Abstract
Gulosibacter molinativorax ON4T is the only known organism to produce molinate hydrolase (MolA), which catalyses the breakdown of the thiocarbamate herbicide into azepane-1-carboxylic acid (ACA) and ethanethiol. A combined genomic and transcriptomic strategy was used to fully characterize the strain ON4T genome, particularly the molA genetic environment, to identify the potential genes encoding ACA degradation enzymes. Genomic data revealed that molA is the only catabolic gene of a novel composite transposon (Tn6311), located in a novel low copy number plasmid (pARLON1) harbouring a putative T4SS of the class FATA. pARLON1 had an ANI value of 88.2% with contig 18 from Agrococcus casei LMG 22410T draft genome. Such results suggest that pARLON1 is related to genomic elements of other Actinobacteria, although Tn6311 was observed only in strain ON4T. Furthermore, genomic and transcriptomic data demonstrated that the genes involved in ACA degradation are chromosomal. Based on their overexpression when growing in the presence of molinate, the enzymes potentially involved in the heterocyclic ring breakdown were predicted. Among these, the activity of a protein related to caprolactone hydrolase was demonstrated using heterologous expression. However, further studies are needed to confirm the role of the other putative enzymes.
Similar content being viewed by others
Introduction
Biocatalysis is a crucial component of white or industrial biotechnology, relying on the broad catalytic activity of enzymes leading to the production of value-added compounds (e.g., detergents, cosmetics), or catalyzing the degradation of several environmental contaminants (e.g., pesticides, hydrocarbons)1,2,3. Genome and transcriptome sequencing projects are critical to the burst of the biocatalysis field, enabling the identification of genes encoding proteins putatively involved in the production/degradation of the target compound(s), which can be further characterized to develop a biocatalytic solution1,4,5.
The biotechnological potential of Actinobacteria is known1,3, yet there is still much to unveil6. Gulosibacter molinativorax ON4T, an actinobacterium of the family Microbacteriaceae7, is an example. It was isolated from a five-membered enrichment culture (strains ON1-ON5) able to mineralize the thiocarbamate herbicide molinate8,9. Strain ON4T produces molinate hydrolase (MolA), responsible for breaking molinate into azepane-1-carboxylic acid (ACA) and ethanethiol8,10. Strain ON4T does not further use ethanethiol, but strains ON1 and ON2 consume this metabolite. In contrast, ACA supports the growth of strain ON4T and other culture members8. Hence, molinate mineralization occurs through the cooperation between strain ON4T and the culture members able to deplete the sulfur metabolite8. Although the putative degradation pathway for molinate has been described8, MolA is the only enzyme involved in this pathway identified and characterized so far10,11,12. Previous studies have shown the capacity of MolA and the recombinant mutant (Arg187Ala) thereof to degrade the thiocarbamate herbicides molinate and thiobencarb10,11,12. The biocatalytic potential of the enzymes involved in the ACA degradation pathway in G. molinativorax ON4T motivated this study.
Genes encoding enzymes involved in a particular metabolic pathway, as in xenobiotic degradation, tend to be close to each other13,14,15,16, often organized in catabolic operons with common regulation mechanisms17,18,19,20. Operons encoding enzymes involved in the degradation of xenobiotics can be located either in plasmids or in the chromosomes. Examples of plasmid operons are those encoding enzymes involved in the degradation of naphthalene, toluene, or xylene, described in pseudomonads (e.g., NAH7, pDTG114,17,18, pWWO13,19). In opposition, the operon involved in 2,4,6-trichlorophenol catabolism (tcpABC genes) is located in the chromosome, as suggested by the ability of R. eutropha JMP134 (pJP4) and of R. eutropha JMP222 (pJP4-cured JMP134 derivative) to degrade this pollutant21. Although primarily described as chromosomal20,22, the operon involved in biphenyl catabolism (bphABC genes) in pseudomonads, and other distant related Betaproteobacteria, is carried by an integrative conjugative element, that can integrate the chromosome and/or plasmid of the host23. In addition, genes involved in the same catabolic pathway are not always organized in catabolic operons and can be located in different regions of the chromosome24, or sometimes in the chromosome and extrachromosomal replicons (e.g., plasmids). An example of complementarity between chromosome and plasmid genes encoding xenobiotic degradation is given for carbaryl (1-naphthyl N-methylcarbamate) by Arthrobacter sp. strain RC10025.
This study aimed at a genomic characterization of G. molinativorax ON4T, particularly at identifying the genes coding for the potential enzymes involved in the ACA degradation pathway. Based on the premise that the genes of this pathway would be in a polycistronic operon unit, we started by searching the molA genetic environment in the publicly available draft genome of G. molinativorax DSM13485T (= LMG 21909T = CCUG 49965T = CIP 108515T = ON4T) generated by the DOE Joint Genome Institute (JGI, GenBank with the accession number AUDX00000000.1). However, it did not report the molA gene. We obtained the complete genome sequence of strain ON4T and, for comparison purposes, the genome of a variant of G. molinativorax ON4T unable to degrade molinate (strain ON4(−), obtained by successively growing in a culture medium without the herbicide for 12 generations). As the genomic data did not reveal other catabolic genes potentially involved in ACA degradation in the vicinity of the molA gene, transcriptomic analyses were further used to identify these genes. Transcripts obtained when strain ON4T grew in the presence and absence of the herbicide were compared and thoroughly analysed. We have identified a novel mobilizable plasmid and seven candidate enzymes involved in ACA mineralization. The future characterization of their in vivo activity will be of great value not only for fundamental microbiology, but to other emerging fields such as biocatalysis and synthetic biology.
Results and discussion
Strain ON4T genome sequencing
The 454 pyrosequencing of the G. molinativorax ON4T genome (ON4T454) generated 305,068 reads, comprising 133.7 million nucleotides (nt), which were assembled in 163 contigs (PXVE00000000). Interested in disclosing the genes in the vicinity of molA, analyses started with a BLAST search of the molA gene sequence against the 163 assembled contigs. The complete molA gene sequence (1398 bp) was located in a single contig (1957 bp) but revealed no other open reading frame (ORF) in its vicinity. The strain ON4T genome was resequenced using PacBio (ON4TPacBio), which generated 102,855 polymerase reads, resulting in 184,306 subreads with a mean subread length of 7254 bp, and 1,337 billion nt. These reads were de novo assembled and two contigs of 3,465,658 bp (linear) (contig A—CP028426) and 37,013 bp (circular) (contig B—CP028427) were generated. The assembled genome had 98.6% completeness, 0.6% contamination (Table S1) and 64% average G+C content for each contig. This in silico G+C content value agrees with previously published data7. The ON4TPacBio genome assembly disclosed 3243 predicted protein-coding genes, most of them assigned to putative functions (75.9%) and matching 94.3% of the predicted protein-coding genes in the assembly of strain DSM13485T (JGI, AUDX00000000.1) (Table 1, Fig. 1, Table S1). Furthermore, forty-nine tRNAs, three 16S rRNA, three 23S rRNA, and three 5S rRNA genes were annotated (Table 1). The occurrence of three 16S rRNA gene copies was further confirmed by determining the ratio between the 16S rRNA and the recA genes (single-copy gene) in both strains ON4T and ON4(−) (Table S2).
As expected, the data retrieved using both sequencing technologies (454 and PacBio) were very similar (Fig. 1). Nevertheless, the long reads obtained by PacBio sequencing allowed to sum up the 163 assembled ON4T454 contigs into two contigs only. In addition, the comparison of the two draft genomes allowed closing the contig A (Fig. S1) and identifying the molA gene in the contig B.
molA is the passenger gene of Tn6311
The molA gene, located in contig B, was surrounded by two identical insertion sequences (IS) of the IS3 group and family (Fig. 2a), whose original transposase belongs to the DDE transposase family26. The primary sequence of this IS was not identical to any IS described so far and was named ISGmo1 (https://www-is.biotoul.fr/). This structure, where a passenger gene (molA), which does not code for a transposition enzyme, is flanked by two IS (ISGmo1), is typical of a composite transposon27,28. This organization was confirmed by Sanger sequencing (data not shown). The novel composite transposon, named Tn6311, contained three copies of molA, each flanked upstream and downstream by ISGmo1 (a total of 4 ISGmo1 copies) (Fig. 2a). This tandem organization was primarily observed in specific PacBio raw reads (data not shown). Further, the three molA copies were confirmed in strain ON4T using quantitative PCR, as indicated by the gene copy ratio between molA and a neighbour single-copy gene (parA) (Table S2).
Mapping of the molA gene genetic vicinity. (a) Representation of the composite transposon Tn6311 in strain ON4T (ON4TPacBio—CP028427), as well as the in silico predicted promoters (P) close to molA gene. (b) Schematic representation of the ISGmo1 without passenger gene in strain ON4T and ON4(−) (ON4TPacBio—CP028426; and ON4T454—PXVE00000000; DSM13485T (= ON4T)—AUDX00000000.1 and ON4(−)—PXVD00000000, http://www.ncbi.nlm.nih.gov/).
As described for other IS3-like sequences, ISGmo1 has a total length of 1287 bp and contains two partially overlapping open reading frames, orfA (291 bp) and orfB (1080 bp), which encode the DNA-binding and the catalytic domain of the transposase, respectively27,29,30,31. Some IS3 family members might assemble different functional protein domains from one DNA segment, using a mechanism known as recoding programmed ribosomal frameshifting. This mechanism involves a ribosomal slippage of 1 nt, occurring at the heptanucleotide sequence A AAA AAG position, between orfA and the downstream fragment, orfB26,31. The slippage generates a −1 frameshift resulting in a fused product of the upstream frame (DNA-binding domain) and the downstream frame (catalytic domain), ORFAB26,31. Thus, ORFAB (396 aa) from ISGmo1 is a putative protein predicted in silico32 based on this mechanism. The three proteins of ISGmo1, ORFA, ORFB, and ORFAB, shared the highest amino acid sequence identity with the respective open reading frames of ISFsp8 (45–49%) found in Frankia sp. and ISAar25 and ISAar4 (43–45% and 44–46%, respectively) both found in Arthrobacter arilaitensis, also belonging to the phylum Actinobacteria (Fig. S2). Although DDE transposases have a highly conserved catalytic structure, they show overall low sequence conservation33, which may explain the low identities found between the ISGmo1 transposase and those found in the ISfinder database (https://www-is.biotoul.fr/). Despite the low identities, ISGmo1 clusters with IS sequences of other Actinobacteria, suggesting a phylogenetic relationship (Fig. S2).
In Tn6311, the inverted repeats on the left side (IRL) includes the start codon of the orfA/orfAB ISGmo1 genes and also two putative transposase promoters according to in silico identification (http://genome2d.molgenrug.nl/) (Fig. 2a). In contrast, the repeats on the right side (IRR) of ISGmo1, just upstream the molA gene, includes the last three codons of orfB and the − 35 region of the molA promoter. The structure of the molA gene regulatory region, i.e., the sequence of the regions − 35, − 10, and the ribosome binding sequence and their relative position towards the molA start codon (Fig. S3), suggests a strong promoter15,34. The existence of such a strong promoter combined with the tandem organization of molA gene in Tn6311 explains the high rate of molinate breakdown by strain ON4T, suggesting a high gene expression response.
Interestingly, the ISGmo1 sequence was also found in contig A (ON4TPacBio), but without any passenger gene (Fig. 2b) and with different direct repeats sequences. Evidence of such a copy of ISGmo1 was also given by the ON4T454, DSM13485T (JGI, AUDX00000000.1), and ON4(−) sequenced genomes and confirmed by primer design and Sanger sequencing (data not shown).
Characterization of contig B: pARLON1
Contigs A and B were presumed, based on size, to be a chromosome and a plasmid, respectively. The detection of a band with approximately 30 kbp by Pulsed-Field Gel Electrophoresis (PFGE) (Fig. S4) supported this hypothesis. Moreover, no reads mapping to contig B were found in the draft genomes of strains DSM13485T (JGI, AUDX00000000.1) and ON4(−) (Fig. 1), an ON4T variant unable to degrade molinate (Fig. S5). Altogether these data corroborate the existence of a mobile element enabling the degradation of molinate, i.e., a plasmid carrying the molA gene, which, most probably, was cured in strain ON4(−) after 12 generations in culture medium without the herbicide. In turn, the absence of reads mapping to contig B in strain DSM13485T (JGI, AUDX00000000.1) suggests that the conditions used to grow the culture collection strain did not favour the maintenance of the plasmid that is quite unstable. Hence, contig B was named pARLON1.
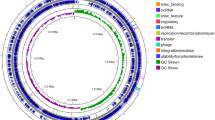
Plasmids are described as having modular structures, where each “module” is dedicated to a particular function (replication, stability, conjugation, establishment and adaption)35. pARLON1 contained 47 identified ORFs from which 36 ORFs (~ 27 kbp) belong to the plasmid backbone, and 11 ORFs belong to the adaptive module (Tn6311, ~ 10 kbp) (Fig. 3). pARLON1 did not share significant nucleotide identity with other described plasmids (< 70%), not even with non-conjugative plasmids harboured by other Microbacteriaceae members (e.g., plasmids pCM1 and pCM2 of Clavibacter michiganensis subsp. michiganensis, closely related with G. molinativorax)36. However, pARLON1 shared a high nucleotide identity (88.2%) with the contig 18 of Agrococcus casei LMG 22410T draft genome (Contig FUHU01000018.1, GenBank FUHU00000000.1), as determined by ANI37 (Table S3). LMG 22410T is the type strain of Agrococcus casei, also a member of the family Microbacteriaceae, with a G+C content (65 mol%) closer to that of G. molinativorax (64 mol%) than to other Agrococcus species7,38. The pARLON1 genes sharing > 70% identity with genes of the A. casei contig were those belonging to the plasmid backbone (36 predicted genes), representing 46.3 and 53.8% of the full coverage of contig B and contig FUHU01000018.1, respectively. In contrast, the predicted genes belonging to the adaptive module of each of these genetic elements were different (Fig. 3, Table S4).
Genetic organization of pARLON1 and scheme of the BLASTn search results between pARLON1 (contig B) and A. casei LMG 22410T (contig FUHU01000018.1 draft genome) using Easyfig software39. Plasmid genes predicted by CONJscan40 and/or oriTDB41 as related to a putative T4SS of the typeFATA are represented by dash lines.
Some ORFs of both pARLON1 and contig FUHU01000018.1 are related to the functional modules of transmissible plasmids, according to the annotation of pARLON1 and the in silico predictions performed by CONJscan40 and by oriTDB41. These analyses predicted the existence of two essential proteins of the single-strand DNA conjugation machinery, the relaxase (MOB) and the type IV coupling protein (T4CP). The former is a critical component of the relaxosome responsible for initiating the DNA transfer in both conjugative and mobilizable plasmids, and the last couples the relaxase-DNA nucleoprotein complex to the type IV secretion system (T4SS)42. A T4SS is a large complex of proteins spanning from the cytoplasm to the cell exterior constituting the mating-pair formation (MPF) module43,44. ORF44 and ORF12 from strains ON4T and A. casei, respectively, showed homology with MOB proteins, whereas ORF46/trsK and ORF10 were related to the TraG/TraD family protein whose members are homologs to T4CP45,46 (Fig. 3, Table S4). However, only CONJscan predicted the existence of an ORF related to VirB4 ATPase (ORF4 and ORF7 from strains ON4T and A. casei, respectively), a marker of a T4SS presence43,44 (Fig. 3, Table S4). Hence, according to CONJscan, both ON4T and A. casei harbour a putative T4SS of the class FATA40, which is a MPF family specific to Firmicutes, Actinobacteria, Tenericutes and Archaea43,44, agreeing with the taxonomic affiliation of both ON4T and A. casei. Because members of these microbial groups lack an outer membrane, some of the core complex T4SS components (e.g., VirB7/10) of Agrococcus tumefaciens are not identified in all the well-characterized conjugation systems of MPFFATA type (e.g., pCF10 and pGO1)44. However, proteins homologous to VirB3, VirB6 and VirB8, proteins of the inner-membrane pore, are common within this system type44. Among these MPF proteins, CONJscan predicted the existence of PrgH in ON4T (ORF2) and A. casei (ORF8), which show homology with VirB644. Also, PrgI, which is suggested to have homology with VirB344, was predicted in ON4T (ORF3). In addition, CONJscan predicted the presence of two other ORFs (1 and 45, and 9 and 11 from strains ON4T and A. casei, respectively) related to accessory genes detected at frequencies higher than 50% within the MPFFATA family (FATA_prgF and FATA_cd411). Although these predictions support the existence of a MPFFATA type, the presence of a functional MPF module in pARLON1 needs to be experimentally validated.
In addition, the pARLON1 backbone seems to carry a stability module. This module is particularly important in low copy number plasmids, where mechanisms, such as the partition (Par) systems, are required to ensure the even partitioning of the DNA molecules among progeny (vertical transmission)35,47,48. ORF22 and ORF28 from strains ON4T and A. casei, respectively, were predicted to be related to the plasmid partition motor protein ParA. However, no homologous to other proteins of this system (e.g., ParB and ParS) were predicted. These results may indicate that the Par system is incomplete/not functional (which could explain the existence of the cured strains DSM13485T and ON4(−)), or that the existing DNA binding proteins have low homology to known Par proteins (lower homology was found for proteins of the ParB group than for group ParA, difficulting the classification of the former49), or that another maintenance mechanism is present. At the population level, conjugative plasmids are described as low copy number, while mobilizable plasmids tend to be high copy number35,50. The ratio between the plasmid (parA) and chromosomal (recA) single-copy genes in strain ON4T was 3:1 (Table S2), suggesting that pARLON1 is a low copy number plasmid51. The presence of ORFs potentially associated with the maintenance and conjugation modules, together with the plasmid size (> 30 kbp) and its low copy number, suggest that pARLON1 can be a conjugative plasmid35. However, further studies are necessary to validate its conjugative activity.
As referred to above, the adaptive module of pARLON1 differed from that of contig 18 from strain A. casei LMG 22410T. However, the similarity of the plasmid backbone and the identification of a putative MPFFATA in strains ON4T and A. casei LMG 22410T strengthens the existence of a common actinobacterial ancestor that, depending on the environment, acquired different adaptive modules. Moreover, plasmid evolution might have been mediated via the activity of the insertion sequences flanking the adaptive module, IS6100 in A. casei LMG 22410T and ISGmo1 in Tn6311 of G. molinativorax ON4T.
Identification of candidate genes involved in ACA mineralization
Given the absence of any other catabolic gene in the vicinity of molA, a transcriptomic approach was used to identify the potential genes involved in ACA mineralization. The gene expression profiles of strain ON4T growing in molinate (mineral medium with the herbicide as the single source of energy, carbon and nitrogen, herein referred to as ON4TMMM) and without molinate (control medium LB, herein referred to as ON4TLB) were compared to identify the genes overexpressed in ON4TMMM.
After quality control, 14,380,135 and 15,330,180 reads in each library (ON4TMMM and ON4TLB, respectively) were retrieved. Analysis of the reads revealed that subtractive hybridization only removed a small amount of the ribosomal RNA, since 86–90% of reads mapped to rRNA. Consequently, approximately 1.4 and 1.0 million mRNA sequence reads were obtained from ON4TMMM and ON4TLB, respectively. After mapping the mRNA reads against the ON4T genome sequence, 95% of the 3243 expected transcripts were expressed under the tested growth conditions (ON4TMMM and/or ON4TLB) (Table S5). From the 3095 expressed transcripts, 2726 were expressed under both growth conditions and 335 were overexpressed in ON4TMMM compared to ON4TLB (Tables S6 and S7). Of these, 149 transcripts were exclusive in ON4TMMM, i.e., no reads were detected in ON4TLB. RT-qPCR validated expression results in eight overexpressed genes in ON4TMMM. The trend of expression level was similar to that of the transcriptomics experiment (Table 2).
Metabolic pathways for Gulosibacter sp. are not available in KEGG. Nevertheless, the predicted gene products from the 335 overexpressed transcripts in ON4TMMM were mapped to the KEGG database52. Some of the overexpressed predicted gene products in ON4TMMM mapped to KEGG Orthology (KO) categories (Fig. S6), namely to metabolism (e.g., amino acid, carbohydrates), environmental information processing (e.g., membrane transport) and Brite hierarchies (e.g., signaling and cellular processes), respectively. However, no complete KEGG pathway appeared to be overexpressed in ON4TMMM, in particular, none that could be related to ACA mineralization. Most of the predicted gene products mapped to more than one metabolic pathway and/or different predicted gene products mapped to the same metabolic function.
Thereby, genes potentially involved in ACA mineralization were identified based on homology to previously described enzymes involved in the degradation of other xenobiotics53,54,55,56 and on the structural similarity of the putative substrates (Fig. 4). Potential candidates involved in the breakdown of the heterocyclic ring containing metabolites are cytochromes P450 (CYP), which act as monooxygenases and are among the most versatile biological catalyst57, being, among others, involved in the degradation of different xenobiotics57,58. In typical class I P450 systems, the electrons required for oxygen activation are indirectly provided by NAD(P)H. The co-enzyme generally reduces a ferredoxin reductase (FDR), which in turn transfers the electrons to the second component of the system, usually a ferredoxin57. Finally, the ferredoxin mediates the electron transport between the FDR and the CYP57, further inducing the incorporation of one oxygen atom into the substrate via the reductive cleavage of oxygen.
Candidate enzymes involved in the five steps (I–V) of the putative molinate degradation pathway by strain ON4T adapted from Barreiros and collaborators8.
Strain ON4T harbours four ORFs with homology to cytochromes P450, namely bioI, cyp104, pipA and cpxP (Fig. 5, Table S8). Among these, bioI and its contiguous ferredoxin reductase (GMOLON4_437 and GMOLON4_436, respectively) were highly expressed in ON4TMMM (Log2FC > 6, Table S8). Also, cyp104 (GMOLON4_1038) and cpxP (GMOLON4_3097) were overexpressed in ON4TMMM (Log2FC > 3, Table S8), whereas pipA and its contiguous ferredoxin reductase (GMOLON4_3051 and GMOLON4_3050, respectively) were not significantly overexpressed in ON4TMMM (Log2FC < 2, Table S8). Noticeably, the cytochromes P450 bioI and pipA of strain ON4T have an amino acid identity of 72% and have a similar genetic neighbourhood (Fig. 5). Indeed, the upstream and downstream enzymes gene sequences seem to be similar, particularly the contiguous ferredoxin reductase GMOLON4_436 and GMOLON4_3050, respectively, which have an amino acid identity of 48% with each other. Also, the proteins potentially involved in the transfer of the electrons from the ferredoxin reductases to the cytochromes P450 bioI and pipA (GMOLON4_435 and GMOLON4_3049, respectively) share an amino acid identity of 69%. In contrast, these cytochromes (bioI and pipA) share less than 26% and 30% amino acid identity with the cytochromes cyp104 and cpxP, respectively, which are also distinct from each other (24% amino acid identity) (Fig. 5).
Curiously, the cytochromes P450 bioI and pipA of strain ON4T have an amino acid identity of approximately 53 and 52% with that of Mycobacterium smegmatis mc2155 (pipA, AAD28344.1) and Mycobacterium sp. HE5 (morA, AAV54064.1), respectively (Table S8). Both are shown to be involved in the hydroxylation of N-heterocycles53,59. M. smegmatis pipA was shown to be induced when strain mc2155 grew in piperidine as the sole source of energy, carbon, and nitrogen53. Mycobacterium sp. HE5 recombinant protein morA in combination with the adjacent pair ferredoxin reductase/ferredoxin was able to oxidize morpholine59. Although the contiguous bioI and pipA ferredoxin reductase (GMOLON4_436 and GMOLON4_3050, respectively) were distinct from those found in Mycobacterium strains (Fig. S7), the inhibition of ACA mineralization under anaerobic conditions8, combined with the similarity between the molecular structure of piperidine/morpholine (six/five membered N-heterocycles), and the predicted seven membered N-heterocycle product from ACA degradation in strain ON4T (hexamethyleneimine)8 (Fig. S7), supports the involvement of one of these systems in the hydroxylation of hexamethyleneimine to hydroxyhexamethyleneimine in strain ON4T8 (step I, Fig. 4). However, further experimental validation is necessary.
According to previous studies, the resulting hydroxyhexamethyleneimine is further oxidized to caprolactam8, which suggests the activity of a dehydrogenase (step II, Fig. 4). Cyclopentanol dehydrogenase (cpnA, GMOLON4_2241) is among the overexpressed dehydrogenases in ON4TMMM (Log2FC > 2, Table S8). It shows 40% amino acid identity with cyclopentanol dehydrogenases from Comamonas sp. NCIMB 987260 and 31–34% identity with cyclohexanol dehydrogenases from Rhodococcus sp. TK661 and Acinetobacter johnsonii62, respectively, known to oxidise cyclic alcohols but not N-heterocyclic alcohols, such as hydroxyhexamethyleneimine63,64,65.Therefore, the role of the GMOLON4_2241 product in the degradation of molinate in strain ON4T needs further experimental validation.
Considering the further step of the putative degradation of molinate, the cleavage of the cyclic metabolite (caprolactam) into 6-aminohexanoic acid (step III, Fig. 4), two possibilities arose from the transcriptomics analysis. These were the gene products of hyuA, GMOLON4_3203, and hyuB, GMOLON4_3204 (hydantoin utilization protein A and hydantoinase B, respectively), and the gene product of chnC (GMOLON4_1493, caprolactone hydrolase). All were overexpressed in ON4TMMM (Log2FC > 2, Table S6). GMOLON4_3203 and GMOLON4_3204 showed the highest amino acid identity (72–90%) with proteins of the Hydantoinase-Hydantoinase B/Oxoprolinase family found in different members of the phylum Actinobacteria. Whereas GMOLON4_1493 showed the highest amino acid identity (75–79%) with Alpha/Beta hydrolases, followed by caprolactone hydrolase (72–75%) of Rhodococcus sp. strains HI-31 and Phi266,67. Both hydantoinases/oxoprolinases and caprolactone hydrolase are described as able to open cyclic organic compounds, by hydrolising C-N bonds in cyclic amides and opening the lactone ring, respectively.
Caprolactam is the raw material for manufacturing Nylon-6 by ring opening polymerization. Attempts to isolate organisms capable of attenuating the environmental pollution caused by caprolactam intensive production and utilization have been made. Up to now, few Gram negative and Gram positive bacterial strains, including Gulosibacter sp. BS4, were described as capable of caprolactam degradation56,68,69,70. Among these organisms, only the enzyme acting upon the cleavage of caprolactam in Pseudomonas jessenii GO3 was described56,71. This caprolactamase, which consists of two subunits with high sequence identity to 5-oxoprolinases, hydrolyzes caprolactam into 6-aminohexanoic acid in an ATP-dependent manner56,71. Such information suggested the possible involvement of the GMOLON4_3203 and GMOLON4_3204 gene products in the breakdown of caprolactam by strain ON4T. However, heterologous expression of both potential candidates demonstrated that only the chnC gene product (GMOLON4_1493) degraded caprolactam in resting cells assays (Fig. 6). Proteins affiliated to this family are involved in the cyclic ε-caprolactone transformation into a linear organic acid, formed during the oxidation of cyclohexanol or cyclohexanone by both Gram staining positive and negative bacteria54,55,66,67. Hence, as far as we know, this is the first report of their involvement in caprolactam degradation.
Evaluation of caprolactam degradation in resting cells assays (DOλ600nm ≈ 6), by E. coli BL21(DE3) strain carrying either pET-30b( +), pET-chnC or pET-hyuA/B over a 24 h incubation period in 50 mM of phosphate buffer (pH 7.2) and 3 mM caprolactam. Values are means ± SE (n = 3, for all biological assays). AB—abiotic control.
Although only 6-oxohexanoate dehydrogenase (GMOLON4_913, chnE) was overexpressed in ON4TMMM (Log2FC > 3, Table S8), both 4-aminobutyrate aminotransferase (GMOLON4_1869, gabT) and 6-oxohexanoate dehydrogenase (GMOLON4_913, chnE) are potentially involved in the transformation of 6-aminohexanoic acid to adipic acid (step IV and V, Fig. 4). Indeed, the 4-aminobutyrate aminotransferase (GMOLON4_1869, gabT) and the 6-oxohexanoate dehydrogenase (GMOLON4_913, chnE) have an amino acid identity of 57 and 80% with the NylD1 6-aminohexanoate aminotransferase and the NylE1 adipate semialdehyde dehydrogenase from the Nylon oligomer-degrading actinobacterium Arthrobacter sp. strain KI7224, respectively (Table S8). In strain KI72, NylD1 in combination with the cofactor pyridoxal phosphate (PLP) catalyse the transference of the amino group from 6-aminohexanoate to an amino acceptor (e.g., α-ketoglutarate, pyruvate, and glyoxylate) generating 6-oxohexanoate and an amino-acid (e.g., glutamate, alanine, and glycine), respectively. Then, NylE1 catalyses the oxidation of 6-oxohexanoate to adipate using NADP+ as a cofactor, enabling the Arthrobacter sp. strain KI72 growth on 6-aminohexanoate as a sole source of carbon and nitrogen24. Similarly, the adipic acid formed through the activity of the 6-oxohexanoate dehydrogenase (GMOLON4_913, chnE) may enter the fatty acid β-oxidation providing further energy for strain ON4T metabolic activity72 (Fig. 4). Indeed, the enzymes of this central metabolic pathway were overexpressed in ON4TMMM (Table S8). The central metabolism activated via succinyl-CoA and acetyl-CoA from fatty acid β-oxidation may be thus related to the ability of strain ON4T to use ACA as the single source of carbon, nitrogen, and energy8.
Also, the overexpression of transcripts related to the metabolism of N-compounds, particularly glutamine synthetase GMOLON4_438/3052 (Fig. 4 and Fig. S6, Table S8), agrees with the ability of strain ON4T to grow on molinate as the single source of nitrogen8. The importance of glutamine synthetase and glutamate synthase cyclic mechanism in ammonium assimilation in prokaryotes is well known. In particular, Harper and collaborators73 reported that either glutamate dehydrogenase or glutamine synthase are very responsive and regulated under high or low nitrogen availability in M. smegmatis.
Among the proteins potentially involved in molinate degradation, only those related with the cytochromes P450 BioI and PipA seem to be found in some strains of the genus Gulosibacter (amino acid identity of up to 72% and 91% respectively, Table S9). These results suggest that some Gulosibacter strains, such as G. chungangensis KCTC 13959T, may have the ability to partially oxidize N-heterocycle compounds.
Interestingly, the products of the genes putatively involved on hexamethyleneimine degradation in strain ON4T showed relatively high amino acid identity with proteins involved in the oxidation of 6 membered N-heterocycle compounds (piperidine) and cycloalkane alcohols (cyclopentanol/cyclohexanol) by a wide diversity of strains, namely actinobacteria (Table S8). All these results suggest that small alterations on the structure of these proteins may have allowed the utilization of caprolactam in strains ON4T and Gulosibacter sp. BS4 and ACA in strain ON4T as energy, carbon, and nitrogen sources.
Conclusions
In summary, the present study, allowed the in silico identification and characterization of a novel transposon (Tn6311), which harbours molA, the enzyme responsible for the breakdown of the molinate to ACA and confers the unique degrading ability of G. molinativorax ON4T. In addition, a novel transmissible plasmid (pARLON1), which is present in strain ON4T and Agrococcus casei LMG 22410T, was characterized in silico. This last finding is of particular importance as the characterization of actinobacterial transmissible plasmids (mobilizable or conjugative) is scarce in the literature, compared to other bacterial phyla, particularly Proteobacteria. The future characterization of the plasmid transmission pathway will enlighten the underrepresented type IV secretion system (T4SS) in the FATA group, which includes only 3 representatives44. Such knowledge would enrich databases such as CONJscan40 and oriTDB41, contributing to better identifying this system, particularly in genomic mining studies.
Additionally, this study identified seven candidate genes involved in ACA mineralization, and validated the activity of the GMOLON4_1493 product, related to the caprolactone hydrolase chnC, in the degradation of caprolactam. The future characterization of these enzymes will contribute to enlightening the molinate degradation pathway in strain ON4T and strengthening their biocatalytic potential.
Material and methods
Culture conditions
Gulosibacter molinativorax ON4T and the variant strain ON4(−) were preserved in 15% (v/v) glycerol suspensions at − 80°C7. Whenever necessary, stored cells were recovered in Luria–Bertani Agar supplemented with 1 mM molinate (LAM). Cultures were incubated at 30 °C for 72 h. For 454 pyrosequencing, strain ON4T was grown on LAM, and for PacBio sequencing in mineral medium B9 supplemented with 0.2 g/L yeast extract and 1.5 mM molinate (MMM). For Illumina sequencing, strain ON4(−) was grown in LAM. For a detailed description see the Supplementary Material.
Genome analysis (sequencing, assembling and annotation)
454 pyrosequencing and PacBio technologies were used to sequence the genome of strain ON4T. For 454 pyrosequencing, five hundred nanograms of high-quality genomic DNA were fragmented by nebulization and the sequencing adapters ligated to create double-stranded DNA libraries. The 454 GS FLX sequencing platform (Roche) at Genoinseq (Cantanhede, Portugal) was used following the standard manufacturer’s protocols (see Supplementary Material). Sequencing reads were quality trimmed and de novo assembled by GS Assembler, version 2.9 (Roche) using default parameters.
Genome resequencing was performed using SMRT technology PacBio RSII on the RS sequencer at GATC Biotech AG (Constance, Germany), according to the manufacturer’s instructions74. Genome assembly was performed on the SMRT analysis software (http://www.pacb.com/products-and-services/analytical-software/smrt-analysis/). The Hierarchical Genome Assembly Process (HGAP, RS HGAP Assembly.3), which includes pre-assembly, de novo assembly with PacBio’s AssembleUnitig and assembly polishing with Quiver, was used with the default parameters, except for the minimum polymerase read quality, where 0.80 was used instead of 0.75. Gene prediction was carried out with GeneMarkS75 using specific parameters for prokaryotes (genetic code 11) and functional annotation was performed using the Sma3s76. The completeness and contamination of the SMRT assembled genome of strain ON4T were calculated with CheckM77.
For the draft genome of strain ON4(-) a DNA library was prepared from one nanogram of high-quality genomic DNA with the Nextera XT DNA Sample Preparation Kit (Illumina, San Diego, USA), which was sequenced using paired-end (PE) 2 × 300 bp on the MiSeq® Illumina® platform at Genoinseq (Cantanhede, Portugal). All procedures were performed according to the standard manufacturer’s protocols. Following demultiplex and quality-filter procedures, the high quality and adapter free reads were assembled with SPAdes, version 3.7.178, using in-house defined parameters.
Confirmation of the molA regulatory region
The 5′-Rapid Amplification of cDNA Ends (5′ RACE) was performed to identify the molA gene regulatory region using RNA extracted from strain ON4T grown in MMM. For a detailed description see the Supplementary Material.
Comparison of different assemblies
The assemblies obtained from all the G. molinativorax genome sequencing projects (PRJNA245014 present study and PRJNA188907) were compared using MAUVE software79 and the RAST server80. In addition, the raw reads of genomes ON4T454 (SRR6825088) and ON4(−) (SRR6825089) were also mapped against the contigs obtained in SMRT analysis software (genome ON4TPacBio) (https://www.pacb.com/products-and-services/analytical-software/smrt-analysis/), using the BWA-MEM algorithm81. The alignments were evaluated using Qualimap v2.2.1 software82 and were visualized in Tablet software83.
A BLASTx search of contig B against Agrococcus casei LMG 22410T draft genome (PRJEB19012) was performed (https://blast.ncbi.nlm.nih.gov/). In addition, the comparison (through BLASTn) between contig B and contig FUHU01000018.1 of Agrococcus casei LMG 22410T was carried out using the Contiguity software84, and their similarity was measured by the Average Nucleotide Identity (ANI) calculator (https://www.ezbiocloud.net/tools/ani).
Growth conditions, RNA extraction and sequencing of cDNA libraries
Given the low biomass yield of strain ON4T in mineral medium supplemented with a single carbon source other than molinate, strain ON4T was grown up to the exponential phase in MMM (ON4TMMM) and Luria–Bertani Broth (ON4TLB) in 250 mL Erlenmeyer flasks to obtain mRNA libraries. Total RNA was extracted using the RNeasy extraction kit (QIAGEN GmbH) (detailed protocol in the Supplementary Material). The RNA quality was assessed using the RNA 6000 Pico Kit with the Prokaryote Total RNA Pico assay (Agilent 2100 Agilent Technologies, Santa Clara, CA, USA).
The subtractive hybridization protocol described by Stewart et al.85 was used (detailed protocol in the Supplementary Material). The resulting rRNA-subtracted products were used to build the cDNA libraries for strand-specific RNA sequencing. The library preparation followed the protocol for whole transcription libraries in the Ion total RNA-Seq kit v2 (Life Technologies), using 500 ng of rRNA-subtracted products, as suggested by the manufacturer. Sequences were obtained in the Ion Proton™ platform at Genoinseq (Cantanhede, Portugal).
Normalization of the data from mRNA libraries
Ion Proton adapter sequences and low-quality bases were trimmed using the Torrent Suite software (Life Technologies). Trimmed reads were aligned to the strain ON4T genome using TMAP version 4.0.6 (Life Technologies). Reads that mapped to the rRNA regions were excluded. The reads count was accessed using eXpress86 and normalized using the Trimmed Mean of M values by edgeR87. Transcript expression differences between ON4TMMM and ON4TLB were considered when |Log2FC|> 2, where Fold Change (FC) is the ratio of normalized expression values of ON4TMMM over those of ON4TLB for each transcript. Thus, transcripts with Log2FC > 2 are overexpressed in ON4TMMM., whereas those with Log2FC > −2 are overexpressed in ON4TLB. The transcripts overexpressed in ON4TMMM were mapped to the KEGG database52 to identify potential overexpressed metabolic pathways related to ACA degradation.
Validation of transcriptomic data by RT-qPCR
For validation of libraries data, strain ON4T was grown in MMM and LB. Total RNA was extracted using kit NucleoSpin® RNA (Macherey–Nagel), and the cDNA from each sample was obtained with the Maxima H Minus First Strand cDNA Synthesis Kit, (ThermoScientific) (see Supplementary Material for detailed protocol).
The potential reference genes were selected among those without differences in expression between ON4TMMM and ON4TLB. Three reference genes (gyrA, gyrB, recA) were selected, and primers were designed using Oligo Explorer (http://www.genelink.com/tools/gl-oe.asp). For each reference gene, the primer pair specificity was assessed in silico, followed by qPCR amplification efficiency checking (Tables S10, S11) using different dilutions of DNA amplicons, and the amplification specificity was assessed using melting curves. The target genes were chosen considering differences in the expression values (|Log2FC|> 2) between ON4TMMM and ON4TLB. The primer choice and design and qPCR amplification efficiency and specificity (Tables S10, S11) for target genes were performed as described for reference genes (see Supplementary Material for detailed protocol).
RT-qPCR was performed in 96-well plates (Thermo Fischer Scientific) on the StepOnePlus system (Applied Biosystems). All RT-qPCR experiments were performed using three biological replicates, two technical replicates, and negative control (without cDNA). For detailed protocol see Supplementary Material.
Evaluation and validation of reference gene expression stability and fold changes of target genes
The candidate reference genes stability was analysed using RefFinder (https://omictools.com/reffinder-tool), where a ranking of genes stability is obtained considering the data obtained from geNorm, NormFinder, BestKeeper and deltaCt method88,89,90,91. The relative expression profile of each evaluated target gene was determined and normalized using a geometric mean value obtained from the amplification of the three best reference genes in all the samples (ON4TMMM and ON4TLB, respectively). Relative fold changes in gene expression were calculated using the Pfaffl method92, and data are displayed as mean ± standard error (SE).
Plasmid construction and cloning
All bacterial strains and plasmids used in the conjugants construction are listed in Table S12. The full sequence of the target genes (chnC and hyuA/hyuB) was amplified using total DNA of strain ON4T as a template and specific primers with a NdeI restriction site (Table S13). An A-tailing was added to the purified PCR products and further inserted into pTZ57R/T vector. Escherichia coli JM109 was transformed with pTZ57R/T with each insert and recombinants were identified by blue/white colour selection and confirmed by colony PCR (Table S14). Recombinant pTZ57R/T vectors were extracted and further digested with the respective Speedy Restriction Enzymes (NZYTech), according to manufacturer’s instructions (Table S13). The pET-30b(+) vector was restricted according to each target. After ligation, E. coli JM109 was transformed with the pET-30b(+) alone or carrying the target genes, and positive clones were selected from LB agar plates containing kanamycin (30 µg/mL) and screened by colony PCR (Table S14). Then, E. coli BL21 (DE3) was transformed with pET, pET-chnC or pET-hyuA/B vectors to further express the targeted genes r. See Supplementary Material for detailed protocol.
Heterologous expression
Resting cells of E. coli BL21(DE3) strain carrying the pET-chnC or pET-hyuA/B vectors were used to analyse the ability of the enzymes caprolactone hydrolase and hydantoin utilisation protein A/B, encoded by chnC and hyuA/hyuB, respectively, to degrade caprolactam. The biomass was resuspended (DOλ600nm ≈ 12) in 50 mM of phosphate buffer (pH 7.2) and mixed with 6 mM of caprolactam in 50 mM of phosphate buffer (1:1 ratio). The resting cells were incubated at 30 ºC for 24 h. E. coli BL21(DE3) with the pET-30b(+) was used as biotic control. Caprolactam was quantified in cell-free supernatants, collected at 0 and 24 h of incubation, by High Performance Liquid Chromatograph (HPLC, VWR Hitachi Chromaster), following the method previously described93. See Supplementary Material for detailed protocol.
Data availability
The datasets generated and analysed during the current study are available in the respective databases. The genomes obtained were deposited in GenBank under the accession numbers PXVE00000000 (genome ON4T454), CP028427 and CP028426 (genome ON4TPacBio) and PXVD00000000 (genome ON4(−)). The ISGmo1 sequence was deposited in the ISfinder database (https://www-is.biotoul.fr/). The composite transposon carrying molA was named Tn6311, according to the Tn number registry (http://www.ucl.ac.uk/eastman/research/departments/microbial-diseases/tn). The data from the cDNA libraries obtained in this work have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession numbers GSM3307305 and GSM3307306 for ON4TMMM and ON4TLB data, respectively.
References
Wiltschi, B. et al. Enzymes revolutionize the bioproduction of value-added compounds: From enzyme discovery to special applications. Biotechnol. Adv. 40, 107520. https://doi.org/10.1016/J.BIOTECHADV.2020.107520 (2020).
Alcalde, M., Ferrer, M., Plou, F. J. & Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. Trends Biotechnol. 24, 281–287 (2006).
Singh, R., Kumar, M., Mittal, A. & Mehta, P. K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 6, 1–15 (2016).
Yang, J. W., Zheng, D. J., Cui, B. D., Yang, M. & Chen, Y. Z. RNA-seq transcriptome analysis of a Pseudomonas strain with diversified catalytic properties growth under different culture medium. Microbiologyopen 5, 626–636 (2016).
Miyazaki, R. et al. Comparative genome analysis of Pseudomonas knackmussii B13, the first bacterium known to degrade chloroaromatic compounds. Environ. Microbiol. 17, 91–104 (2015).
Tischler, D., van Berkel, W. J. H. & Fraaije, M. W. Editorial: Actinobacteria, a source of biocatalytic tools. Front. Microbiol. 10, 800. https://doi.org/10.3389/fmicb.2019.00800 (2019).
Manaia, C. M., Nogales, B., Weiss, N. & Nunes O. C. Gulosibacter molinativorax gen. nov., a molinate-degrading bacterium, and classification of ‘Brevibacterium helvolum’ DSM 20419 as Pseudoclavibacter helvolus gen. nov., sp. Nov. Int. J. Syst. Evol. Microbiol. 54, 783–789 (2004).
Barreiros, L. et al. New insights into a bacterial metabolic and detoxifying association responsible for the mineralization of the thiocarbamate herbicide molinate. Microbiology 154, 1038–1046 (2008).
Barreiros, L. et al. A novel pathway for mineralization of the thiocarbamate herbicide molinate by a defined bacterial mixed culture. Environ. Microbiol. 5, 944–953 (2003).
Duarte, M. et al. Gulosibacter molinativorax ON4T molinate hydrolase, a novel cobalt-dependent amidohydrolase. J. Bacteriol. 193, 5810–5816 (2011).
Sugrue, E. et al. Evolutionary expansion of the amidohydrolase superfamily in bacteria in response to the synthetic compounds molinate and diuron. Appl. Environ. Microbiol. 81, 2612–2624 (2015).
Leite, J. P. et al. Structure-guided engineering of molinate hydrolase for the degradation of thiocarbamate pesticides. PLoS ONE 10, 1–18 (2015).
Greated, A., Lambertsen, L., Williams, P. A. & Thomas, C. M. Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol. 4, 856–871 (2002).
Simon, M. J. et al. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene 127, 31–37 (1993).
Rocha, E. P. C. The organization of the bacterial genome. Annu. Rev. Genet. 42, 211–233 (2008).
Habe, H. et al. Phthalate catabolic gene cluster is linked to the angular dioxygenase gene in Terrabacter sp. strain DBF63. Appl. Microbiol. Biotechnol. 61, 44–54 (2003).
Dennis, J. J. & Zylstra, G. J. Complete sequence and genetic organization of pDTG1, the 83 kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4. J. Mol. Biol. 341, 753–768 (2004).
Sota, M. et al. Genomic and functional analysis of the IncP-9 naphthalene-catabolic plasmid NAH7 and its transposon Tn4655 suggests catabolic gene spread by a tyrosine recombinase. J. Bacteriol. 188, 4057–4067 (2006).
Harayama, S. & Rekik, M. The meta cleavage operon of TOL degradative plasmid pWWO comprises 13 genes. MGG Mol. Gen. Genet. 221, 113–120 (1990).
Ohtsubo, Y. et al. BphS, a key transcriptional regulator of bph genes involved in polychlorinated biphenyl/biphenyl degradation in Pseudomonas sp. KKS102. J. Biol. Chem. 276, 36146–36154 (2001).
Matus, V., Sánchez, M. A., Martínez, M. & González, B. Efficient degradation of 2,4,6-trichlorophenol requires a set of catabolic genes related to tcp genes from Ralstonia eutropha JMP134(pJP4). Appl. Environ. Microbiol. 69, 7108–7115 (2003).
Furukawa, K., Hayase, N., Taira, K. & Tomizuka, N. Molecular relationship of chromosomal genes encoding biphenyl/poychlorinated biphenyl catabolism: Some soil bacteria possess a highly conserved bph operon. J. Bacteriol. 171, 5467–5472 (1989).
Hirose, J. et al. Biphenyl/PCB Degrading bph genes of ten bacterial strains isolated from biphenyl-contaminated soil in Kitakyushu, Japan: Comparative and dynamic features as integrative conjugative elements (ICEs). Genes (Basel) 10, 404 (2019).
Takehara, I. et al. Metabolic pathway of 6-aminohexanoate in the nylon oligomer-degrading bacterium Arthrobacter sp. KI72: identification of the enzymes responsible for the conversion of 6-aminohexanoate to adipate. Appl. Microbiol. Biotechnol. 102, 801–804 (2018).
Hayatsu, M., Hirano, M. & Nagata, T. Involvement of two plasmids in the degradation of carbaryl by Arthrobacter sp. strain RC100. Appl. Environ. Microbiol. 65, 1015–1019 (1999).
Siguier, P., Gourbeyre, E. & Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 38, 865–891 (2014).
Darmon, E. & Leach, D. R. F. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 78, 1–39 (2014).
Tsuda, M., Tan, H. M., Nishi, A. & Furukawa, K. Mobile catabolic genes in bacteria. J. Biosci. Bioeng. 87, 401–410 (1999).
Habe, H. et al. Characterization of the upper pathway genes for fluorene metabolism in Terrabacter sp. strain DBF63. J. Bacteriol. 186, 5938–5944 (2004).
Mahillon, J. & Chandler, M. Insertion sequences. Microbiol. Mol. Biol. Rev. 62, 725–774 (1998).
Polard, P., Prère, M. F., Chandler, M. & Fayet, O. Programmed translational frameshifting and initiation at an AUU codon in gene expression of bacterial insertion sequence IS911. J. Mol. Biol. 222, 465–477 (1991).
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J. & Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36 (2006).
Guérillot, R., Siguier, P., Gourbeyre, E., Chandler, M. & Glaser, P. The diversity of prokaryotic DDE transposases of the mutator superfamily, insertion specificity, and association with conjugation machineries. Genome Biol. Evol. 6, 260–272 (2014).
Mccarthy, J. E. G. & Gualerzl, C. Translational control of prokaryotic gene expression. Reviews 6, 78–85 (1990).
Garcillán-Barcia, M. P., Alvarado, A. & De la Cruz, F. Identification of bacterial plasmids based on mobility and plasmid population biology. FEMS Microbiol. Rev. 35, 936–956 (2011).
Gartemann, K. H. et al. The genome sequence of the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. J. Bacteriol. 190, 2138–2149 (2008).
Yoon, S. H., Ha, S. M., Lim, J., Kwon, S. & Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 110, 1281–1286 (2017).
Bora, N. et al. Agrococcus casei sp. nov., isolated from the surfaces of smear-ripened cheeses. Int. J. Syst. Evol. Microbiol. 57, 92–97 (2007).
Sullivan, M. J., Petty, N. K. & Beatson, S. A. Easyfig: A genome comparison visualizer. Bioinformatics 27, 1009–1010 (2011).
Abby, S. S. et al. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 6, 1–14 (2016).
Li, X. et al. oriTfinder: A web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 46, W229–W234 (2018).
Francia, M. V. et al. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28, 79–100 (2004).
Guglielmini, J., De La Cruz, F. & Rocha, E. P. C. Evolution of conjugation and type IV secretion systems. Mol. Biol. Evol. 30, 315–331 (2013).
Guglielmini, J. et al. Key components of the eight classes of type IV secretion systems involved in bacterial conjugation or protein secretion. Nucleic Acids Res. 42, 5715–5727 (2014).
Grohmann, E., Muth, G. & Espinosa, M. Conjugative plasmid transfer in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 277–301 (2003).
Alvarez-Martinez, C. E. & Christie, P. J. Biological diversity of prokaryotic Type IV secretion systems. Microbiol. Mol. Biol. Rev. 73, 775–808 (2009).
Guynet, C., Cuevas, A., Moncalián, G. & de la Cruz, F. The stb operon balances the requirements for vegetative stability and conjugative transfer of plasmid R388. PLoS Genet. 7, e1002073 (2011).
Bignell, C. & Thomas, C. M. The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91, 1–34 (2001).
Cegłowski, P. & Zielenkiewicz, U. Mechanisms of plasmid stable maintenance with special focus on plasmid addiction systems. Acta Biochim. Pol. 48, 1003–1023 (2001).
Fernández-López, R. et al. Dynamics of the IncW genetic backbone imply general trends in conjugative plasmid evolution. FEMS Microbiol. Rev. 30, 942–966 (2006).
Münch, K., Münch, R., Biedendieck, R., Jahn, D. & Müller, J. Evolutionary model for the unequal segregation of high copy plasmids. PLoS Comput. Biol. 15, e1006724. https://doi.org/10.1371/journal.pcbi.1006724 (2019).
Moriya, Y., Itoh, M., Okuda, S., Yoshizawa, A. C. & Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35, W182–W185. https://doi.org/10.1093/nar/gkm321 (2007).
Poupin, P., Ducrocq, V., Hallier-Soulier, S. & Truffaut, N. Cloning and characterization of the genes encoding a cytochrome P450 (PipA) involved in piperidine and pyrrolidine utilization and its regulatory protein (PipR) in Mycobacterium smegmatis mc2155. J. Bacteriol. 181, 3419–3426 (1999).
Brzostowicz, P. C., Blasko, M. S. & Rouvière, P. E. Identification of two gene clusters involved in cyclohexanone oxidation in Brevibacterium epidermidis strain HCU. Appl. Microbiol. Biotechnol. 58, 781–789 (2002).
Cheng, Q., Thomas, S. M., Kostichka, K., Valentine, J. R. & Nagarajan, V. Genetic analysis of a gene cluster for cyclohexanol oxidation in Acinetobacter sp. strain SE19 by in vitro transposition. J. Bacteriol. 182, 4744–4751 (2000).
Otzen, M., Palacio, C. & Janssen, D. B. Characterization of the caprolactam degradation pathway in Pseudomonas jessenii using mass spectrometry-based proteomics. Appl. Microbiol. Biotechnol. 102, 6699–6711 (2018).
Hannemann, F., Bichet, A., Ewen, K. M. & Bernhardt, R. Cytochrome P450 systems-biological variations of electron transport chains. Biochim. Biophys. Acta Gen Subj. 1770, 330–344 (2007).
Besse, P. et al. Degradation of morpholine and thiomorpholine by an environmental Mycobacterium involves a cytochrome P450. Direct evidence of intermediates by in situ 1H NMR. J. Mol. Catal. B Enzym. 5, 403–409 (1998).
Sielaff, B. & Andreesen, J. R. Kinetic and binding studies with purified recombinant proteins ferredoxin reductase, ferredoxin and cytochrome P450 comprising the morpholine mono-oxygenase from Mycobacterium sp. strain HE5. FEBS J. 272, 1148–1159 (2005).
Iwaki, H., Hasegawa, Y., Wang, S., Kayser, M. M. & Lau, P. C. K. Cloning and characterization of a gene cluster involved in cyclopentanol metabolism in Comamonas sp. strain NCIMB 9872 and biotransformations effected by Escherichia coli-expressed cyclopentanone 1,2-monooxygenase. Appl. Environ. Microbiol. 68, 5671–5684 (2002).
Kim, A., Tae-Kang, A., Choi, J. H. & Rhee, I. K. Purification and characterization of a cyclohexanol dehydrogenase from Rhodococcus sp. TK6. J. Microbiol. Biotechnol. 12, 39–45 (2002).
Chen, Y. C., Peoples, O. P. & Walsh, C. T. Acinetobacter cyclohexanone monooxygenase: Gene cloning and sequence determination. J. Bacteriol. 170, 781–789 (1988).
Tully, B. J., Graham, E. D. & Heidelberg, J. F. The reconstruction of 2,631 draft metagenome-assembled genomes from the global oceans. Sci. Data 5, 1–8 (2018).
Mehrshad, M. et al. The enigmatic SAR202 cluster up close: Shedding light on a globally distributed dark ocean lineage involved in sulfur cycling. ISME J. 12, 655–668 (2018).
Tully, B. J., Wheat, C. G., Glazer, B. T. & Huber, J. A. A dynamic microbial community with high functional redundancy inhabits the cold, oxic subseafloor aquifer. ISME J. 12, 1–16 (2018).
Brzostowicz, P. C., Walters, D. M., Thomas, S. M., Nagarajan, V. & Rouvière, P. E. mRNA differential display in a microbial enrichment culture: Simultaneous identification of three cyclohexanone monooxygenases from three species. Appl. Environ. Microbiol. 69, 334–342 (2003).
Mirza, I. A. et al. Crystal structures of cyclohexanone monooxygenase reveal complex domain movements and a sliding cofactor. J. Am. Chem. Soc 131, 8848–8854 (2009).
Baxi, N. N., Patel, S. & Hansoti, D. An Arthrobacter citreus strain suitable for degrading ε-caprolactam in polyamide waste and accumulation of glutamic acid. AMB Express 9, 1–11 (2019).
Esikova, T. Z. & Taran, S. A. A novel strain Gulosibacter sp. BS4 degrading epsilon-caprolactam and Nylon-6 oligomers. Microbiology (Russian Federation) 85, 642–645 (2016).
Baxi, N. N. & Shah, A. K. ε-caprolactam-degradation by Alcaligenes faecalis for bioremediation of wastewater of a nylon-6 production plant. Biotechnol. Lett 24, 1177–1180 (2002).
Marjanovic, A. et al. Catalytic and structural properties of ATP-dependent caprolactamase from Pseudomonas jessenii. Proteins Struct. Funct. Genet. 89, 1079–1098 (2021).
Fujita, Y., Matsuoka, H. & Hirooka, K. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 66, 829–839 (2007).
Harper, C. J., Hayward, D., Kidd, M., Wiid, I. & van Helden, P. Glutamate dehydrogenase and glutamine synthetase are regulated in response to nitrogen availability in Myocbacterium smegmatis. BMC Microbiol. 10, 138. https://doi.org/10.1186/1471-2180-10-138 (2010).
Eid, J. et al. Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138 (2009).
Besemer, J. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29, 2607–2618 (2001).
Casimiro-Soriguer, C. S., Muñoz-Mérida, A. & Pérez-Pulido, A. J. Sma3s: A universal tool for easy functional annotation of proteomes and transcriptomes. Proteomics 17, 1700071 (2017).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Bankevich, A. et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Darling, A. E., Mau, B. & Perna, N. T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5, e11147 (2010).
Aziz, R. K. et al. The RAST server: Rapid annotations using subsystems technology. BMC Genomics 9, 1–15 (2008).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26, 589–595 (2010).
Okonechnikov, K., Conesa, A. & García-Alcalde, F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 32, 292–294 (2016).
Milne, I. et al. Using tablet for visual exploration of second-generation sequencing data. Brief. Bioinform. 14, 193–202 (2013).
Sullivan, M., Zakoura, N., Fordea, B., Stanton-Cooka, M. & Beatson, S. Contiguity: Contig adjacency graph construction and visualisation. PeerJ Prepr. 3, e1037v1. https://doi.org/10.7287/peerj.preprints.63v1 (2015).
Stewart, F. J., Ottesen, E. A. & Delong, E. F. Development and quantitative analyses of a universal rRNA-subtraction protocol for microbial metatranscriptomics. ISME J. 4, 896–907 (2010).
Roberts, A. & Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods 10, 71–73 (2013).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. Determination of most stable housekeeping genes, differentially regulated target genes and sample integrity : BestKeeper. Biotechnol. Lett. 26, 509–515 (2004).
Silver, N., Best, S., Jiang, J. & Thein, S. L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 1–9 (2006).
Andersen, C. L., Jensen, J. L. & Ørntoft, T. F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250 (2004).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–12 (2002).
Pfaffl, M. W. Quantification strategies in real-time PCR in The Real-time PCR Encyclopaedia A–Z of Quantitative PCR (ed. Bustin, S.A.) 87–120 (International University Line, 2004).
Yagoubi, N., Guignot, C. & Ferrier, D. Simultaneous determination of polyamide 6 monomers: Amino 6 hexanoic acid and ε-caprolactam by RP HPLC. J. Liq. Chromatogr. Relat. Technol. 21, 2633–2643 (1998).
Acknowledgements
Authors gratefully acknowledge Mafalda Paiva, Márcia Duarte, Miguel Pinheiro and Miguel Vaz for their technical support. We also acknowledge Ana Catarina Ferreira and Célia Manaia from Universidade Católica Portuguesa, CBQF–Centro de Biotecnologia e Química Fina, Laboratório Associado, Escola Superior de Biotecnologia (ESB) and to Nuno Pereira from Instituto Superior de Engenharia do Porto (ISEP) and António Múrias from CIBIO—Research Centre in Biodiversity and Genetic Resources, InBIO, University of Porto for their valuable help in the PFGE analysis and informatics support, respectively. We are also indebted to Patricia Siguier from the ISFinder database for her valuable help in the identification and characterization of ISGmo1. This work was financially supported by: Base Funding LA/P/0045/2020 (ALiCE), and UIDB/00511/2020 (LEPABE) UIDB/00511/2020, funded by national funds through the FCT/MCTES (PIDDAC) and GenomePT: National Laboratory for Genome Sequencing and Analysis (POCI-01-0145-FEDER-022184).
Author information
Authors and Affiliations
Contributions
Study concept and design: O.C.N., C.E., A.R.L.; acquisition of data: A.R.L., A.T.V., C.B., D.P., J.F.; data analysis: A.R.L., E.B., A.T.V., C.B., D.P., H.F., A.M-M.; interpretation of data: O.C.N., C.E., A.R.L., A.T.V., X.B., E.B., I.V.-M.; Wrote the manuscript: O.C.N., C.E., and A.R.L. Critical revision of the manuscript: All authors reviewed the manuscript. Obtained funding: O.C.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopes, A.R., Bunin, E., Viana, A.T. et al. In silico prediction of the enzymes involved in the degradation of the herbicide molinate by Gulosibacter molinativorax ON4T. Sci Rep 12, 15502 (2022). https://doi.org/10.1038/s41598-022-18732-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18732-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.