Abstract
Recent studies suggest that sensory impairment is related to cognitive function at older ages. Therefore, we aimed to investigate the impact of sensory impairment on cognitive function in the Korean population. We used the Korean Longitudinal Study of Aging data from 2006 to 2018. Cognitive function was measured by the Korean version of the Mini-Mental State Examination scale. A score < 24 at the time of assessment was defined as cognitive impairment. Sensory impairment was assessed according to the self-reported levels of hearing or vision, and the development of sensory impairment was investigated using records of prior survey. We used the generalized estimating equation model to determine association between cognitive function and sensory impairment. A total of 4844 participants (age range: 47–95 years; mean age: 58) were included in the study. Compared to people without sensory impairment, people with a single sensory impairment of hearing or vision had a higher risk of cognitive impairment (odds ratio (OR) = 1.65 [95% confidence interval (CI), 1.49–1.82]). People with dual sensory impairment had the greatest risk of cognitive impairment (OR = 3.23 [95% CI, 2.52–4.12]). The findings suggested the need for timely assessment of sensory function in older persons, which may be useful in identifying individuals at risk for cognitive impairment.
Similar content being viewed by others
Introduction
The prevalence of hearing or visual impairment, and dual sensory impairment (DSI) increases with advancing age. The impairment of sensory function that affects health-related problems in older adults may be the result of aging, but may be the result of accumulation of genetic, environmental, and lifestyle factors1,2 Of the Americans aged 70 years and over, 33% are affected by hearing loss and 18% by vision impairment3. Dual sensory impairment, defined as a combination of hearing and vision loss, has a prevalence of about 5%–21%4. Sensory impairment is considered a chronic condition in an aging society5,6. Hearing and visual impairments are likely to be overlooked compared to the burden of other health conditions7. However, the quality of life for people with sensory impairment may be lower than for people without5,8,9.
Sensory impairments are not only related to functional problems in older adults, but also affect mental health conditions10,11. Sensory impairment is associated with an increased mortality rate and an increased risk of physical and social functional difficulties12. Additionally, several studies have shown that hearing and vision impairments in older adults increase the risk of cognitive dysfunction and dementia13,14,15.
Cognitive impairment often occurs with sensory impairment in older adults16,17. While cognitive function may decline with age, it may also indicate the onset of geriatric neurodegenerative diseases. Cognitive impairment has adverse health consequences, including diminished health-related quality of life18, frailty19, and high mortality risk20. Therefore, screening to prevent cognitive impairment is important for the mental health and successful aging of older adults.
There are various pathways by which sensory impairments affect cognitive function. Sensory impairment could isolate individuals from society, which can affect cognitive function by causing a decrease in cognitive stimulation. In addition, changes in hearing and vision that decrease with age are mostly peripheral and can affect central processing21. A prospective cohort study suggests that central auditory dysfunction may be used as an early risk marker for dementia22. Another study has also found that visual impairment is associated with an increased risk and severity of Alzheimer’s disease23. As such, cognitive impairment may be modifiable and preventable rather than an inevitable health outcome.
Most previous studies have focused on single sensory disorders, and there is a paucity of evidence on the effects of dual sensory disorders on cognitive function when compared with single sensory impairment14,24,25. When one sensory function impaired, the other sensory function may offset for it. However, in older age, both hearing and vision are likely to decrease, which may limit this offset21. Moreover, there is a lack of research on the relationship between sensory disorders and cognitive function among Koreans.
We hypothesized that onset of single or dual sensory impairment would be associated with cognitive impairment. Therefore, this study aimed to investigate the effects of sensory impairment on cognitive function among middle-aged and older Koreans, using a population-based longitudinal data.
Methods
Data collection and study population
We used data from the first (2006) to the seventh (2018) waves of the Korea Longitudinal Study of Aging (KLoSA). The KLoSA is a prospective population-based study designed to investigate the factors related to aging in community-dwelling Korean adults, including demographics, family and social networks, physical and mental health status, employment and retirement, income, and wealth. The surveyed participants were aged 45 years or older and were selected by multistage stratified probability sampling method. The study was initiated by the Korea labor institute in 2006, and the sample has been followed up at 2-yearly intervals for a total of seven waves. A more detailed description of the KLoSA can be found on the Korea Employment Information Service (KEIS) website (http://survey.keis.or.kr).
In 2006, the original panel sample was composed of 10,254 adults aged 45 years and over (born in 1961 or earlier) who resided in South Korea. We excluded those with cognitive impairment (n = 2686) from the first wave. In order to target only those who have newly developed sensory impairment, people with sensory impairment at baseline (n = 1391) were excluded from the study. We also excluded participants who contained missing values for all the variables from first wave to seventh wave (n = 2004). The total number of participants was 4844 in the final sample at the baseline (2008). Only subjects who participated from the first wave were used, and new participants during follow-up were not considered. The sample size for each wave is as follows; 2008 = 4844, 2010 = 4300, 2012 = 4181, 2014 = 4293, 2016 = 3954, 2018 = 3700.
Cognitive function
The main objective of this study was to analyze the association of sensory impairment on cognitive function. Cognitive function was measured by Korean version of the Mini-Mental State Examination (K-MMSE) scale26,27. The K-MMSE comprised 19 questions in five cognitive function areas (orientation in time and place, registration, attention and calculation, memory recall, and visual construction). The subscale scores for these areas were summed up to derive an overall K-MMSE score ranging from 0 to 30, with higher scores indicating better cognitive function. In this study, K-MMSE score < 24 at the time of assessment was defined as cognitive impairment. Participants were categorized into two groups of either cognitive impairment (K-MMSE score < 24) or normal cognition (K-MMSE score ≥ 24)26.
Sensory impairment
The main exposure of interest was the onset of sensory impairment. The survey required the participants to report their perception of hearing and vision. Individuals who use hearing aids/glasses reported their hearing/vision with those aids. That is, hearing was investigated on the basis of hearing ability when using hearing aids in the case of hearing aid users, and vision was investigated on the basis of corrected vision. The level of self-reported hearing and vision were rated on a 5-point scale (excellent, very good, good, fair, or poor). We categorized the levels as ‘normal’ (excellent, very good, or good) or ‘poor’ (fair or poor). This type of self-reported evaluation and classification has also been used in previous longitudinal studies of aging10,28. Onset of sensory impairment was measured by changes in hearing or visual status in the previous and subsequent years. We applied a lag-time option across all 2-year units in the overall period to detect changes in sensory impairment compared to that of prior survey year. Therefore, the baseline for this study was 2008. Compared to the prior wave, the case where the sensory function changed from ‘normal’ to ‘poor’ was defined as the onset of sensory impairment. When one of the hearing or visual impairments developed, this was defined as single sensory impairment. When hearing and visual impairment occurred at the same time, this was defined as dual sensory impairment. Therefore, we categorized the respondents into three groups according to their development of sensory impairment over the two years, as follows: No → No, No → Single impairment, No → Dual sensory impairment. ‘No → No’ was set as a reference group. Also, for the additional analysis to analyze the association between sensory impairment types and cognitive impairment, the single impairment was stratified into hearing and visual impairment.
Covariates
In this study, demographic, socioeconomic, and health-related characteristics were included as covariates in the fully adjusted models. The included covariates were age (45–54, 55–64, 65–74, ≥ 75 years); sex; educational level (middle school or below, high school, college or above); region (metropolitan, urban, rural); economic activity (active, inactive); equivalized household income (divide into quartiles); marital status (married, divorced, separated, or widowed, unmarried); the number of chronic disease (0, 1, ≥ 2; hypertension, diabetes, cancer or malignant tumors, chronic lung disease, liver disease, heart disease, cerebrovascular disease, psychiatric disease, arthritis and rheumatism); limitations in activities of daily living (ADL; disabled, normal); limitations in instrumental ADL (IADL; disabled, normal); body mass index (BMI; normal or underweight [BMI < 23]; overweight [23 ≤ BMI < 25], obese [BMI ≥ 25]); smoking status (current, past, never); alcohol consumption (current, past, never); regular exercise (yes, no); depressive symptom using CES-D 10 scores (yes, no); and year.
Statistical analysis
Chi-square test was used to evaluate and compare the general characteristics of the study population. The statistical significance level was defined as a two-tailed p value of < 0.05. We also evaluated the relationship between sensory impairment and cognitive function using a generalized estimating equation (GEE) model that is useful for analyzing longitudinal data. The GEE model could account for time variations and correlations among repeated measurements observed in a longitudinal study29. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to compare parameters between those who had cognitive impairment and those with normal cognition. In addition, in this statistical model, multicollinearity was tested using the variance inflation factors that were all below the generally accepted threshold of 5–10, used to indicate collinearity30. All the statistical analyses were performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA).
Ethical standards
Ethical approval was not required as the Korean Longitudinal Study of Aging Data provides anonymous, secondary data that is publicly available for scientific use.
Results
Table 1 shows the general characteristics of participants at baseline, which is the first point of change (2006 → 2008). The baseline for this study is 2008 as the lag-time option is applied to detect changes in sensory impairment compared to previous survey wave. A total of 4844 participants were included in the study. Their age range was 47 to 95 years old and the mean age was 58. Of which 11.4% (n = 551) and 88.6% (n = 4293) were classified with cognitive impairment and normal cognitive function, respectively. At baseline, out of 4844 participants, 16.3% (n = 788) had single sensory impairment such as hearing or vision, and 1.1% (n = 54) had dual sensory impairment. In 788 cases of single sensory impairment, 10.2% (n = 80) with hearing problems and 89.8% (n = 708) with visual problems. All covariates, such as demographic, socioeconomic, and health-related characteristics were significantly associated with cognitive function (all p values < 0.05).
Table 2 presents the results of the GEE model for the impact of sensory impairment on cognitive function that were repeatedly measured in the two-yearly units from 2006 to 2018 as ORs. All analyses were performed after adjusting for covariates. The “No → No” group that maintained normal sensory function, was the reference group. The ORs for cognitive impairment was 1.65 times higher in the single sensory impairment group than in the reference group (OR = 1.65 [95% CI, 1.49–1.82]). The ORs for cognitive impairment was highest in the DSI group, where hearing and visual impairment occurred simultaneously (OR = 3.23 [95% CI, 2.52–4.12]). The results did not change even when a sensitivity analysis was performed on the entire sample, including all missing values.
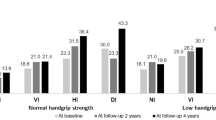
In order to analyze the association between the onset of sensory impairment by types and cognitive impairment, all the independent variables were adjusted for, and GEE analysis was performed (Fig. 1, Supplementary Table S1). We observed that the odds of cognitive impairment were 1.55 times and 1.67 times higher, respectively, when hearing impairment and visual impairment occurred newly (OR = 1.55 [95% CI, 1.25–1.92] for hearing; 1.67 [95% CI, 1.50–1.86] for visual). The values were statistically significant.
Discussion
In this study, we examined the impact of changes in sensory impairment on cognitive function in Koreans aged 45 years or older using the KLoSA data. We found that those who reported the development of sensory impairment were more likely to experience cognitive impairment than older adults without these impairments. Moreover, older adults with dual sensory impairment had a higher risk of cognitive impairment than those with single sensory impairment such as hearing or vision.
Several previous studies also found a relationship between sensory impairment and cognitive functions. A study found that individuals with hearing loss have accelerated cognitive decline of 30–40% and increased risk of incident cognitive impairment of 24% compared to individuals with normal hearing25. One longitudinal study examined 253 individuals aged 45–64 years at baseline and found that hearing impairment was associated with faster cognitive decline over 20 years31. As a result of meta-analysis of 36 studies, age-related hearing loss was significantly associated with multi-domain cognitive decline, cognitive impairment, and dementia acceleration32. One community-based prospective study33 found visual impairment to be predictive of subsequent functional impairment in older persons, while other studies showed a decline in cognition and functional activities34,35,36. A meta-analysis of 40 studies found that people with visual impairments were associated with an approximately 2-fold odds of prevalent or incident cognitive impairment37.
Our results on changes in sensory impairment types showed that those with dual sensory impairment had higher risk of cognitive impairment, which is in line with several previous studies. According to a prospective cohort study, dual sensory impairment was associated with 86% increased risk for all‐cause dementia and a 112% increased risk for Alzheime’s disease compared with having no sensory impairment14. A study of women aged 69 years and over reported that dual sensory impairment increased the risk of cognitive decline twofold compared to no impairment over a 4-year period38. Another study reported that those with dual sensory impairment had approximately twofold increased odds of cognitive decline, even after adjusting for other factors such as age, medical comorbidities, smoking, and walking speed38. A study of older Australians also found a higher rate of cognitive impairment among those with dual sensory impairment compared to those without24. Similar results have been reported in Iceland showing approximately 30% higher rate of cognitive impairment among older adults with dual sensory impairment39. A longitudinal study in China also found that cognitive function declined even more with dual sensory impairment13.
Sensory impairment may lead to cognitive impairment by cumulative effect of decreased sensory stimulation. This has causal effects on sensory loss, social isolation, depression, and decreased physical activity. Impaired communication due to sensory impairment limits participation in social activities and increase the difficulty of maintaining social networks40. In addition, protracted lack of sense can reduce the chances of intellectually stimulating exchanges, eventually reducing the general level of cognitive ability41. Sensory impairment and cognitive impairment could be caused by common pathological processes such as vascular disease. For example, β-amyloid pathology, pathological features of Alzheimer’s disease, could damage both sensory and cognitive abilities. Sensory disorders can increase cognitive load, limiting neural resources required for optimal performance of cognitive tasks, and loss of senses can affect changes in brain structure and function42.
The limitations of this study should be considered when interpreting our results. First, the information for sensory function data used in this study were self-reported, so there may lead to biases in the respondents’ responses. Specifically, we used self-reported measures of hearing and visual functions. Although self-reported assessments of hearing or vision have been widely used in population-based surveys10,13,28, it may not represent the subject’s health condition as is, whether intended or not. Previous studies have shown that older adults participants overestimate the adequacy of their vision when asked for a self‐evaluation43, and underestimate their hearing impairment44. Another study found that older adults tend to overestimate their hearing and report fewer hearing problems45. Therefore, studies should seek to replicate our findings using objective measures of hearing and vision. Second, only MMSE was used as a screening indicator for cognitive impairment. In the KLoSA data, MMSE was the only indicator that could check cognitive function. Further studies need to use clinical tools to assess cognitive function. Third, we could not measure the cause of sensory impairment in the participants due to data limitations. To minimize this limitation, we adjusted for variables that could affect the occurrence of sensory impairments or changes in sensory function state (e.g., chronic disease). It is also necessary to consider missing data due to non-response. Participants who recovered from sensory impairment were also not considered. Finally, this study used an observational study design, which prevented us from directly drawing causal inference. Although we adjusted for numerous potential confounders, some residual confounding may still persist. Despite these limitations, this study had a relatively large sample size, is representative of the community-dwelling Korean population aged 45 years and over, and documented an understudied association.
Conclusion
This study highlighted that developing sensory impairment was related with cognitive function in middle-aged and older adults in South Korea. Specifically, the findings of our study provide further evidence implying a greater relationship between dual sensory impairment and cognitive function. Considering Korea’s rapidly aging population, this is a salient topic with important public health implications. Timely assessment of sensory function in older persons may be useful in identifying individuals at risk of cognitive impairment. Our research findings could provide health policy makers and professionals with valuable information about the development of intervention strategies to minimize negative consequences for cognitive ability by preventing sensory loss or optimal treatment of sensory impairment.
Data availability
The Korea Employment Information Service (KEIS) website (http://survey.keis.or.kr).
References
Schaie, K. W. & Willis, S. L. Handbook of the psychology of aging (Academic Press, 2010).
Kim, S. et al. Age-related hearing loss in the Korea national health and nutrition examination survey. PLoS ONE 15, e0243001 (2020).
Crews, J. E. & Campbell, V. A. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. Am. J. Public Health 94, 823–829 (2004).
Brennan, M., Su, Y.-P. & Horowitz, A. Longitudinal associations between dual sensory impairment and everyday competence among older adults. J. Rehabil. Res. Dev. 43, 777 (2006).
Park, S. J., Ahn, S. & Park, K. H. Burden of visual impairment and chronic diseases. JAMA Ophthalmol. 134, 778–784. https://doi.org/10.1001/jamaophthalmol.2016.1158 (2016).
Gordon-Salant, S. Hearing loss and aging: New research findings and clinical implications. J. Rehabil. Res. Dev. 42, 9–24 (2005).
Chiang, P., Keeffe, J., Le Mesurier, R. & Taylor, H. Global burden of disease and visual impairment. Lancet 368, 365 (2006).
Davis, A. C. & Hoffman, H. J. Hearing loss: Rising prevalence and impact. Bull. World Health Organ. 97, 646 (2019).
Mathers, C., Smith, A. & Concha, M. Global burden of hearing loss in the year 2000. Glob. Burd. Dis. 18, 1–30 (2000).
Han, J., Lee, H., Jung, J. & Park, E.-C. Effects of self-reported hearing or vision impairment on depressive symptoms: A population-based longitudinal study. Epidemiol. Psychiatric Sci. 28, 343 (2019).
Shakarchi, A. F. et al. Dual sensory impairment and perceived everyday discrimination in the United States. JAMA Ophthalmol. 138, 1227–1233. https://doi.org/10.1001/jamaophthalmol.2020.3982 (2020).
Schubert, C. R. et al. Sensory impairments and risk of mortality in older adults. J. Gerontol. Ser. A 72, 710–715. https://doi.org/10.1093/gerona/glw036 (2016).
Rong, H. et al. Association of sensory impairments with cognitive decline and depression among older adults in China. JAMA Netw. Open 3, e2014186–e2014186. https://doi.org/10.1001/jamanetworkopen.2020.14186 (2020).
Hwang, P. H. et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimer’s Dement Diagn. Assess Dis. Monit. 12, e12054 (2020).
Livingston, G. et al. Dementia prevention, intervention, and care. Lancet 390, 2673–2734 (2017).
Maharani, A. et al. Associations between self-reported sensory impairment and risk of cognitive decline and impairment in the health and retirement study cohort. J. Gerontol. Ser. B 75, 1230–1242. https://doi.org/10.1093/geronb/gbz043 (2019).
Mitoku, K., Masaki, N., Ogata, Y. & Okamoto, K. Vision and hearing impairments, cognitive impairment and mortality among long-term care recipients: A population-based cohort study. BMC Geriatr. 16, 112. https://doi.org/10.1186/s12877-016-0286-2 (2016).
Pan, C.-W. et al. Cognitive dysfunction and health-related quality of life among older Chinese. Sci. Rep. 5, 1–8 (2015).
Raji, M. A., Al Snih, S., Ostir, G. V., Markides, K. S. & Ottenbacher, K. J. Cognitive status and future risk of frailty in older Mexican Americans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 65, 1228–1234 (2010).
Bae, J. B. et al. Impact of mild cognitive impairment on mortality and cause of death in the elderly. J. Alzheimers Dis. 64, 607–616. https://doi.org/10.3233/JAD-171182 (2018).
Roberts, K. L. & Allen, H. A. Perception and cognition in the ageing brain: A brief review of the short-and long-term links between perceptual and cognitive decline. Front. Aging Neurosci. 8, 39 (2016).
Gates, G. A., Beiser, A., Rees, T. S., D’Agostino, R. B. & Wolf, P. A. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J. Am. Geriatr. Soc. 50, 482–488 (2002).
Uhlmann, R. F., Larson, E. B., Koepsell, T. D., Rees, T. S. & Duckert, L. G. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J. Gen. Intern. Med. 6, 126–132 (1991).
Gopinath, B. et al. Dual sensory impairment in older adults increases the risk of mortality: A population-based study. PLoS ONE 8, e55054 (2013).
Lin, F. R. et al. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299 (2013).
Kang, Y., Na, D. L. & Hahn, S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 15, 300–308 (1997).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Chou, K.-L. Combined effect of vision and hearing impairment on depression in older adults: Evidence from the english longitudinal study of ageing. J. Affect. Disord. 106, 191–196. https://doi.org/10.1016/j.jad.2007.05.028 (2008).
Twisk, J. W. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide (Cambridge University Press, 2013).
Midi, H. & Bagheri, A. In: Proceedings of the 4th international conference on applied mathematics, simulation, modeling. 138–142 (World Scientific and Engineering Academy and Society (WSEAS).).
Deal, J. A. et al. Hearing impairment and cognitive decline: A pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am. J. Epidemiol. 181, 680–690 (2015).
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S. & Lawlor, B. A. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol.-Head Neck Surg. 144, 115–126. https://doi.org/10.1001/jamaoto.2017.2513 (2018).
Reuben, D. B., Mui, S., Damesyn, M., Moore, A. A. & Greendale, G. A. The prognostic value of sensory impairment in older persons. J. Am. Geriatr. Soc. 47, 930–935 (1999).
West, C. G. et al. Is vision function related to physical functional ability in older adults?. J. Am. Geriatr. Soc. 50, 136–145 (2002).
Clemons, T. E., Rankin, M. W. & McBee, W. L. Cognitive impairment in the age-related eye disease study: AREDS report no. 16. Archives of Ophthalmology (Chicago, Ill.: 1960) 124, 537–543 (2006).
Marsiske, M., Klumb, P. & Baltes, M. M. Everyday activity patterns and sensory functioning in old age. Psychol. Aging 12, 444 (1997).
Vu, T. A. et al. The bidirectional relationship between vision and cognition: A systematic review and meta-analysis. Ophthalmology 128, 981–992. https://doi.org/10.1016/j.ophtha.2020.12.010 (2021).
Lin, M. Y. et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J. Am. Geriatr. Soc. 52, 1996–2002 (2004).
Fisher, D. et al. Impairments in hearing and vision impact on mortality in older people: The AGES-Reykjavik study. Age Ageing 43, 69–76 (2014).
Kramer, S. E., Kapteyn, T. S., Kuik, D. J. & Deeg, D. J. H. The association of hearing impairment and chronic diseases with psychosocial health status in older age. J. Aging Health 14, 122–137. https://doi.org/10.1177/089826430201400107 (2002).
Lindenberger, U. & Baltes, P. B. Sensory functioning and intelligence in old age: A strong connection. Psychol. Aging 9, 339–355. https://doi.org/10.1037/0882-7974.9.3.339 (1994).
Whitson, H. E. et al. American geriatrics society and national institute on aging bench-to-bedside conference: Sensory impairment and cognitive decline in older adults. J. Am. Geriatr. Soc. 66, 2052–2058. https://doi.org/10.1111/jgs.15506 (2018).
Long, C., Holden, R., Mulkerrin, E. & Sykes, D. Opportunistic screening of visua acuity of elderly patients attending outpatient clinics. Age Ageing 20, 392–395 (1991).
Kamil, R. J., Genther, D. J. & Lin, F. R. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear 36, 164–167. https://doi.org/10.1097/AUD.0000000000000075 (2015).
Bainbridge, K. E. & Wallhagen, M. I. Hearing loss in an aging American population: Extent, impact, and management. Annu. Rev. Public Health 35, 139–152 (2014).
Acknowledgements
We would like to thank the Korea Employment Information Service, which conducted and provided data based on a nationwide survey. In addition, we would like to thank our colleagues at the Institute of Health Services Research of Yonsei University, who provided their advice on intellectual content. We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.J.J. and J.H.J. designed the study, collected the data, performed the statistical analysis, and drafted the manuscript. H.J.J., J.H.J., S.H.K., J.K. and E.C.P. contributed to the discussion and reviewed and edited the manuscript. E.C.P. is the guarantor of this work and as such, has full access to all the study data. E.C.P. assumes the responsibility for the integrity of the data and accuracy of the data analysis. All authors have approved the final article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joo, H.J., Joo, J.H., Kim, S.H. et al. The impact of self-reported sensory impairment on cognitive function using the Korean longitudinal study of aging survey data. Sci Rep 12, 17907 (2022). https://doi.org/10.1038/s41598-022-22840-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22840-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.