Abstract
Downregulation of human leukocyte antigen (HLA) class I is one mechanism of escaping anti-tumor immunity by tumor cells. This study was conducted to compare HLA class I expression in ductal carcinoma in situ (DCIS) and invasive breast carcinoma (IBC) and to evaluate its association with immune cell infiltration of the tumors and clinical outcome of the patients. A total of 830 cases comprising 288 DCIS and 542 IBC were included in this study. Immunohistochemistry for HLA class I expression was performed using HLA-ABC in tissue microarrays and was analyzed in relation to clinicopathologic characteristics of tumors and infiltration of CD4+, CD8+, and FOXP3+ tumor-infiltrating lymphocyte (TIL) subsets and PD-L1+ immune cells. As a whole, there was no difference in HLA class I expression between DCIS and IBC when dichotomized into high or low expression. However, in the HR-negative group, a high level of HLA class I expression was more frequent in IBC than DCIS. On the contrary, in the HR-positive group, a complete loss of HLA class I expression was more frequently observed in IBC than DCIS. High HLA class I expression level was generally associated with aggressive clinicopathologic features of IBC and was associated with high CD4+, CD8+, and FOXP3+ TIL and PD-L1+ immune cell infiltration in both DCIS and IBC. In survival analyses, HLA class I expression was not associated with clinical outcome in DCIS and IBC as a whole; however, low HLA class I expression was associated with poor clinical outcome in HR-negative IBC, especially in triple-negative subtype. In conclusion, this study showed that HLA class I expression increased in association with increased immune cell infiltration during in situ to invasive transition of HR-negative breast cancer, and HLA class I down-regulation had a prognostic value in HR-negative breast cancer.
Similar content being viewed by others
Introduction
Tumor immune microenvironment is regarded as an important element in cancer development, progression, and control1. Immune reactions mediated by CD8+ and CD4+ Th1 lymphocytes play a crucial role in anti-tumor immunity by eliciting specific immune responses to tumors. While regulatory T cells (Tregs) have an essential role in maintaining immune tolerance, Tregs also have a strong immunosuppressive function by inhibiting activation and differentiation of CD4+ and CD8+ T lymphocytes2. In addition to these immune cells, programmed cell death-ligand 1 (PD-L1) is an important immune checkpoint molecule expressed on both cancer cells and immune cells3 that serves as a brake in an immune reaction by interfering with activation of T lymphocytes. Thus, the composition and quantity of these immune cell subsets affect the balance between their pro- and anti-tumor effects, which in turn, modulate the course of cancer4,5.
Ductal carcinoma in situ (DCIS), which is characterized by proliferation of tumor cells within the ductal-lobular system, is a non-obligate precursor of invasive breast carcinoma (IBC)6. Although previously uncommon, it now comprises up to 25% of all breast cancers, and up to 40% can progress to IBC7. However, it remains ambiguous how DCIS progresses to IBC and what factors determine the transition to invasive carcinoma although it is thought to be a complex process driven by tumor cells and tumor microenvironment including myoepithelial cells, stromal fibroblasts, and immune infiltrates8.
DCIS commonly shows a significant amount of immune cell infiltration which is frequently found in the periductal stroma9. Dense tumor-infiltrating lymphocyte (TIL) infiltration is more prevalent in high-grade DCIS, especially in the hormone receptor (HR)-negative subgroup10,11. Our previous study demonstrated the different composition of immune cell subsets between DCIS and invasive carcinoma depending on the HR status11. Interestingly, more immune cell infiltration has been associated with tumor recurrence12,13. These findings support a hypothesis that immunological composition and immune-related dynamic changes are strongly related to subsequent tumor progression in DCIS.
Evasion of immune surveillance is essential in tumor development14, and down-regulation of human leukocyte antigen (HLA) class I molecule is a well-established mechanism of immune evasion in cancer15. HLA class I is expressed on the cell membrane in most cells and triggers important activities of immune surveillance, presenting cellular peptides to cytotoxic T lymphocytes16. Thus, deficient HLA class I expression can lead to inactivation of T-cell-based immune surveillance resulting in tumor development and progression16. Various mechanisms underlying loss or down-regulation of HLA class I have been reported: promoter methylation, mutations in the HLA class I heavy chains, ꞵ2-microglobulin or antigen presentation machinery components, loss of heterozygosity of HLA gene loci, and transcriptional regulation17. Downregulation of HLA class I has been reported in 40–70% of invasive cancer18,19,20, and it has often shown correlations with worse prognoses17,18,19,21,22,23,24.
To our knowledge, however, there exists no previous study comparing HLA class I expression in in situ and invasive carcinoma of the breast from the standpoint of balance in immune response. Tumor cells in DCIS, being confined to the ductal-lobular system, are physically separated from the stromal immune cells and become directly intermingled with these immune cells during invasive progression. We supposed that invasive tumor cells, which are inevitably recognized by the immune cells in the stroma, require an immune escape for survival via mechanisms such as alteration of HLA class I expression, and that this would lead to a difference in HLA class I expression between DCIS and IBC. This study was aimed at comparing HLA class I expression in DCIS and IBC and evaluating the associations between HLA class I expression, immune cell subset infiltration, and clinical outcome of the patients with DCIS and IBC.
Results
Comparison of HLA class I expression between DCIS and IBC
HLA class I expression was mostly detected in the tumor cell membrane showing a complete membranous staining pattern; however, occasional incomplete membranous staining or cytoplasmic staining was observed. Only complete or incomplete membranous staining was evaluated in this study.
First, HLA class I expression was compared between DCIS and IBC using 830 breast cancer samples comprising 288 cases of DCIS and 542 cases of IBC (Table 1; Fig. 1). As a whole, there was no difference in HLA class I expression in DCIS and invasive carcinoma when it was dichotomized into high and low expression. Low HLA class I expression, defined as strong positivity in < 50% of the tumor cells or weak positivity in ≥ 50% of tumor cells, was found in 145 cases (50.3%) of DCIS and 269 cases (49.6%) of IBC. In the HR-positive group, low HLA class I expression tended to be more frequent in IBC compared to DCIS (53.4% vs. 46. 9%; p = 0.122). On the contrary, in the HR-negative group, low HLA class I expression was more frequent in DCIS than in IBC (62.9% vs. 40.5%); high HLA class I expression was more frequently found in IBC than in DCIS (59.5% vs. 37.1%; p = 0.003).
Representative images of immunostaining with human leukocyte antigen (HLA) class I in ductal carcinoma in situ (DCIS) and invasive breast carcinoma (IBC). In the low level expression group, tumor cells exhibit a complete loss of membranous expression or weak, focal membranous expression of HLA class I compared to the stromal cells that show strong expression. Conversely, tumor cells in the high level expression group show mostly diffuse and strong, complete membranous expression in both DCIS and IBC.
A complete loss of HLA class I expression was more frequently observed in IBC than in DCIS (17.0% vs. 9.4%; p = 0.003). In subgroup analysis by HR status, the difference was also apparent in the HR-positive group (17.7% vs. 9.7%; p = 0.007) but with borderline significance in the HR-negative subgroup (15.2% vs. 8.1%; p = 0.160).
Next, we compared HLA class I expression in DCIS and invasive components within same tumors using 90 cases of IBC with a DCIS component (Table 2). HLA class I expression level was not statistically different between DCIS and invasive components of the same tumor in the whole group, and HR-positive and HR-negative subgroups (p = 0.238, p = 0.302 and p = 1.000, respectively). A complete loss of HLA class I expression tended to be more frequent in the invasive component than in the DCIS component with borderline significance in the whole group (p = 0.092).
The difference in HLA class I expression between DCIS and DCIS associated with invasive carcinoma (DCIS-INV) was also examined (Supplementary Table S1). HLA class I expression level was not significantly different between DCIS and DCIS-INV in the whole group and in the HR-positive group (p = 0.809 and p = 0.443, respectively). However, in the HR-negative group, a high level of HLA class I expression tended to be more frequently found in DCIS-INV than in DCIS (60.9% vs. 37.1%; p = 0.050). The frequency of HLA class I loss was not different between DCIS and DCIS-INV in all groups.
Correlation of HLA class I expression with clinicopathologic features of tumor
In DCIS, HLA class I expression level was associated with only ER status: low HLA class I expression was associated with ER-negative tumors (p = 0.020; Table 3). Low HLA class I expression level was more common in HER2-positive and triple-negative subtypes compared to luminal A and luminal B subtypes.
However, the relationship between HLA class I expression level and clinicopathologic features of IBCs revealed different patterns (Table 4). Interestingly, high HLA class I expression was associated with aggressive clinicopathologic features including high histologic grade (p < 0.001), ER negativity (p = 0.002), positive HER2 status (p = 0.032), high Ki-67 labeling index (p < 0.001) and p53 overexpression (p < 0.001). Accordingly, a high level of HLA class I expression was predominant in HER2+ and triple-negative subtypes of IBCs with a significant difference between DCIS and IBC in triple-negative subtype (p = 0.004).
A complete loss of HLA class I expression did not show correlations with clinicopathologic features of tumors except for absence of microinvasion (p = 0.005) in DCIS (Supplementary Table S2) and low Ki-67 proliferation index (p = 0.033) as well as absence of p53 overexpression (p = 0.020) in IBCs (Supplementary Table S3). Thus, further analyses were focused on HLA class I expression levels thereafter.
Comparison of immune cell subsets between DCIS and invasive carcinoma according to the expression level of HLA class I
In the next step, we compared immune cell subset infiltration according to HLA class I expression levels using 288 cases of DCIS and 339 cases of IBC to check whether immune cell infiltration is associated with HLA class I expression in tumor cells (Table 5). All TIL subset infiltration and the presence of PD-L1+ immune cells were significantly higher in IBC compared to DCIS as previously have been shown11. As a whole, in both DCIS and IBC, tumors with high HLA class I expression contained significantly more CD4+, CD8+, and FOXP3+ TILs and PD-L1+ immune cells than those with low HLA class I expression (all p < 0.05). The difference was also evident in HR-positive subgroup except for CD4+ TIL infiltration in IBC. In HR-negative group, high infiltration of CD4+, CD8+ and FOXP3+ TILs and PD-L1+ immune cells were also associated with high HLA class I expression in both DCIS and IBC, albeit with borderline significance for CD4+ and CD8+ TIL infiltration in DCIS and CD4+ TIL infiltration in IBC.
Prognostic significance of HLA class I expression level in breast carcinoma
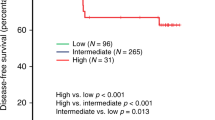
In DCIS, expression level of HLA class I did not show a statistically significant association with recurrence-free survival of patients with DCIS, irrespective of HR status (p = 0.415, whole group; p = 0.524, HR-positive group; p = 0.929, HR-negative group; Supplementary Fig. 1). In IBC, HLA class I expression level was not associated with disease-free survival of the patients as a whole. However, in subgroup analysis, low expression of HLA class I was associated with significantly worse disease-free survival in the HR-negative subgroup (p = 0.011), but not in the HR-positive subgroup (p = 0.186; Fig. 2). Furthermore, in multivariate analysis, expression level of HLA class I was revealed as an independent prognostic factor (Hazard ratio, 2.238; 95% CI, 1.021–4.907; p = 0.044; Table 6).
Kaplan–Meier survival curves according to the expression level of human leukocyte antigen (HLA) class I in patients with invasive breast carcinoma. Low HLA class I expression is associated with shorter disease-free survival of patients in the hormone receptor (HR)-negative subgroup. There is no survival difference in the whole group and in the HR-positive subgroup.
We also performed survival analyses according to subtype of breast cancer including luminal A, luminal B, HER2+ and triple-negative. Low expression of HLA class I was associated with decreased disease-free survival of the patients with triple-negative breast cancer (TNBC) (p = 0.021; Fig. 3). Furthermore, in multivariate analysis, low HLA class I expression level was found to be an independent adverse prognostic factor for disease-free survival in TNBC (Hazard ratio, 2.654; 95% CI, 1.027–6.858; p = 0.044; Table 6).
Kaplan–Meier survival curves stratified by expression level of human leukocyte antigen (HLA) class I in different subtypes of invasive breast carcinoma. Low HLA class I expression is associated with decreased disease-free survival in triple-negative subtype, but not in luminal A, luminal B, and HER+ subtypes.
Discussion
As HLA class I down-regulation is a well-known mechanism of immune evasion in cancer and is associated with tumor progression15, we had expected that IBC would show decreased HLA class I expression compared to DCIS. While a complete loss of HLA class I was more frequent in IBC than in DCIS, its expression level was not different between IBC and DCIS. Interestingly, in the HR-negative subgroup, high HLA class I expression was more frequent in IBC than in DCIS, and it also tended to be higher in DCIS-INV than in DCIS. Although HLA class I molecules are known to be expressed at the basal state in almost all nucleated cells, this expression can be induced or repressed through various mechanisms including interferon signaling and transcription factors of nuclear factor-κB (NF-κB) and NLRC5, etc. by the cell itself or its surrounding immune cells25. Thus, we suppose that increased HLA class I expression in IBC compared to DCIS in HR-negative tumors would be related to the degree of immune cell infiltration and induction of HLA class I by the immune cells as IBC generally shows higher immune cell infiltration than DCIS, which is a finding more prominent in HR-negative tumors.
In breast cancer, expression of HLA class I has been linked to aggressive features of tumor. Shin et al.17 observed that HLA class I expression correlated with lymph node metastasis and high histopathological grade. Park et al.23 reported a positive correlation between HLA class I expression and high histologic and nuclear grades. Lee et al.26 demonstrated that HLA class I expression was negatively correlated with ER expression. Similarly, we observed that high HLA class I expression was associated with aggressive features of tumor such as high histologic grade, ER negativity, positive HER2 status, high Ki-67 proliferation index, and p53 overexpression. Moreover, in this study, high HLA class I expression was associated with high TIL subset and PD-L1+ immune cell infiltration. These associations could be explained by the study by Takahashi and colleagues who demonstrated the counterbalance among aggressive tumor features, accumulation of mutation, and strong immunogenicity in breast cancer27. They also showed that aggressive tumor behaviors were associated with a high mutational burden, which was more frequent in the ER-negative subgroup and TNBC, and to increased neo-antigen loads27. Several studies demonstrated that neo-antigens attract effective immune cells in their microenvironments such as CD4+ T cells or M1 macrophages27,28. These immune cells, as anti-cancer immune cells, release cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor (TNF)-alpha which are major effectors upregulating HLA class I molecules29,30. Thus, in HR-negative group, high levels of HLA class I expression in IBC compared to DCIS might be associated with increased immune cell infiltration caused by accumulation of genetic alterations during invasive tumor progression.
Considering the role of HLA class I in antigen presentation31, high expression of HLA class I would increase cancer cell recognition, and thus, result in increased CD8+ T cell infiltration. Several studies have demonstrated that HLA class I expression is associated with increased TILs. Na et al.19 reported that the density of CD3+ and CD8+ T cell infiltration was significantly correlated with higher expression of HLA class I in colorectal cancer. While Lee and colleagues26 reported that expression level of HLA class I related to higher TIL infiltration, especially CD8+ T cells in TNBC, Park et al.23 showed that increased HLA class I expression was associated with a higher number of Tregs in breast cancer. Our study revealed that a high level of HLA class I was associated with increased immune cell infiltration of not only CD8+ T cells but also immune-suppressive FOXP3+ Tregs and PD-L1+ immune cells in both DCIS and IBC. This finding also supports the possibility of induction of HLA class I expression by infiltrating immune cells rather than increased immune cell infiltration caused by increased HLA class I expression in tumor cells.
In this study, HLA class I expression showed a statistically significant impact on patient survival in HR-negative IBC, especially in TNBC. Although the connection between HLA class I down-regulation and patient prognosis is unclear, studies on the evolution of HLA class I expression during cancer progression may help explain this finding32,33. Genetic diversity caused by the accumulation of mutations may lead to the selection of the subclones that survive anti-cancer immunity. Cancer cells with alteration of HLA class I may be selected and they could escape T-cell-mediated immune destruction, leading to cancer progression. HR-negative breast cancers, especially TNBCs, are more immunogenic than the other subtypes, and thus, HLA class I down-regulation in TNBC might have a greater impact on immune surveillance resulting in poor clinical outcome of patients. However, there were conflicting results regarding the prognostic impact of HLA class I expression. Shin et al. reported that HLA class I heavy chain expression was associated with poor disease-free survival in patients with HR-positive breast cancer17. Kruijf and colleagues reported that HLA class I was not associated with clinical outcome in patients with breast cancer34. Nevertheless, loss of HLA class I has generally been reported to be related to worse survival in various cancers including breast cancer, non-small cell lung cancer, and colorectal cancer19,23,35. These contradictory results might be partially due to the use of different antibodies recognizing different HLA loci, various cutoffs for positive or negative staining, and different compositions of study populations. Further studies would be needed to validate the prognostic effect of HLA class I down-regulation in breast cancer.
In conclusion, our study showed that high expression of HLA class I expression was associated with aggressive clinicopathologic features of IBC. In addition, high HLA class I expression appeared to be related to increased immune cell infiltration including CD8+ T cells, CD4+ T cells, Treg and PD-L1+ immune cells. HLA class I was lost, but paradoxically its expression increased in association with increased immune cell infiltration during in situ to invasive progression of HR-negative breast cancers. Prognostic significance of HLA class I down-regulation was dependent on HR status, and it was found to affect patient prognosis in HR-negative breast cancers, especially in TNBCs.
Materials and methods
Tissue samples
A total of 830 breast cancer cases comprising 288 cases of DCIS (including 211 pure DCIS and 77 DCIS with microinvasion) and 542 cases of IBC that had been diagnosed between 2003 and 2011 at Seoul National University Bundang Hospital were included in this study. Of the 542 IBC cases, 90 cases with a sufficient DCIS component were selected for comparative analysis of the invasive and DCIS components within the same tumor. Patient details and clinicopathologic information were collected from the medical records and reviewing hematoxylin and eosin (H&E)-stained slides and immunohistochemical slides for the standard biomarkers. For DCIS, the following information was collected: age at diagnosis, tumor extent, nuclear grade, presence of comedo-type necrosis, microinvasion, the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki-67 proliferation index, P53 overexpression, treatment modality, and clinical outcome. For IBC, clinicopathologic data including age at diagnosis, pT stage, pN stage, histologic subtype, histologic grade, biomarker status, adjuvant therapy, recurrence, and follow-up information were collected. The clinicopathological features of DCIS and IBC are summarized in Supplementary Tables S4 and S5, respectively. This study was approved by the institutional review board (IRB) of Seoul National University Bundang Hospital (IRB No B-1803/450–305), and informed consent was waived by the IRB. All experiments and procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Tissue microarrays (TMAs)
The most representative areas on H&E stained slides were selected, and the corresponding areas were taken from the formalin-fixed, paraffin-embedded tissue blocks in each case for tissue microarray (TMA) construction. One to three cores of 4-mm-diameter depending on the extent of the tumor from DCIS and three cores of 2-mm-diameter from IBC were arranged in TMA blocks using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea).
Evaluation of HLA class I expression by immunohistochemistry and scoring
Immunohistochemical staining of HLA class I was performed on TMAs using HLA-ABC (Clone EMR8-5; 1:200 dilution; Abcam) with Dako EnVision detection kit (Dako, Carpinteria, CA, USA). Some of the IBC cases had been evaluated for HLA-ABC expression in the previous study36, and those cases were re-evaluated during the study. HLA-ABC expression was evaluated in the tumor cell membrane for both intensity and proportion of positive cells. We adopted the interpretation method of HLA class I expression from the study by Na et al.19. The staining intensity was classified into negative, weak positive, and strong positive. Lymphocytes and endothelial cells were used as internal control, and weak positivity was defined as staining weaker than internal control. Staining equivalent or stronger than internal control was considered strong positive. For statistical analysis, high expression was defined as 50% or more tumor cells showing strong positivity. Low expression was defined as < 50% of the tumor cells revealing strong positivity or ≥ 50% showing weak positivity.
Data of immune cell subsets
The data for the CD4+, CD8+, and FOXP3+ TILs and PD-L1+ immune cells were adopted from our previous studies11,36 for 288 cases of DCIS and 339 cases of IBC. Immunohistochemical staining had been performed with a BenchMark XT autostainer (Ventana Medical Systems) using an UltraView detection kit (Ventana Medical Systems). The following antibodies were used: CD4 (clone SP35; ready to use; Dako), CD8 (clone C8/144B; ready to use; Dako), FOXP3 (clone 236A/E7; 1:100; Abcam) and PD-L1 (clone E1L3N; 1:100; Cell Signaling, Danvers, MA, USA).
CD4+, CD8+, and FOXP3+ T cells had been counted in intratumoral and stromal areas as absolute numbers per high-power field. Detailed information on the counting method of TILs is described in the previous studies11,36. PD-L1+ immune cells were considered to be present when at least 1% of the tumor stromal area was occupied by PD-L1+ immune cells.
Evaluation of standard biomarkers and determination of breast cancer subtypes
Expression of the basic biomarkers including ER, PR, HER2, p53, and Ki-67 was evaluated at the time of pathologic diagnosis in the surgical specimens. The antibodies stained were as follows; ER (clone SP1; 1:100 dilution; LabVision, Fremont, CA), PR (clone PgR 636; 1:70 dilution; Dako, Carpinteria, CA), HER2 (clone 4B5; ready to use; Ventana Medical Systems, Tuscon, AZ), p53 (clone D07; 1:600 dilution; Dako), and Ki-67 (clone MIB-1; 1:250 dilution; Dako). ER and PR staining in ≥ 1% of the tumor cells was determined as positive. Patients with ER- or PR-positive tumors were regarded as hormonal receptor (HR)-positive. HER2 positivity was defined as an immunohistochemical score of 3+ or the presence of HER2 gene amplification on fluorescence/silver in situ hybridization. For p53, staining in 10% or more of the tumor cells was considered positive. High Ki-67 proliferation index was defined as staining in ≥ 10% of tumor cells for DCIS and ≥ 20% for IBC.
Breast cancer subtype was determined with standard biomarker profiles according to 2011 St Gallen International Expert Consensus37. Each subtype was defined as follows: luminal A (ER+ and/or PR+, HER2−, Ki-67 < 14%), luminal B (ER+ and/or PR+, HER2−, Ki-67 ≥ 14%; ER+ and/or PR+, HER2+), HER2+ (ER−, PR−, HER2+), and triple-negative (ER−, PR−, HER2−).
Statistical analysis
All statistical analysis was performed using Statistical package, SPSS version 22.0 for Windows (SPSS Inc, Chicago, IL). The Chi-square test or Fisher’s exact test was used to analyze the association between HLA class I expression and clinicopathologic characteristics of DCIS and IBC or status of PD-L1+ immune cells. Comparison of HLA class I expression between the invasive and in situ components within the same tumor was performed using McNemar test. Difference in CD4+, CD8+, and FOXP3+ TILs was analyzed by the Mann–Whitney U test between the two groups. Recurrence-free survival for DCIS and disease-free survival for IBC were assessed by the Kaplan–Meier method with the log-rank test. Factors that affected survival were identified using univariate analysis, which were then used for multivariate analysis using a backward stepwise selection method in the Cox proportional hazard model. Statistical significance was based on p < 0.05 with all reported p values being two-sided.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Li, C., Jiang, P., Wei, S., Xu, X. & Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 19, 116 (2020).
Zhu, X. & Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 8, 97671–97682 (2017).
Lei, X. et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 470, 126–133 (2020).
Garner, H. & de Visser, K. E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat. Rev. Immunol. 20, 483–497 (2020).
Tan, P. H. et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 77, 181–185 (2020).
McCormick, B. et al. RTOG 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J. Clin. Oncol. 33, 709–715 (2015).
Choi, Y. et al. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum. Pathol. 44, 2581–2589 (2013).
Hendry, S. et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 24, 235–251 (2017).
Hendry, S. et al. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin. Cancer Res. 23, 5210–5217 (2017).
Kim, M. et al. Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res. 22, 32 (2020).
Chen, X. Y. et al. Breast ductal Carcinoma in situ associated with microinvasion induces immunological response and predicts ipsilateral invasive recurrence. Virchows Arch. 478, 679–686 (2021).
Toss, M. S. et al. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod. Pathol. 31, 1226–1236 (2018).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Bubenik, J. Tumour MHC class I downregulation and immunotherapy (Review). Oncol. Rep. 10, 2005–2008 (2003).
Chang, C. C., Campoli, M. & Ferrone, S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv. Cancer Res. 93, 189–234 (2005).
Sinn, B. V. et al. Human leucocyte antigen class I in hormone receptor-positive, HER2-negative breast cancer: Association with response and survival after neoadjuvant chemotherapy. Breast Cancer Res. 21, 142 (2019).
Dusenbery, A. C. et al. MHC class I loss in triple-negative breast cancer: A potential barrier to PD-1/PD-L1 checkpoint inhibitors. Am. J. Surg. Pathol. 45, 701–707 (2021).
Na, H. Y. et al. Expression of human leukocyte antigen class I and beta2-microglobulin in colorectal cancer and its prognostic impact. Cancer Sci. 112, 91–100 (2021).
Garrido, M. A. et al. HLA class I alterations in breast carcinoma are associated with a high frequency of the loss of heterozygosity at chromosomes 6 and 15. Immunogenetics 70, 647–659 (2018).
Najafimehr, H. et al. The role of Human leukocyte antigen class I on patient survival in Gastrointestinal cancers: a systematic review and meta- analysis. Sci. Rep. 10, 728 (2020).
Kaneko, K. et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer 11, 454 (2011).
Park, H. S. et al. Loss of human leukocyte antigen class I expression is associated with poor prognosis in patients with advanced breast cancer. J. Pathol. Transl. Med. 53, 75–85 (2019).
Tsang, J. Y. et al. Co-expression of HLA-I loci improved prognostication in HER2+ breast cancers. Cancer Immunol. Immunother. 69, 799–811 (2020).
Sznarkowska, A., Mikac, S. & Pilch, M. MHC class I regulation: The origin perspective. Cancers 12, 1155 (2020).
Lee, H. J. et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget 7, 30119–30132 (2016).
Takahashi, H. et al. Biologically aggressive phenotype and anti-cancer immunity counterbalance in breast cancer with high mutation rate. Sci. Rep. 10, 1852 (2020).
Oshi, M. et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 10, 16554 (2020).
Neumann, H., Schmidt, H., Cavalie, A., Jenne, D. & Wekerle, H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J. Exp. Med. 185, 305–316 (1997).
Castro, F., Cardoso, A. P., Goncalves, R. M., Serre, K. & Oliveira, M. J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 9, 847 (2018).
Shiina, T., Hosomichi, K., Inoko, H. & Kulski, J. K. The HLA genomic loci map: Expression, interaction, diversity and disease. J. Hum. Genet. 54, 15–39 (2009).
Garrido, F., Ruiz-Cabello, F. & Aptsiauri, N. Rejection versus escape: The tumor MHC dilemma. Cancer Immunol. Immunother. 66, 259–271 (2017).
Garrido, F. & Aptsiauri, N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology 158, 255–266 (2019).
de Kruijf, E. M. et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin. Cancer Res. 16, 1272–1280 (2010).
Datar, I. J. et al. Spatial analysis and clinical significance of HLA class-I and class-II subunit expression in non-small cell lung cancer. Clin. Cancer Res. 27, 2837–2847 (2021).
Chung, Y. R., Kim, H. J., Jang, M. H. & Park, S. Y. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res. Treat. 161, 409–420 (2017).
Goldhirsch, A. et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 22, 1736–1747 (2011).
Acknowledgements
This study was supported by a grant from Seoul National University Bundang Hospital, Seongnam, Korea (Grant No. 02-2020-0043) to Park SY and a grant from Seoul National University Foundation funded by HUNKIM FAMILY CHARITABLE FOUNDATION to Park SY.
Author information
Authors and Affiliations
Contributions
S.H.H. participated in the interpretation and analysis of data and drafted the manuscript. M.K. and Y.R.C. participated in the acquisition and interpretation of the data. J.W.W. participated in the acquisition and interpretation of pathologic data. H.Y.C. carried out the experiments. S.Y.P. conceived of the study, and participated in its design and was responsible for preparation of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, SH., Kim, M., Chung, Y.R. et al. Expression of HLA class I is associated with immune cell infiltration and patient outcome in breast cancer. Sci Rep 12, 20367 (2022). https://doi.org/10.1038/s41598-022-24890-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24890-3
This article is cited by
-
Prognostic and Clinical Significance of Human Leukocyte Antigen Class I Expression in Breast Cancer: A Meta-Analysis
Molecular Diagnosis & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.