Abstract
Cancer-related systemic inflammation influences postoperative outcomes in cancer patients. Although the relationship between inflammation-related markers and postoperative outcomes have been investigated in many studies, their clinical significance remains to be elucidated in rectal cancer patients. We focused on the lymphocyte count/C-reactive protein ratio (LCR) and its usefulness in predicting short- and long-term outcomes after rectal cancer surgery. Patients with rectal cancer who underwent curative resection at our institution between 2010 and 2018 were enrolled in this study. We comprehensively compared the effectiveness of 11 inflammation-related markers, including LCR and other clinicopathological characteristics, in predicting postoperative complications and survival. Receiver operating characteristic curve analysis indicated that LCR had the highest area under the curve value for predicting the occurrence of postoperative complications. In the multivariate analysis, male sex (odds ratio [OR]: 2.21, 95% confidence interval [CI] 1.07–4.57, P = 0.031), low tumor location (OR: 2.44, 95% CI 1.23–4.88, P = 0.011), and low LCR (OR: 3.51, 95% CI 1.63–7.58, P = 0.001) were significantly and independently associated with the occurrence of postoperative complications. In addition, multivariate analysis using Cox’s proportional hazard regression model for the prediction of survival showed that low LCR (≤ 12,600) was significantly associated with both poor overall survival (hazard ratio [HR]: 2.07, 95% CI 1.03–4.15, P = 0.041) and recurrence-free survival (HR: 2.21, 95% CI 1.22–4.01, P = 0.009). LCR is a useful marker for predicting both short- and long-term postoperative outcomes in rectal cancer patients who underwent curative surgery.
Similar content being viewed by others
Introduction
Colorectal cancer is one of the most common cancers worldwide. Surgery is the standard treatment for resectable colorectal cancer. Although the postoperative outcome of colorectal cancer has improved, preventing postoperative complications remains challenging. Anastomotic leakage and urogenital and sexual dysfunctions are common complications after rectal surgery, and the risk is much higher than that associated with colon surgery. Postoperative complications can result in increased overall inpatient costs and sometimes lead to delayed initiation of postoperative treatments, including adjuvant chemotherapy. Furthermore, they negatively affect long-term survival1. Therefore, determining a biomarker to identify patients at a high risk of postoperative complications is important.
Cancer-related systemic inflammation was first reported by Rudolf Virchow in 18632. Inflammatory responses can proliferate residual cancer cells and stimulate micrometastases, leading to a risk of recurrence. Recently, preoperative systemic inflammation has been reported to be associated with poor prognosis after surgery in several types of cancer, including colorectal cancer. A review of previous research showed that combinations of inflammation-related variables, including serum neutrophil count, lymphocyte count, monocyte count, platelet count, C-reactive protein (CRP) concentration, and albumin concentration, the values of which are available from preoperative routine blood examinations can be useful biomarkers3.
CRP is a useful preoperative and postoperative parameter that reflects the inflammatory status in patients and is routinely evaluated in patients undergoing surgery in Japan. The combinations of CRP and other inflammatory variables, namely CRP/albumin ratio (CAR) and lymphocyte count/CRP ratio (LCR), have been reported to affect short-term and long-term postoperative outcomes4,5,6,7,8,9,10,11,12,13. However, only few studies have focused on patients with rectal cancer who underwent curative resection; moreover, the optimal cut-off value of each biomarker for rectal cancer has not been thoroughly scrutinized.
In the present study, we comprehensively compared the potential of various inflammation-related markers to predict postoperative short- and long-term outcomes in patients with rectal cancer who underwent curative resection. Furthermore, we evaluated the relationship between LCR and postoperative outcomes.
Materials and methods
Patients
A total of 202 patients with rectal cancer who underwent curative surgery at Kitano Hospital between 2010 and 2018 were enrolled in this study. Patients who underwent non-curative resection, recurrent tumor resection, or had distant metastasis were excluded. The tumor was located below the lower margin of the second sacral vertebra in all the patients. Based on tumor location, patients were categorized into the high (above the peritoneal reflection) and low (below the peritoneal reflection including the anal canal) groups. The clinical stage was determined using colonoscopy, computed tomography (CT), magnetic resonance imaging, and contrast-enhanced colonography.
Treatment protocol
Treatment strategies, including surgical procedures and perioperative chemotherapy or chemoradiotherapy, were determined for individual cases by a multidisciplinary team. Neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy was administered in cases where there was high risk for recurrence, such as for bulky tumors and swelling of multiple lymph nodes. All surgeries were performed or managed by board-certified colorectal surgeons.
Postoperative follow-up
Patients were postoperatively followed up according to the guidelines of the Japanese Society for Cancer of the Colon and Rectum14; physical examination and blood tests were performed every 3 months in the first 3 years and every 6 months thereafter. CT was performed every 6 months during the first 3 years after the operation and every year thereafter. Overall survival (OS) was defined as the period from the date of surgery to the date of death from any cause, and recurrence-free survival (RFS) as that from the date of surgery to the date of recurrence or death from any cause.
Inflammation-related markers
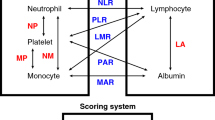
We reviewed the following 11 inflammation-related markers: lymphocyte count (/µL)/CRP (mg/dL) ratio (LCR), lymphocyte count/monocyte count (/µL) ratio (LMR), monocyte count (/µL)/albumin (g/dL) ratio (MAR), neutrophil count (/µL)/albumin (g/dL) ratio (NAR), neutrophil count/lymphocyte count (/µL) ratio (NLR), platelet count (/µL)/albumin (g/dL) ratio (PAR), platelet count/lymphocyte count (/µL) ratio (PLR), CRP (mg/dL)/albumin (g/dL) ratio (CAR), prognostic nutritional index (PNI), Glasgow prognostic score (GPS), and systemic inflammation score (SIS). All marker data were generated by routine preoperative blood examinations conducted within 2 weeks before the operation.
Regarding GPS, patients with both high serum CRP concentration (> 1.0 mg/dL) and low albumin concentration (< 3.5 g/dL) were assigned a score of 2; those with either of these two abnormal values, a score of 1; and those with neither, a score of 015. Regarding SIS, patients with both low albumin concentration (< 4.0 g/dL) and low LMR (< 4.44) were assigned a score of 2; those with either of these two abnormal values, a score of 1; and those with neither, a score of 016,17. PNI was calculated using the following formula: albumin concentration (g/L) + 0.005 × lymphocyte count (/µL)18
Prediction of postoperative complications
Postoperative complications were categorized according to the Clavien-Dindo classification19. We analyzed the correlation between the values of preoperative inflammation-related markers and the occurrence of postoperative complications (≥ Clavien-Dindo grade II) and compared the predictive performance of the markers with other clinical characteristics of the patients. The optimal cut-off value of each inflammation-related marker was determined using receiver operating characteristic (ROC) curve analysis.
Prognostic analysis
For the prognostic analysis, we focused on LCR. The patients were divided into high LCR and low LCR groups. We compared the clinical impact of LCR and other clinicopathological characteristics (such as age, sex, tumor location, carcinoembryonic antigen [CEA], pT category and pN category, and histology) on OS and RFS.
Statistical analysis
Continuous variables were presented as median [range] or mean ± standard deviation. The Fisher’s exact test or chi-square test was used to compare categorical variables. Prognostic analysis was performed using the log-rank test and the Kaplan–Meier method. Variables with a P value < 0.05 in the preceding univariate analysis were subjected to multivariate analysis using the Cox’s proportional hazard regression model. All analyses were two-sided, and a P value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro, version 16 (SAS Institute Inc., Cary, NC, USA).
Ethics approval
This retrospective study protocol was approved by the institutional review board of Kitano Hospital (reference no. 2201003) and conformed to the provisions of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained in the form of opt-out on the website. Those who rejected were excluded.
Results
Patient characteristics and outcomes
The clinicopathological characteristics of the study participants are shown in Table 1. A total of 202 patients (121 men and 81 women) with a median age of 67 years (range 34–93 years) were included. The mean body mass index was 22.4 ± 3.5 kg/m2. The tumor location was high in 98 patients (49%) and low in 104 patients (51%). Postoperative complications ≥ Clavien-Dindo grade II occurred in 52 patients (infectious complications that required the use of antibiotics in 26 patients, anastomotic leakage in 10, ileus in 9, anastomotic bleeding in 4, and other complications in 6). Pathologically positive regional lymph node metastases were observed in 56 patients (28%). Table 2 lists the test results of inflammation-related variables. The median follow-up duration was 62.8 months. The 5-year OS and RFS rates of all 202 patients were 82.9% and 70.6%, respectively (Fig. 1).
Comparison among the inflammation-related markers
The study participants were divided into the complication and non-complication groups based on the occurrence of postoperative complications ≥ Clavien-Dindo grade II. We performed ROC curve analysis for continuous variables to determine the optimal cut-off value to predict the occurrence of postoperative complications. The area under the curve value of LCR, LMR, and PNI was 0.57, which was higher than that of any other marker (Supplementary Fig. S1). We compared the values of all the 11 inflammation-related markers between the complication and non-complication groups (Table 3). LCR, LMR, NLR, PAR, CAR, PNI, and GPS were significantly associated with the occurrence of postoperative complications, and further analysis was conducted with a focus on the LCR ≤ 12,600 (P < 0.001).
Risk for postoperative complications
Table 4 shows the comparison between LCR and other clinicopathological characteristics in terms of their association with the occurrence of postoperative complications. The univariate analysis showed that male sex, low tumor location, and LCR ≤ 12,600 were significantly associated with the occurrence of postoperative complications. Similarly, in the multivariate analysis, male sex (odds ratio [OR]: 2.21, 95% confidence interval [CI] 1.07–4.57, P = 0.031), low tumor location (OR: 2.44, 95% CI 1.23–4.88, P = 0.011), and LCR ≤ 12,600 (OR: 3.51, 95% CI 1.63–7.58, P = 0.001) were independent risk factors for postoperative complications.
Predictive value of LCR for prognosis
The prognostic effects of the patient characteristics including LCR were investigated (Table 5). During the study period, 39 deaths (19%) and 41 recurrences (20%) occurred.
We first investigated potential prognostic factors for OS (Table 5, left). Univariate analysis showed that age > 70 years, CEA > 5 ng/mL, pT3-4, pN+, intraoperative blood loss > 100 mL, and LCR ≤ 12,600 were significantly associated with poor OS. In multivariate analysis using Cox’s proportional hazard regression model, age > 70 years (hazard ratio [HR]: 2.94, 95% CI 1.49–5.77, P = 0.002), intraoperative blood loss > 100 mL (HR: 2.74, 95% CI 1.38–5.43, P = 0.004), and LCR ≤ 12,600 (HR: 2.07, 95% CI 1.03–4.15, P = 0.041) were significantly associated with poor OS. The Kaplan–Meier curve depicting the effect of LCR on OS is shown in Fig. 2a.
We also investigated potential prognostic factors for RFS (Table 5, right). Univariate analysis revealed that age > 70 years, CEA > 5 ng/mL, pT3-a, pN+, intraoperative blood loss > 100 mL, and LCR ≤ 12,600 were significantly associated with poor RFS. In multivariate analysis, age > 70 years (HR: 2.04, 95% CI 1.20–3.47, P = 0.008), pT3–4 (HR: 3.00, 95% CI 1.54–5.85, P = 0.001), pN+ (HR: 2.32, 95% CI 1.33–4.03, P = 0.003), intraoperative blood loss > 100 mL (HR: 2.38, 95% CI 1.36–4.18, P = 0.003), and LCR ≤ 12,600 (HR: 2.21, 95% CI 1.22–4.01, P = 0.009) were significantly associated with poor RFS. The Kaplan–Meier curve representing the effect of LCR on RFS is shown in Fig. 2b.
Discussion
Recently, many reports have indicated the prognostic potential of inflammation-related biomarkers in patients with colorectal cancer. NLR is one of the most representative biomarkers, and the relationship between NLR and postoperative prognosis in patients with resectable colorectal cancer has been analyzed in many studies20,21,22,23,24,25,26. Similarly, several papers on the analysis of GPS or modified GPS27,28,29, LMR30,31,32, and PLR32,33 in the same population have been reported.
In the present study, we revealed that a low LCR (≤ 12,600) could be associated with both short- and long-term outcomes in patients with rectal cancer who underwent curative resection. The number of patients categorized as having low LCR was 38 (19%), and this cut-off value was appropriate for identifying patients with poor outcomes.
A few studies have revealed a relationship between CRP-related parameters and survival in patients with colorectal cancer. For example, Koike et al. reported that preoperative CRP > 0.5 mg/dL was significantly associated with poor prognosis in patients with stage I and II colorectal cancer34. There are also several reports on combinations of CRP and other variables. Dolan et al. indicated that CAR > 0.22 was a marker for poor prognosis in their retrospective analysis of the data of 801 patients with colon cancer6. Similarly, Ishizuka et al. analyzed the data of 627 patients with colorectal cancer patients and reported that CAR > 0.038 was associated with poor prognosis4. In both these studies, the cut-off values were determined using ROC curve analysis.
The prognostic potential of LCR has also been reported. Suzuki et al. analyzed 16 inflammation-related markers, including NLR, LMR, PLR, CAR, PNI, LCR, NAR, MAR, and PAR, in 1303 patients with colorectal cancer9. They concluded that an LCR ≤ 12,980 was significantly associated with a poor prognosis after surgery. Similarly, Okugawa et al. also investigated seven combinations and reported that LCR ≤ 6676 was the most significant predictor of survival in patients with colorectal cancer12. In both these studies, the cut-off value for LCR was determined using ROC curve analysis. Nakamura et al. categorized 756 patients with unresectable metastatic colorectal cancer into low, intermediate, and high LCR groups13. This stratification was significantly correlated with prognosis and a lower LCR independently affects survival.
Lymphocytes can promote cytotoxic immune responses in cases of malignancies. Decreased serum lymphocyte count can reflect impaired immunity to cancer. Some previous studies have indicated that decreased lymphocyte count itself could be associated with poor survival outcomes in patients with colorectal cancer35,36,37,38. Low LCR indicates a low lymphocyte count and high CRP concentration, which reflects elevated systemic inflammation and decreased immune function.
No reports have focused on the impact of LCR on postoperative outcomes in patients with rectal cancer. The tumor microenvironment and mechanism of tumor progression and metastasis can differ between the colon and rectum; therefore, it is mandatory to focus specifically on rectal cancer to identify the optimal inflammation-related marker and cut-off value. The present study showed that an LCR ≤ 12,600 could be an important biomarker in patients with resectable rectal cancer.
Importantly, no study has analyzed the predictive potential of inflammation-related markers for short-term outcomes after rectal cancer surgery. The rates of postoperative complications, including anastomotic leakage, intra-abdominal infectious disease, and urogenital, sexual, and anal dysfunctions, are generally higher in patients with rectal cancer than those in patients with colon cancer. This can be attributed to the immunological vulnerability of these patients and correlated with preoperative cancer-related systemic inflammation.
Recently, Artinyan et al. indicated a significant relationship between postoperative complications and poor prognosis in a large-scale database study that included 12,075 patients with colorectal cancer1. Therefore, it is important to identify biomarkers associated with short-term outcomes in order to improve long-term outcomes. Okugawa et al. reported that a preoperative LCR ≤ 6000 was significantly correlated with the rate of postoperative infectious complications as well as long-term survival, although they enrolled both patients with colon and rectal cancer7.
In the present study focusing on patients with rectal cancer, preoperative LCR ≤ 12,600 was significantly and independently associated with the rate of postoperative complications. Therefore, meticulous postoperative management is necessary for patients with a low preoperative LCR in order to improve short-term outcomes.
Preoperative treatments are important for advanced rectal cancer with high risk for recurrence, and 29 patients underwent neoadjuvant chemoradiotherapy (nCRT) and six patients underwent neoadjuvant chemotherapy (NAC) in the present study. In our dataset, there was no significant difference in LCR between patients with and without preoperative treatments (48,888 [1567–620,444] vs. 65,664 [4168–452,676], P = 0.162). We consider that LCR can independently affect postoperative outcomes regardless of the preoperative treatments.
To the best of our knowledge, this is the first report to indicate the predictive value of LCR for both short- and long-term outcomes, specifically in patients with rectal cancer. The assessment of preoperative LCR can help physicians identify subpopulations at high risk for postoperative complications and poor prognosis, and this can lead to improvement of short- and long-term outcomes after rectal cancer surgery.
This study has some limitations. First, the data were collected over a long time, and the results might have been affected by chronological changes in pre- and postoperative management, including chemotherapy regimens. Second, the optimal timing of blood sample collection was not evaluated. In the present study, the timing differed within the 2-week period before the operation depending on the patients. It is necessary to standardize the timing of blood tests to establish robust evidence regarding LCR. Third, owing to the retrospective nature of this single-center study involving a relatively small population, there might have been selection bias. Larger studies are necessary to establish the clinical utility of LCR as a biomarker for predicting short- and long-term survival of patients with rectal cancer.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author.
References
Artinyan, A. et al. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann. Surg. 261, 497–505. https://doi.org/10.1097/SLA.0000000000000854 (2015).
Balkwill, F. & Mantovani, A. Inflammation and cancer: Back to Virchow?. Lancet 357, 539–545. https://doi.org/10.1016/S0140-6736(00)04046-0 (2001).
Yamamoto, T., Kawada, K. & Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22158002 (2021).
Ishizuka, M. et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann. Surg. Oncol 23, 900–907. https://doi.org/10.1245/s10434-015-4948-7 (2016).
Ide, S. et al. Clinical significance of C-reactive protein-to-albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res. 37, 5797–5804. https://doi.org/10.21873/anticanres.12022 (2017).
Dolan, R. D. et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: Comparison of composite ratios and cumulative scores. Br. J. Cancer 119, 40–51. https://doi.org/10.1038/s41416-018-0095-9 (2018).
Okugawa, Y. et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003239 (2019).
Matsuoka, H. et al. Postoperative C-reactive protein/albumin ratio is a biomarker of risk of recurrence and need for adjuvant chemotherapy for stage III colorectal cancer. Int. J. Clin. Oncol. 25, 1318–1326. https://doi.org/10.1007/s10147-020-01672-3 (2020).
Suzuki, S. et al. Comprehensive comparative analysis of prognostic value of systemic inflammatory biomarkers for patients with stage II/III colon cancer. Ann. Surg. Oncol. 27, 844–852. https://doi.org/10.1245/s10434-019-07904-9 (2020).
Yasui, K. et al. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br. J. Cancer 124, 933–941. https://doi.org/10.1038/s41416-020-01189-6 (2021).
Taniai, T. et al. The prognostic significance of C-reactive protein-to-lymphocyte ratio in colorectal liver metastases. J. Surg. Res. 258, 414–421. https://doi.org/10.1016/j.jss.2020.08.059 (2021).
Okugawa, Y. et al. Cumulative perioperative lymphocyte/C-reactive protein ratio as a predictor of the long-term outcomes of patients with colorectal cancer. Surg. Today 51, 1906–1917. https://doi.org/10.1007/s00595-021-02291-9 (2021).
Nakamura, Y. et al. Lymphocyte-to-C-reactive protein ratio is the most sensitive inflammation-based prognostic score in patients with unresectable metastatic colorectal cancer. Dis. Colon Rectum 64, 1331–1341. https://doi.org/10.1097/DCR.0000000000002059 (2021).
Hashiguchi, Y. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 25, 1–42. https://doi.org/10.1007/s10147-019-01485-z (2020).
Forrest, L. M., McMillan, D. C., McArdle, C. S., Angerson, W. J. & Dunlop, D. J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 89, 1028–1030. https://doi.org/10.1038/sj.bjc.6601242 (2003).
Suzuki, Y. et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann. Surg. 267, 527–531. https://doi.org/10.1097/SLA.0000000000002115 (2018).
Chang, Y. et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br. J. Cancer 113, 626–633. https://doi.org/10.1038/bjc.2015.241 (2015).
Onodera, T. G. N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85, 1001–1005 (1984).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 250, 187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2 (2009).
Malietzis, G. et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann. Surg. 260, 287–292. https://doi.org/10.1097/SLA.0000000000000216 (2014).
Choi, W. J. et al. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann. Surg. Oncol. 22(Suppl 3), S603-613. https://doi.org/10.1245/s10434-015-4571-7 (2015).
Li, Z., Zhao, R., Cui, Y., Zhou, Y. & Wu, X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I–III colon cancer. Sci. Rep. 8, 9453. https://doi.org/10.1038/s41598-018-27896-y (2018).
Feliciano, E. M. C. et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C SCANS study. JAMA Oncol. 3, e172319. https://doi.org/10.1001/jamaoncol.2017.2319 (2017).
Dimitriou, N. et al. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer 18, 1202. https://doi.org/10.1186/s12885-018-5042-x (2018).
Mazaki, J. et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: A propensity score analysis. BMC Cancer 20, 922. https://doi.org/10.1186/s12885-020-07429-5 (2020).
Yoshida, D. et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in stage I–II rectal cancer patients. J. Surg. Res. 245, 281–287. https://doi.org/10.1016/j.jss.2019.07.072 (2020).
Toiyama, Y. et al. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp. Ther. Med. 2, 95–101. https://doi.org/10.3892/etm.2010.175 (2011).
Park, J. H. et al. Tumour invasiveness, the local and systemic environment and the basis of staging systems in colorectal cancer. Br. J. Cancer 116, 1444–1450. https://doi.org/10.1038/bjc.2017.108 (2017).
Tokunaga, R. et al. Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int. J. Clin. Oncol. 22, 740–748. https://doi.org/10.1007/s10147-017-1102-5 (2017).
Stotz, M. et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 110, 435–440. https://doi.org/10.1038/bjc.2013.785 (2014).
Chan, J. C. et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann. Surg. 265, 539–546. https://doi.org/10.1097/SLA.0000000000001743 (2017).
Ward, W. H. et al. Predictive value of leukocyte- and platelet-derived ratios in rectal adenocarcinoma. J. Surg. Res. 232, 275–282. https://doi.org/10.1016/j.jss.2018.06.060 (2018).
Dudani, S. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer 19, 664. https://doi.org/10.1186/s12885-019-5892-x (2019).
Koike, Y. et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J. Surg. Oncol. 98, 540–544. https://doi.org/10.1002/jso.21154 (2008).
Yang, J. et al. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci. Rep. 7, 17166. https://doi.org/10.1038/s41598-017-17130-6 (2017).
Dou, X. et al. Circulating lymphocytes as predictors of sensitivity to preoperative chemoradiotherapy in rectal cancer cases. Asian Pac. J. Cancer Prev. 14, 3881–3885. https://doi.org/10.7314/apjcp.2013.14.6.3881 (2013).
Kitayama, J., Yasuda, K., Kawai, K., Sunami, E. & Nagawa, H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat. Oncol. 5, 47. https://doi.org/10.1186/1748-717X-5-47 (2010).
Noh, O. K., Oh, S. Y., Kim, Y. B. & Suh, K. W. Prognostic significance of lymphocyte counts in colon cancer patients treated with FOLFOX chemotherapy. World J. Surg. 41, 2898–2905. https://doi.org/10.1007/s00268-017-4104-6 (2017).
Author information
Authors and Affiliations
Contributions
T.Y. planned the concept and design of the study. T.Y. and M.F. contributed to the analysis and interpretation of the data. T.Y., Y.O., and M.F. acquired the data. T.Y. drafted the manuscript. All the authors critically reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamamoto, T., Fukuda, M., Okuchi, Y. et al. Clinical impact of lymphocyte/C-reactive protein ratio on postoperative outcomes in patients with rectal cancer who underwent curative resection. Sci Rep 12, 17136 (2022). https://doi.org/10.1038/s41598-022-21650-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21650-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.