Abstract
TREML4 and other members of the triggering receptor expressed in the myeloid cell family are associated with a risk of atherosclerosis and progression in coronary artery disease, acute coronary syndrome, and coronary artery calcification. Herein, the relationship between TREML4 expression and its polymorphisms (rs2803495 and rs280396) was evaluated in patients with subclinical atherosclerosis (n = 340) and heart failure post-acute myocardial infarction (MI) (n = 68) for the first time. TREML4 variants rs2803495 (A > G) and rs2803496 (T > C) and leukocyte mRNA expression was analyzed by qRT–PCR. The rs2803495 G allele was associated with TREML4 expression (OR 8.01, CI 3.78–16.99, p < 0.001). Patients carrying the rs2803496 C minor allele (TC/CC genotypes) were more likely to express TREML4 than those without the C allele (OR 10.42, CI 4.76–22.78, p < 0.001), as well as having higher levels of TREML4 expression (OR 4.88, CI 2.35–10.12, p < 0.001). Thus, we report for the first time that TREML4 is not associated with the early stages of atherosclerotic plaque formation and later stages after MI. In conclusion, TREML4 mRNA expression in blood leukocytes is influenced by minor alleles (G and C) and may regulate differently during the atherosclerosis progression stages, but not in asymptomatic atherosclerosis disease and post-MI.
Similar content being viewed by others
Introduction
Cardiovascular diseases are common, have high lethality, and are increasing worldwide1. Atherosclerosis is a progressive disease with a prolonged latent phase and clinically unapparent lesions, so its acute or chronic manifestations demonstrate a more significant atherosclerotic burden or more advanced stages of the disease2.
The atherosclerotic process begins with the migration and retention of lipids, especially LDL cholesterol, recruitment of inflammatory cells, and formation of foam cells with subsequent apoptosis, necrosis, calcification, and arterial remodeling, which may result in plaque inflammation, rupture, thrombosis, and other cardiovascular diseases. Due to the complex interaction between these processes, most plaques remain asymptomatic (subclinical atherosclerosis, SA); others become obstructive (stable angina) or even cause acute thrombosis leading to acute coronary syndrome (ACS)3. The ACS range from unstable angina to non–ST-segment elevation myocardial infarction (MI), ST-segment elevation myocardial infarction, and sudden cardiac death. The consequence of extensive myocardial damage and impairment of myocardial contractility, modifying the left ventricular (LV) structure, in a process known as LV remodeling (LVR), which reduce LV function4.
Genome-wide association studies have identified 164 gene loci that confer an increased risk of coronary artery disease and Atherosclerosis, and the role of many of these genes and pathways has not yet been adequately investigated5. However, many genes are already well described in the literature as being associated with the development of Atherosclerosis, such as genes for lipid metabolism (APOA5, LDLR, APOB), platelets and blood coagulation (PCSK9, TGFB1), and cell migration and adhesion (NCK, IRS1)5. Furthermore, new genes have shown a promising role in developing cardiovascular diseases in the last decade, such as TREML46,7,8.
TREML4 is a member of the Triggering receptor family expressed on myeloid cells (TREM). It is a single immunoglobulin (IgV) variable, with domain activating receptors on the surface of neutrophils/monocytes and dendritic cells9. The Triggering Receptor Expressed on Myeloid cells-Like 4 (TREML4) belongs to the triggering receptor expressed on the myeloid cell (TREM) family located on chromosome 6, which is composed of receptors of a single extracellular variable-type immunoglobulin-like domain and are structurally similar9. In previous studies, we showed that the expression of TREML4 and its polymorphisms (rs2803495 and rs2803496) are associated with different clinical outcomes of atherosclerosis, such as coronary artery disease6, ACS7, and Coronary Artery Calcium (CAC)8. Based on our previous results, TREML4 is likely involved in the atherosclerotic progress to plaque disruption ending up in MI and might be a potential biomarker. However, no studies have investigated its relationship with stages that precede or succeed atherosclerotic progress to MI and after the rupture in heart failure post-MI.
We previously investigated the relationship between gene expression and TREML4 polymorphisms in patients with coronary artery disease undergoing coronary angiography. We demonstrated that TREML4 mRNA expression in leukocytes is influenced by the extent of coronary artery lesions and genetic polymorphisms (RS2803495 and rs2803496), so the more significant the atherosclerotic burden, the greater the expression of TREML46. In 2013, we were the first group to demonstrate the relationship between TREML4 expression and patients with ACS. Through transcriptomic analysis by microarray technology, we demonstrated that the differential expression of TREML4 is related to the early stages of ACS and that TREML4 can be used to monitor early cardiac ischemic recovery7.
The role of TREML4 in CAC was initially described by Sen et al.8 through an integrative analysis using omics technologies and suggested that TREML4 functions as a modifier that plays a role in the conversion of soft plaque to calcified plaque rather than to be the cause of the presence of the plaque itself. These data support the hypothesis that the TREM Family locus, especially TREML4, may be responsible for various phenotypes in coronary artery disease and that gene expression can sometimes be related to different clinical outcomes.
Thus, in seeking to assess the TREML4 expression profile throughout the progress of ischemic heart disease, our study aimed to investigate relationships between TREML4 mRNA expression in blood leukocytes and its polymorphisms (rs2503495 and rs2803496) in asymptomatic patients and in patients who developed left ventricular (LV) dysfunction after MI.
Results
Clinical and biochemical laboratory data
Subclinical Atherosclerosis (SA) was detected in 60.88% of subjects (n = 207). Therefore, 39.12% (133) of the subjects did not present SA in the carotid doppler ultrasound (CDU) or Coronary tomography with calcium score (CAC) and were set as a control group. Clinical and biochemical laboratory data of SA are shown in Table 1. The mean age was higher in the SA group (p < 0.001). Patients with dyslipidemia and hypertension were more frequent in the SA group (p = 0.002 and p = 0.004, respectively) than controls. Laboratory data revealed that urea, creatinine, and tumor necrosis factor-alpha (TNF-α) significantly differed between groups (p = 0.015, p = 0.005, and 0.027, respectively). The use of medications in this sample is described in Supplementary Table 1. There was a higher usage frequency of statins (73.4%, p = 0.001) and diuretics (49.8%, p = 0.028) in SA patients.
All subjects with previous MI were matched for biodemographic characteristics, laboratory data, and some cardiac risk factors such as smoking, alcohol consumption, family history of MI, high blood pressure, and dyslipidemia, which were present in all of them (Table 2). Diabetes was a recurrent comorbidity in patients with LV dysfunction (p = 0.012) and consequently antidiabetic treatment (p = 0.008) (Supplementary Table 2).
TREML4 mRNA expression and polymorphisms
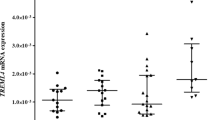
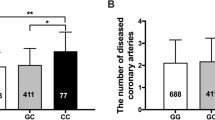
A total of 47.1% of SA (n = 160) and 64.3% of LV dysfunction (n = 9) subjects had TREML4 expression values detected by quantitative reverse transcription (qRT)-polymerase chain reaction (PCR), whereas 52.9% and 35.7% tested positive for the ACTB gene alone, respectively. In addition, 50.0% of the SA (80) and 55.6% of the LV dysfunction subjects with positive TREML4 on qRT-PCR had values above the group mean. No association was observed between TREML4 expression and SA (p > 0.05) or LV dysfunction (Tables 3, 4).
The genotype and allele frequencies were similar between the SA and Control groups (p > 0.05, Table 5). The genotype frequencies of TREML4 polymorphisms (rs2803495 and rs2803496) were in Hardy–Weinberg equilibrium, indicating no genotyping errors in breeding and evolutionary pressure. Linkage disequilibrium between rs2803495 and rs2803496 was not detected (D′ = 0.99, p = 0.006).
We previously described that not every patient with clinical Atherosclerosis could express TREML4 in blood leukocytes, and this expression is associated with the presence of the rs2803496 C allele (TC/CC genotypes)6. In this cohort, the genotype rs2803495 (AG) was more frequent (31.2%, p < 0.001) in the group that showed TREML4 expression than the one that did not express this gene (Table 6). Genotypes (CT + CC) were also more frequent (34.4%, p < 0.001) for rs2803496 in the TREML4 expressing group (Table 6). Subjects with the G allele (2,803,495) were more likely to express TREML4 (OR 8.01, 95% CI 3.78–16.99, p < 0.001). The C allele (rs2803496) also confers greater chances of expressing TREML4 (OR 10.42, 95% CI 4.76–22.78, p < 0.001). Moreover, subjects with the C allele were more likely to have high TREML4 mRNA expression (OR 4.88, 95% CI 2.35–10.12, p < 0.001) (Table 7).
Discussion
Herein we report for the first time that TREML4 is not associated with the early stages of atherosclerotic plaque formation and later stages after MI. In a previous study, we analyzed the expression of TREML4 and its polymorphisms in blood leukocytes from 137 patients with coronary artery disease. We observed that patients with higher levels of atherosclerotic lesions were associated with higher TREML4 expression levels and that this expression is influenced by the presence of the rs2803495 and rs2803496 variants6. Furthermore, similar to our previous findings, only some associations between classic risk factors with subclinical Atherosclerosis and heart failure post-MI were observed in our study, as shown in Tables 1, 2. Together, our observations support the hypothesis that classical risk factors for cardiovascular diseases are not sensitive enough to assess atherosclerotic lesions or to stimulate coronary lesions and strengthen the importance of new, more sensitive, and minimally invasive biomarkers to assess the presence and burden of atherosclerotic lesions.
Thus, we also observed that TREML4 is more expressed during acute events such as ACS through a transcriptomic study using a microarray model and validated by a real-time polymerase chain reaction in patients with ACS7. Similarly, it was possible to observe that the presence of minor alleles of polymorphisms rs2803495 (G) and rs2803496 (C) confer greater cardiovascular risk and may favor the expression of TREML4 through two cis-eQTL single nucleotide polymorphism (SNP) analyses based on a multi-omics analysis in patients with coronary artery calcification8.
Through transcriptomic analysis in human and murine macrophages, Gonzalez-Cotto et al.17 recently demonstrated that TREML4 is more preferentially expressed in inflammatory macrophages and favors disease development by increasing the lesion load and macrophage content. Their transcriptome results report that the presence of lower frequency alleles can increase TREML4 expression up to 300-fold. In order to obtain more information about the expression profile of genes possibly controlled by TREML4 expression, the same group performed RNA sequencing analysis in human blood macrophages that were carriers of haplotypes considered permissive (carriers of the minor alleles G and C) for TREML4 expression and observed that TREML4 regulates genes from several pathways, primarily related to the inflammatory response (TLR410, TLR1311, MMP2512, RUNX313, lipid regulation EHD114, LIPG15, ABCA516 and carbohydrate metabolism17.
In a previous study, we demonstrated that TREML4 seems to be associated with the regulation of pathways involving carbohydrate metabolism and that patients who have Diabetes mellitus type 2 and are carriers of the minor allele (c) (rs2803496) may have an increase of up to 20.9 times in the expression of TREML48. Interestingly, a recent study analyzed the transcriptional profile of genes in Apoe −/− /Treml4 −/− mice Macrophages and demonstrated that genes in the Glycolysis/gluconeogenesis pathways are regulated by TREML417.
Our current and previous results show that the transcriptional profile of TREML4 is present between the initial to intermediate stages of atherosclerotic disease progression, as illustrated in Fig. 1. As it is a multifactorial and inflammatory disease, more advanced stages of this disease are characterized by the presence of an inflammatory microenvironment18, with the presence of calcification8 which commonly progresses to ACS7, and its advance is influenced by the presence of polymorphisms 19.
Of the subjects with SA in our study, 18.2% have the rs2803495 AG + GG genotype, and 20.6% have the rs2803496 CT + CC genotype. These results are similar to those in a European population in phase 3 of the 1000 Genomes project (http://www.internationalgenome.org/). Additionally, we observed that subjects who carry the minor alleles G (AG + GG) or C (CT + CC) are more likely to express TREML4. SNP can impact mRNA in several ways, whether in mRNA splicing, nucleo-cytoplasmic export, stability, or translation20. rs2803495 and rs2803496 are variants in the regulatory region and the 5'-untranslated region (UTR) of chromosome 621. The 5’UTR regions contain key elements in transcriptional and translational regulation, such as the upstream open reading frames (uORFs), which can control the selection of translation-initiation sites or even contribute to the translatability of mRNA22, which may precisely impact the regulation of TREML4 expression. Although we did not find a relationship between SNPs and TREML4 with SA (early stage) and LV dysfunction after MI (a final consequence of MI), we hypothesize that TREML4 polymorphisms, expression, and other regulatory molecules such as microRNAs, may be critical to compress the progression of atherosclerotic burden6 and the rupture plate7.
Conclusion
In conclusion, this study evaluated the relationship between gene expression and TREML4 polymorphisms in patients with subclinical atherosclerosis compared to a control group and LV dysfunction after MI. Our results in this cohort and our previous results6,7 suggest that genetic polymorphisms in TREML4, including in asymptomatic patients, influence mRNA expression in leukocytes and may regulate differently during the atherosclerosis progression stages, but not in symptomatic Atherosclerosis disease and post-MI.
Methods
Subclinical atherosclerosis sample
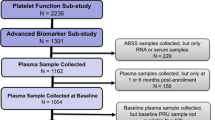
A total of 340 subjects who had subclinical atherosclerosis (SA) as verified in their hospital records were enrolled for screening in this cross-sectional study between February 2010 and March 2013. The patients were recruited during their routine appointment at the Medical Sections of Dyslipidemia, Arterial Hypertension, and Nephrology of the Dante Pazzanese Cardiology Institute (DPCI), São Paulo, SP, Brazil.
The clinical outcome of the SA patients was according to protocols presented by Bertolami et al.23. Carotid Doppler ultrasound (CDU) and Coronary tomography with calcium score (CAC) were used to detect SA. Subjects with CAC > 0 or the presence of plaque and/or increased intima thickness above the 75th percentile for age, gender and race in the CDU according to the criteria of the MESA study were included in the SA group. The control group consisted of individuals who did not present SA in any of the methods.
The Research Ethics Committee of DPCI approved the study of this sample, which complies with the Declaration of Helsinki, under protocol number 3852/2009. Written informed consent was obtained from each participant before sample collection, and all experiments were performed following relevant guidelines and regulations. Clinical data were assessed from each patient in their hospital records.
Heart failure post-MI sample
A total of 65 subjects who had suffered previous STEMI at least two months prior to inclusion in the study as verified in their hospital records were enrolled for screening in this cross-sectional study between July 2018 and December 2019. The patients were recruited during their routine appointment at the Cardiology Outpatient Unit, Onofre Lopes University Hospital of the Federal University of Rio Grande do Norte (UFRN), Natal, RN, Brazil.
The clinical outcome of the STEMI patients was determined by LVEF measured at ± 3 months from the date of venous blood collection. Since ejection fraction is widely used to determine LV dysfunction, patients were classified into two groups: LV dysfunction and symptoms of HF (LVEF ≤ 40%, n = 14) and those with normal LV function (LVEF > 40%, n = 14), according to the American Heart Association Guideline for the Management of HF17. The exclusion criteria included other cardiac diseases that could lead to HF such as congenital cardiomyopathies, dilated cardiomyopathy, atrial fibrillation, Chagas disease, and non-treated hypertension; patients forwarded to cardiac transplants were not enrolled.
The Research Ethics Committee of UFRN approved the study of this sample, which complies with the Declaration of Helsinki under protocol number 2.017.026. Written informed consent was obtained from each participant before sample collection, and all experiments were performed following relevant guidelines and regulations. Clinical data were assessed from each patient in their hospital records.
Biochemical measurements
Fasting serum glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL)-cholesterol, Urea, creatinine, uric acid, alanine aminotransferase, and aspartate aminotransferase (AST) were measured using colorimetric and enzymatic colorimetric assays. Low-density lipoprotein (LDL)-cholesterol levels were calculated according to the Friedewald formula. The CKD-EPI equation obtained creatinine clearance. High sensitivity C-reactive protein (hs-CRP) was analyzed by Nephelometry. CPK measurements were performed using the modified Szasz method. The high-performance liquid chromatography (HPLC) method was used to measure HbA1c. Insulin was performed by chemiluminescence. A “sandwich”-type enzyme immunoassay was performed using the CHEMICON® Adiponectin Sandwich ELISA Kit (Chemicon International, CA, USA). TNFα and Lp-PLA2 were measured by Luminex™ xMAP for resistin, and radioimmunoassay was used for leptin.
Insulin resistance was evaluated using the Homeostasis Model Assessment Insulin Resistance (HOMA-IR)11, calculated by applying the formula: (FG*fasting insulin)/405. Beta-cell function was evaluated utilizing Homeostasis Model Assessment Beta (HOMA-B) by the formula: (20 × fasting insulin)/[(FG/18) − 3.5].
RNA isolation and quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from leukocytes stored in RNAlater® stabilization solution (Life Technologies, Carlsbad, CA, USA) using a RiboPureTM Blood kit (Life Technologies) for SA sample and Trizol protocol (Invitrogen, Massachusetts, USA) for HF. RNA integrity was assessed by the TapeStation® system (Agilent Technologies, Santa Clara, USA) or gel with GelRed (Uniscience, São Paulo, SP, Brazil), revealing the presence of two sharp bands at approximately 5 and 1.8 Kb, corresponding to 28S and 18S ribosomal RNA, respectively. RNA concentration and purity (260/280) were measured using a Qubit® 2.0 Fluorometer (Life Technologies) and NanoDrop® ND1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, USA), respectively. RNA samples were stored at − 80 °C.
mRNA expression was analyzed by qRT-PCR. cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) in a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA). qPCRs were run using TaqMan assay (TREML4, Hs01080584_g1; Life Technologies). The reference gene was selected from a normalization study of three endogenous candidate genes: GAPDH (Hs.592355_g1), ACTB (Hs.520640_g1), and 18S rRNA (Hs.626362_g1) using geNorm and NormFinder software. qPCRs were run in the Rotor-Gene Q Real-Time PCR Detection System (QIAGEN GmbH, Hilden, Germany) for SA, and 7500 Fast Real-Time PCR instrument (Applied Biosystems, CA, USA) for HF. Relative mRNA expression was calculated using the 2–ΔCT method, with ACTB as a reference gene.
DNA isolation and genotyping
According to the manufacturer’s instructions, the genomic DNA was isolated from whole blood collected in EDTA tubes using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). DNA quantification was measured by a UBIT® 2.0 fluorometer (Life Technologies, Forest City, USA). Purity (260/280) was performed using a NanoDrop® ND1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, USA) and Integrity 2200 TapeStation® system (Agilent Technologies, Santa Clara, USA). DNA samples were stored at − 20 °C until analysis. TREML4 polymorphisms rs2803495 (A > G) and rs2803496 (C > T) were genotyped by qRT-PCR using TaqMan SNP Genotyping Assays (C_27302616_10 and C_27302614_10) (Life Technologies) in a Rotor-Gene Q Real-Time PCR Detection System (QIAGEN GmbH, Hilden, Germany), according to the manufacturer’s protocol. Ten percent of randomly selected DNA samples were assayed in duplicate, and SNPs were 100% confirmed and concordant in the duplicate pairs. Genotyping analysis was not performed for the patients who had suffered previous MI.
Statistical analysis
Statistical analysis was performed using the SPSS® 22.0 software program (SPSS, Inc., Chicago, IL, USA) (www.ibm.com/br-pt/products/spss-statistics). Normal distribution was evaluated using the Kolmogorov–Smirnov test. Continuous variables with normal distributions are presented as the mean and standard deviation and were compared using t-tests or analysis of variance followed by Tukey’s test. Variables without parametric distributions are presented as the median and were analyzed using the Kruskal–Wallis test followed by the Mann–Whitney test. The chi-squared test compared categorical variables. Independent variables possibly affecting TREML4 mRNA expression were determined by univariate logistic regression analysis. A p-value < 0.05 was considered significant. Linkage disequilibrium was evaluated using the HAPLOVIEW® 4.2 software program (www.broadinstitute.org/haploview/haploview)24.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020).
Ahmadi, A., Argulian, E., Leipsic, J., Newby, D. E. & Narula, J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J. Am. Coll. Cardiol. 74(12), 1608–1617 (2019).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114(12), 1852–1866. https://doi.org/10.1161/CIRCRESAHA.114.302721 (2014).
Maciejak, A. et al. Circulating miR-30a-5p as a prognostic biomarker of left ventricular dysfunction after acute myocardial infarction. Sci. Rep. 8(1), 9883. https://doi.org/10.1038/s41598-018-28118-1 (2018).
Schunkert, H. et al. Genetics of coronary artery disease in the light of genome-wide association studies. Clin. Res. Cardiol. 107(2), 2–9. https://doi.org/10.1007/s00392-018-1324-1 (2018).
Duarte, V. H. R. et al. TREML4 mRNA expression and polymorphisms in blood leukocytes are associated with atherosclerotic lesion extension in coronary artery disease. Sci. Rep. 9(1), 1–8 (2019).
Silbiger, V. N. et al. Novel genes detected by transcriptional profiling from whole-blood cells in patients with early onset of acute coronary syndrome. Clin. Chim. Acta 421, 184–190. https://doi.org/10.1016/j.cca.2013.03.011 (2013).
Sen, S. K. et al. Integrative DNA, RNA, and protein evidence connects TREML4 to coronary artery calcification. Am. J. Hum. Genet. 95(1), 66–76 (2014).
Allcock, R. J. N., Barrow, A. D., Forbes, S., Beck, S. & Trowsdale, J. The human TREM gene cluster at 6p21. 1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur. J. Immunol. 33, 567–577 (2003).
Li, W. et al. TLR4 promotes liver inflammation by activating the JNK pathway. Eur. Rev. Med. Pharmacol. Sci. 23(17), 7655–7662 (2019).
Diniz, M. F. H. S. et al. Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study. Cad. Saude Publica 36(8), e00072120. https://doi.org/10.1590/0102-311X00072120 (2020).
Starr, A. E., Bellac, C. L., Dufour, A., Goebeler, V. & Overall, C. M. Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25): Chemokine and vimentin cleavages enhance cell migration and macrophage phagocytic activities. J. Biol. Chem. 287(16), 13382–13395 (2012).
Lotem, J. et al. Runx3 in immunity, inflammation and cancer. Adv. Exp. Med. Biol. 962, 369–393 (2017).
Naslavsky, N., Rahajeng, J., Rapaport, D., Horowitz, M. & Caplan, S. EHD1 regulates cholesterol homeostasis and lipid droplet storage. Bone 23(1), 1–7 (2008).
Hutter, C. M. et al. Association of endothelial lipase gene (LIPG) haplotypes with high-density lipoprotein cholesterol subfractions and apolipoprotein AI plasma levels in Japanese Americans. Atherosclerosis 185(1), 78–86 (2006).
Ray, A. G. et al. Novel mechanism of cholesterol transport by ABCA5 in macrophages and its role in dyslipidemia: Compensatory role of ABCA5 in cholesterol efflux. J. Mol. Biol. 432(17), 4922–4941. https://doi.org/10.1016/j.jmb.2020.07.006 (2020).
Gonzalez-Cotto, M. et al. TREML4 promotes inflammatory programs in human and murine macrophages and alters atherosclerosis lesion composition in the apolipoprotein E deficient mouse. Front. Immunol. 11, 397 (2020).
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of Atherosclerosis. Nature 473(7347), 317–325 (2011).
Franceschini, N. et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat. Commun. 9(1), 1–14 (2018).
Robert, F. & Pelletier, J. Exploring the impact of single-nucleotide polymorphisms on translation. Front Genet. 9(October), 1–11 (2018).
Yates, A. D. et al. Ensembl 2020. Nucl. Acids Res. 48(D1), D682–D688 (2020).
Jia, L. et al. Decoding mRNA translatability and stability from the 5′ UTR. Nat. Struct. Mol. Biol. 27(9), 814–821. https://doi.org/10.1038/s41594-020-0465-x (2020).
Bertolami, A. et al. Adiponectin concentration data improve the estimation of atherosclerotic risk in normal and in overweight subjects. Clin. Endocrinol. https://doi.org/10.1111/cen.13540 (2017).
Barrett, J. C., Fry, B., Maller, J. & Daly, M. J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 21(2), 263–265 (2005).
Acknowledgements
This study was partly financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Financing Code 001, the Fundação de Apoio à Pesquisa do RN (FAPERN) grant number 005/2011, the Conselho Nacional de Desenvolvimento Científco e Tecnológico (CNPq) grant number (483031/2013-5) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2010/18095-0), Brazil. V.H.R.D. and M.S.C. are recipients of a fellowship from CAPES, Brazil. M.H.H. and R.D.C.H. are recipients of fellowships from CNPq, Brazil.
Author information
Authors and Affiliations
Contributions
V.N.S., A.D.L., and V.H.R.D. were responsible for the original study concept and design. V.H.R.D., A.B., M.S.C. were responsible for the recruitment of the patients, collection of the samples, and laboratory analysis. V.H.R.D., A.D.L., and S.V.N. interpreted the results and performed the statistical analyses. V.H.R.D., M.S.C, A.D.L. and V.N.S. wrote the manuscript. M.H.H., R.D.C.H., A.D.L. and V.N.S. were responsible for the critical revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duarte, V.H.R., Cruz, M.S., Bertolami, A. et al. TREML4 polymorphisms increase the mRNA in blood leukocytes in the progression of atherosclerosis. Sci Rep 12, 18612 (2022). https://doi.org/10.1038/s41598-022-22040-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22040-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.