagia experts carefully examined the FEES videos multiple times to prevent false positives. Finally, FEES-CAD is more sensitive than the expert panel. Therefore, FEES-CAD can provide complementary information to improve the aspiration and penetration detection performance of clinicians.
Tables 4 and 5 show the evaluation of FEES-CAD for each classification task. Penetration and aspiration classifications are the main tasks. A patient is classified into penetration or aspiration only when the examiner can find a test bolus entering the laryngeal vestibule or the subglottis in the FEES video. FEES-CAD achieved 100\(\%\) on penetration classification, which is significantly better than that of the expert panel. In addition, FEES-CAD reached the same level of performance on aspiration classification. Residue in the vallecula and residue in the hypopharynx classification are also important because the residue may enter the vestibule and subglottis after finishing FEES and thus increases the risk of penetration and aspiration. FEES-CAD can perfectly classify the residue in the vallecula. Among all tasks, residue in the hypopharynx classification is the only one for which the expert panel attains higher sensitivity.
Discussion
The shortage of dysphagia experts and raters to rate FEES for diagnostic purposes has become a serious social problem68,69. The lack of timely diagnosis and treatment of dysphagia results in longer hospital lengths of stay, longer rehabilitation time, higher mortality, and higher healthcare expense69,70,71. Figure 6 illustrates confusion matrices of classification by expert panel and FEES-CAD. From the comparison between FEES-CAD and the expert panel shown in Tables 3–5, we found that the FEES-CAD achieves satisfactory results and outperforms the expert panel on several tasks. Because FEES-CAD guarantees a high sensitivity with reasonable specificity, it can be adopted in clinical practice to provide a timely and accurate diagnosis.
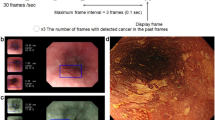
FEES-CAD demonstrated higher sensitivities and outperformed the expert panel on several tasks. The major problem for the expert panel in improving the classification performance is that they sometimes lose tracking. Figure 7 shows an example of two consecutive FEES images in Fig. 7a,b and their corresponding segmentation results in Fig. 7c,d. These images were extracted from a test video where FEES-CAD outperformed the expert panel. In this example, a small amount of the test bolus is stuck in the hypopharynx and covered by saliva. The expert panel could not detect penetration and aspiration in this case. Because the motion of swallowing was so fast and only took about 0.3 s, the expert panel hardly performed reliable test bolus trajectory tracking. The expert panel sometimes showed lower performance in reliable test bolus trajectory tracking compared with AI. FEES-CAD alleviates the lost tracking problem by using various colors to make objects stand out and therefore enhance the boundaries of interest. FEES-CAD is proven to have great potential in penetration and aspiration detection. More specifically, FEES-CAD achieves 100.00\(\%\) sensitivity, which is significantly better than that of the expert panel, on penetration detection.
FEES-CAD demonstrated lower performance in the detection of residue in the hypopharynx compared with the expert panel. As shown in Fig. 8, we use two consecutive FEES images in Fig. 7a,b and their corresponding segmentation results in Fig. 7c,d to analyze how the expert panel outperformed FEES-CAD. There were residues in the vallecula and the hypopharynx in these consecutive FEES images. Contrast mediums were added to test bolus during FEES to increase the contrast of fluids in the video recording during FEES and thus enhance the detection of residue and airway invasion (aspiration and penetration). However, residue in the hypopharynx might be covered by saliva, and the hypopharynx may be not properly illuminated because of changing angle and lightning from the FEES. UNet learns features pixel-wise and is sensitive to color contrast when distinguishing different objects because UNet can not maintain global spatial and multilevel semantic representations. Thus, UNet has difficulty classifying the liquid in the hypopharynx in some frames. When the network of FEES-CAD wrongly segments some pixels in the hypopharynx as residue, the FEES-CAD is misled to wrongly classify the case as residue in the hypopharynx. In FEES videos, the expert panel could avoid such mistakes based on clinical experience and demonstrates 0\(\%\) false positives in all classification tasks. Therefore, the expert panel outperforms FEES-CAD by a large margin on the residue in the hypopharynx classification.
In contrast to residue in the hypopharynx, residue in the vallecula is more likely to be properly illuminated. Therefore, the network of FEES-CAD can segment the residue in the vallecula with a high color contrast. The expert panel cannot recognize all residue in the vallecula caused by the fast motion of swallowing. Therefore, FEES-CAD achieves higher sensitivity and accuracy than the expert panel on the residue in the hypopharynx classification. Similarly, FEES-CAD achieves better performance on penetration because the vestibule is properly illuminated while the aspiration area, especially the subglottis, is often not properly illuminated. FEES-CAD is proven to have great potential in penetration and residue in the hypopharynx classification.
Limitations and future works
The proposed networks can distinguish test bolus, i.e., the most frequently used jelly-like test food in FEES72. The segmentation of other test boluses, such as colorful water, may improve the generalization and clinical usefulness of the proposed method. We still have to tackle the problem of oral intake of saliva, which may influence the observation of test bolus. The customized UNet is the most suitable network for our FEES video segmentation task. However, the segmentation performance of the customized UNet decreases when it is trained with more liquid classes. We can modify the customized UNet to enhance the segmentation performance in more liquid classes.
The proposed FEES-CAD should be improved to automatically classify aspiration and penetration. During the swallow, the pharynx and larynx are invisible when a white-out takes place. White-out is related to the decreasing distance between the pharyngeal walls and the light from the endoscope (pharyngeal constrictor muscle contraction)73. Therefore, in the two-dimensional FEES video, the test bolus can generally be observed easily if it appears in the hypopharynx and vallecula but cannot be detected if it enters the airway. In addition, in the proposed network it is difficult to judge whether the test bolus is stuck in the aspiration and penetration areas only using two-dimensional FEES videos. To overcome this problem, three-dimensional reconstruction from two-dimensional images may ease the detection procedure74.
The proposed FEES-CAD may be further improved in detecting residues in the hypopharynx. The structures of laryngeal and pharyngeal reduce endoscopy performance when the hypopharynx, vallecula, aspiration areas, and penetration areas are simultaneously illuminated. However, without proper illumination, it is also difficult to label residues in the hypopharynx to train the network. Therefore, we should perform the FEES with proper illumination on the hypopharynx when the test bolus is mixed with other liquids.
Conclusion
In this paper, we proposed FEES-CAD, a deep learning-based CAD, to analyze the FEES video for the detection of penetration, aspiration, residue in the vallecula, and residue in the hypopharynx. We collected and annotated the FEES videos from patients who underwent FEES between December 2016 and August 2019 at Fukushima Medical University Hospital to train and test the performance of FEES-CAD. Comprehensive experiments on multiple classification tasks show that FEES-CAD is effective in analyzing the FEES video. We also discussed the influence of the changing angle and lightning from the FEES on the performance of the FEES-CAD. FEES-CAD analyzes images pixel-wise and is sensitive to color contrast when distinguishing objects. Therefore, FEES-CAD yields better results when FEES videos are recorded in proper illumination.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the restrictions of Institutional Review Board but are available from a corresponding author (Mitsuyoshi Imaizumi, ima-mitu@fmu.ac.jp) on reasonable request.
References
Gordon, C., Hewer, R. L. & Wade, D. T. Dysphagia in acute stroke. Br. Med. J. (Clin. Res. Ed.) 295, 411–414 (1987).
Mann, G., Hankey, G. J. & Cameron, D. Swallowing function after stroke: Prognosis and prognostic factors at 6 months. Stroke 30, 744–748 (1999).
Kidd, D., Lawson, J., Nesbitt, R. & MacMahon, J. Aspiration in acute stroke: A clinical study with videofluoroscopy. QJM Int. J. Med. 86, 825–829 (1993).
Finegold, S. M. Aspiration pneumonia. In Seminars in respiratory and critical care medicine 16, 475–483 (1995).
Crausaz, F. M. & Favez, G. Aspiration of solid food particles into lungs of patients with gastroesophageal reflux and chronic bronchial disease. Chest 93, 376–378 (1988).
Han, H. et al. The relation between the presence of aspiration or penetration and the clinical indicators of dysphagia in poststroke survivors. Ann. Rehabil. Med. 40, 88 (2016).
Langmore, S. E., Kenneth, S. & Olsen, N. Fiberoptic endoscopic examination of swallowing safety: A new procedure. Dysphagia 2, 216–219 (1988).
Onofri, S. M. M., Cola, P. C., Berti, L. C., da Silva, R. G. & Dantas, R. O. Correlation between laryngeal sensitivity and penetration/aspiration after stroke. Dysphagia 29, 256–261 (2014).
Chih-Hsiu, W., Tzu-Yu, H., Jiann-Chyuan, C., Yeun-Chung, C. & Shiann-Yann, L. Evaluation of swallowing safety with fiberoptic endoscope: Comparison with videofluoroscopic technique. Laryngoscope 107, 396–401 (1997).
Leder, S. B., Sasaki, C. T. & Burrell, M. I. Fiberoptic endoscopic evaluation of dysphagia to identify silent aspiration. Dysphagia 13, 19–21 (1998).
Leder, S. B. & Karas, D. E. Fiberoptic endoscopic evaluation of swallowing in the pediatric population. Laryngoscope 110, 1132–1136 (2000).
Jean, A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol. Rev. 81, 929–969 (2001).
Gunn, S. R. et al. Support vector machines for classification and regression. ISIS technical report 14, 5–16 (1998).
Veropoulos, K., Cristianini, N. & Campbell, C. The application of support vector machines to medical decision support: A case study. Adv. Course Artif. Intell. 1–6 (1999).
Rawat, W. & Wang, Z. Deep convolutional neural networks for image classification: A comprehensive review. Neural Comput. 29, 2352–2449 (2017).
Ahsan, M. M. E., Alam, T., Trafalis, T. & Huebner, P. Deep mlp-cnn model using mixed-data to distinguish between covid-19 and non-covid-19 patients. Symmetry 12, 1526 (2020).
Islam, F., Hoq, M. N. & Rahman, C. M. Application of transfer learning to detect potato disease from leaf image. In 2019 IEEE International Conference on Robotics, Automation, Artificial-intelligence and Internet-of-Things (RAAICON), 127–130 (2019).
Danala, G. et al. Comparison of computer-aided diagnosis schemes optimized using radiomics and deep transfer learning methods. Bioengineering 9, 256 (2022).
Huang, Z. et al. Ccnet: Criss-cross attention for semantic segmentation. In Proceedings of the IEEE/CVF International Conference on Computer Vision, 603–612 (2019).
Jaderberg, M., Simonyan, K., Zisserman, A. et al. Spatial transformer networks. iN Advances in neural information processing systems 28 (2015).
Zhang, Y., Liu, H. & Hu, Q. Transfuse: Fusing transformers and cnns for medical image segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention, 14–24 (2021).
Karimi, D., Vasylechko, S. D. & Gholipour, A. Convolution-free medical image segmentation using transformers. In International Conference on Medical Image Computing and Computer-Assisted Intervention, 78–88 (2021).
Wang, W. et al. Transbts: Multimodal brain tumor segmentation using transformer. In International Conference on Medical Image Computing and Computer-Assisted Intervention, 109–119 (2021).
Islam, W. et al. Improving performance of breast lesion classification using a resnet50 model optimized with a novel attention mechanism. Tomography 8, 2411–2425 (2022).
Rajpurkar, P. et al. Chexnet: Radiologist-level pneumonia detection on chest x-rays with deep learning. arXiv preprint arXiv:1711.05225 (2017).
Tschandl, P. et al. Expert-level diagnosis of nonpigmented skin cancer by combined convolutional neural networks. JAMA Dermatol. 155, 58–65 (2019).
Raghu, M., Zhang, C., Kleinberg, J. & Bengio, S. Transfusion: Understanding transfer learning for medical imaging. In Advances in neural information processing systems 32 (2019).
Deng, J. et al. Imagenet: A large-scale hierarchical image database. In 2009 IEEE Conference on Computer Vision and Pattern Recognition, 248–255 (2009).
Ma, C., Ji, Z. & Gao, M. Neural style transfer improves 3d cardiovascular mr image segmentation on inconsistent data. In International Conference on Medical Image Computing and Computer-Assisted Intervention, 128–136 (2019).
Qin, X. Transfer learning with edge attention for prostate mri segmentation. arXiv preprint arXiv:1912.09847 (2019).
Karimi, D., Warfield, S. K. & Gholipour, A. Transfer learning in medical image segmentation: New insights from analysis of the dynamics of model parameters and learned representations. Artif. Intell. Med. 116, 102078 (2021).
Ghafoorian, M. et al. Transfer learning for domain adaptation in mri: Application in brain lesion segmentation. In International Conference on Medical Image Computing and Computer-assisted Intervention, 516–524 (2017).
Zhu, J.-Y., Park, T., Isola, P. & Efros, A. A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proceedings of the IEEE International Conference on Computer Vision, 2223–2232 (2017).
Tajbakhsh, N. et al. Surrogate supervision for medical image analysis: Effective deep learning from limited quantities of labeled data. In 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), 1251–1255 (2019).
Pathak, D., Krahenbuhl, P., Donahue, J., Darrell, T. & Efros, A. A. Context encoders: Feature learning by inpainting. In Proceedings of the IEEE conference on Computer Vision and Pattern Recognition, 2536–2544 (2016).
Zhang, R., Isola, P. & Efros, A. A. Colorful image colorization. In European Conference On Computer Vision, 649–666 (2016).
Doersch, C., Gupta, A. & Efros, A. A. Unsupervised visual representation learning by context prediction. In Proceedings of the IEEE International Conference on Computer Vision, 1422–1430 (2015).
Noroozi, M. & Favaro, P. Unsupervised learning of visual representations by solving jigsaw puzzles. In European Conference on Computer Vision, 69–84 (2016).
Gidaris, S., Singh, P. & Komodakis, N. Unsupervised representation learning by predicting image rotations. arXiv preprint arXiv:1803.07728 (2018).
Hadsell, R., Chopra, S. & LeCun, Y. Dimensionality reduction by learning an invariant mapping. In 2006 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’06), vol. 2, 1735–1742 (2006).
Buchler, U., Brattoli, B. & Ommer, B. Improving spatiotemporal self-supervision by deep reinforcement learning. In Proceedings Of The European Conference On Computer Vision (eccv), 770–786 (2018).
Chang, Y. et al. Clinical application and improvement of a cnn-based autosegmentation model for clinical target volumes in cervical cancer radiotherapy. J. Appl. Clin. Med. Phys. 22, 115–125 (2021).
Shin, H.-C. et al. Deep convolutional neural networks for computer-aided detection: Cnn architectures, dataset characteristics and transfer learning. IEEE Trans. Med. Imaging 35, 1285–1298 (2016).
Li, L. et al. A large-scale database and a cnn model for attention-based glaucoma detection. IEEE Trans. Med. Imaging 39, 413–424 (2019).
Min, J. K., Kwak, M. S. & Cha, J. M. Overview of deep learning in gastrointestinal endoscopy. Gut Liver 13, 388 (2019).
Yadav, S. S. & Jadhav, S. M. Deep convolutional neural network based medical image classification for disease diagnosis. J. Big Data 6, 1–18 (2019).
Saxe, A. M., McClelland, J. L. & Ganguli, S. Exact solutions to the nonlinear dynamics of learning in deep linear neural networks. arXiv preprint arXiv:1312.6120 (2013).
Garza, A. The aging population: The increasing effects on health care. Pharm. Times 82, 36–41 (2016).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Bejnordi, B. E. et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318, 2199–2210 (2017).
Chen, J. et al. Transunet: Transformers make strong encoders for medical image segmentation. arXiv preprint arXiv:2102.04306 (2021).
Zhou, Z., Sodha, V., Pang, J., Gotway, M. B. & Liang, J. Models genesis. Med. Image Anal. 67, 101840 (2021).
Abdar, M. et al. A review of uncertainty quantification in deep learning: Techniques, applications and challenges. Inf. Fusion 76, 243–297 (2021).
Bahdanau, D., Cho, K. & Bengio, Y. Neural machine translation by jointly learning to align and translate. arXiv preprint arXiv:1409.0473 (2014).
Cheng, J., Dong, L. & Lapata, M. Long short-term memory-networks for machine reading. arXiv preprint arXiv:1601.06733 (2016).
Imaizumi, M. et al. Implementing a flexible endoscopic evaluation of swallowing at elderly care facilities to reveal characteristics of elderly subjects who screened positive for a swallowing disorder. Auris Nasus Larynx 47, 602–608 (2020).
Ioffe, S. & Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In International Conference on Machine Learning, 448–456 (2015).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. In Proceedings of the IEEE International Conference on Computer Vision, 1026–1034 (2015).
Sudre, C. H., Li, W., Vercauteren, T., Ourselin, S. & Jorge Cardoso, M. Generalised dice overlap as a deep learning loss function for highly unbalanced segmentations. In Deep learning in medical image analysis and multimodal learning for clinical decision support, 240–248 (2017).
Bertels, J., Robben, D., Vandermeulen, D. & Suetens, P. Optimization with soft dice can lead to a volumetric bias. In International MICCAI Brainlesion Workshop, 89–97 (2019).
Taghanaki, S. A. et al. Combo loss: Handling input and output imbalance in multi-organ segmentation. Comput. Med. Imaging Graph. 75, 24–33 (2019).
Kingma, D. P. & Ba, J. Adam: A method for stochastic optimization. arXiv preprint arXiv:1412.6980 (2014).
Tieleman, T. et al. Lecture 6.5-rmsprop: Divide the gradient by a running average of its recent magnitude. COURSERA Neural Netw Mach Learn. 4, 26–31 (2012).
Ruder, S. An overview of gradient descent optimization algorithms. arXiv preprint arXiv:1609.04747 (2016).
Meyer, T. K., Pisegna, J. M., Krisciunas, G. P., Pauloski, B. R. & Langmore, S. E. Residue influences quality of life independently of penetration and aspiration in head and neck cancer survivors. Laryngoscope 127, 1615–1621 (2017).
Martino, R. et al. The toronto bedside swallowing screening test (tor-bsst) development and validation of a dysphagia screening tool for patients with stroke. Stroke 40, 555–561 (2009).
van den Berg, M. G. et al. Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: A cross-sectional study. Head Neck 36, 60–65 (2014).
Sánchez-Sánchez, E. et al. Knowledge and practice of health professionals in the management of dysphagia. Int. J. Environ. Res. Public Health 18, 2139 (2021).
Nacci, A. et al. Fiberoptic endoscopic evaluation of swallowing (fees): Proposal for informed consent. Acta Otorhinolaryngol. Ital. 28, 206 (2008).
Martino, R. et al. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 36, 2756–2763 (2005).
Sura, L., Madhavan, A., Carnaby, G. & Crary, M. A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 7, 287 (2012).
Kamarunas, E. E., McCullough, G. H., Guidry, T. J., Mennemeier, M. & Schluterman, K. Effects of topical nasal anesthetic on fiberoptic endoscopic examination of swallowing with sensory testing (feesst). Dysphagia 29, 33–43 (2014).
Langmore, S. E. History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dysphagia: Changes over the years. Dysphagia 32, 27–38 (2017).
Lin, C.-H., Kong, C. & Lucey, S. Learning efficient point cloud generation for dense 3d object reconstruction. In Proceedings of the AAAI Conference on Artificial Intelligence, vol. 32 (2018).
Acknowledgements
This study was partially supported by the Competitive Research Fund, The University of Aizu (2022-P-12). I confirm that I have mentioned all organizations that funded my research in the Acknowledgements section of my submission, including grant numbers where appropriate.
Author information
Authors and Affiliations
Contributions
W.W., M.I., and S.M. carried out the experiments; W.W. wrote the manuscript with support from M.I. and X.Z.; W.W., M.I., and X.Z. conceived the original idea; W.W., M.I., S.M., and X.Z. analyzed the results; M.I. and X.Z. supervised the project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video S1.
Supplementary Video S2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weng, W., Imaizumi, M., Murono, S. et al. Expert-level aspiration and penetration detection during flexible endoscopic evaluation of swallowing with artificial intelligence-assisted diagnosis. Sci Rep 12, 21689 (2022). https://doi.org/10.1038/s41598-022-25618-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25618-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.