Abstract
Carex buekii is a highly adaptive species showing a fairly wide ecological spectrum. It belongs to the group of river corridor plants which are vulnerable to any human activity directed at transformation of river valley habitats worldwide. This study was aimed at: determining the phenotypic variability of the species in the central part of its range, examining effects of soil conditions on the sedge’s morphological traits, and finding out whether the phenotypic plasticity observed may have taxonomic implications. A total of 487 specimens from 26 populations were collected in Hungary, Poland and Slovakia and tested by univariate, bivariate, and multivariate statistical methods. The analysis involved 16 morphological traits and 7 soil parameters (organic matter, pH, potassium, phosphorus, nitrogen, magnesium, calcium). Soil conditions were shown to affect the C. buekii morphology; particularly important was potassium, the only soil parameter that was indicated as a factor affecting intra-specific variability. Sites with lower contents of bioavailable potassium hosted C. buekii individuals which were generally smaller than those at sites showing higher soil potassium contents. The relationship held true also with respect to generative traits important in sedge taxonomy, i.e. utricle and beak lengths. Consideration of morphological differences only, without analysing relationships between morphology and soil conditions, could have resulted in distinguishing new entities at the level of species, subspecies or variety. Thus, knowledge on the range of phenotypic plasticity in field populations seems to be of a key importance in taxonomic studies.
Similar content being viewed by others
Introduction
Diversity of soil conditions may affect the morphology of plants growing in heterogeneous habitats1,2. Usually, the culm height, number of leaves, leaf surface area, and biomass were observed to increase with increasing nutrient contents3,4. Phenotypic variability associated with habitat conditions may lead to a distinct intra-specific differentiation between morphotypes, and even to separation of new taxa5. On one hand, the morphological traits used to identify and describe organisms are major practical criteria in plant taxonomy, as numerous taxonomic descriptions are based on morphological data6. On the other hand, using morphological traits alone for taxon delimitation, with no consideration for its plasticity, may lead to misidentifications5. Plasticity is considered to be a major source of phenotypic variation as it affects natural selection and, consequently, patterns of diversification among populations and species7.
Carex buekii Wimm., as a highly adaptive species of a fairly wide ecological spectrum, is a suitable model species for the research on phenotypic variability against ecological background. This sedge from the section Phacocystis Dumort. occurs in central-eastern Europe, in the northern part of the Balkan Peninsula and in south-eastern Asia8. C. buekii is a perennial plant more than 100 cm tall, with long and thick rhizomes. The species is distinguished based on, inter alia, dark reddish-brown basal leaf sheaths which display a characteristic reticulate-fibrous structure, the shiny upper side of the broad leaves (more than 1 cm) and nerveless or indistinctly nerved utricles with very short beaks9.
C. buekii is associated mainly with wetlands in river valleys and belongs to the so-called river corridor plants10. It is a group of species vulnerable to any human activity directed at transformation of river valley habitats worldwide. C. buekii grows both on river floodplains and in areas located at a considerable distance away from the river, including man-made habitats such as ditch and canal banks, bridgeheads and river embankments as well as roadsides. It usually occurs in nutrient-rich habitats, but is also capable of colonising relatively nutrient-poor ones; it grows on both acidic and alkaline soils (pH 3.3–7.4) with diverse concentrations of assimilable elements11.
Floodplains are relatively nutrient-rich10,12, particularly in valleys of large rivers, with a higher mineralisation rate and higher amounts of available macroelements13,14. On central-European plains, the corridors of large rivers are covered mainly by clay-rich Holocene deposits, surrounded by predominantly sandy material10. In addition, regular flooding of river valleys leads to distinct differences in nutrient contents, with floodplains representing relatively nutrient-rich corridors in a nutrient-poor landscape15.
The present study was aimed at: (1) determining the phenotypic variability of C. buekii in the central part of its range, (2) examining effects of soil conditions on the sedge’s morphological traits, and (3) finding out whether the observed phenotypic plasticity may have taxonomic implications.
Material and methods
Field studies and specimen collection
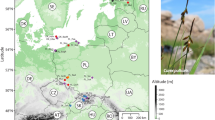
A total of 487 specimens from 26 wild populations (10–29 specimens per population, depending on the population size) of C. buekii in three Central European countries were examined. Field studies were conducted in Poland (119 specimens from 10 populations), Slovakia (262 specimens from 10 populations), and Hungary (106 specimens from 6 populations) (Fig. 1; Table S1). Specimens from a population were collected 3–6 m apart from one another to reduce the chance of collecting individuals from the same clone. The formal identification of the plant material was carried out by H. Więcław and J. Koopman. Voucher specimens for each population were deposited in the publicly available Herbarium Stetinensis at the University of Szczecin (SZUB)16.
Location of Carex buekii collection sites in Poland, Slovakia and Hungary. The map was made in QGIS version 3.22 available at https://qgis.org.
No official permits for our research were required because (i) all field research was conducted outside protected areas, at sites where sedges were abundant, (ii) the study did not concern protected taxa (Zákon/the Law No. 15/2005 Z.z. and Vyhláška/the Edict No. 24/2003 Z.z., Regulation of the Minister of the Environment of 2014, item 1409). Only a negligible part of each Carex buekii population was collected (always without root systems); therefore, no negative effects on the population were induced.
Morphological traits and measurements
A total of 16 morphological traits were determined (Table 1). Utricles, beaks and glumes were measured to 0.01 mm under a stereomicroscope. Five utricles and glumes, each from the middle part of a spike of each specimen, were isolated for measuring and the results were averaged. Other measurements were taken with a vernier calliper (to 0.05 cm: the spike size, widths of bracts and leaves, the peduncle length) and a ruler (to 0.1 cm: the culm height, leaf and bract lengths). The culm height was measured from the soil level to the top of the uppermost male spike. For each specimen, the length and width of the uppermost cauline leaf were measured. The leaf width was measured in the leaf’s central part.
Soil analysis
Soil samples were collected at each site, from the depth of 0–25 cm, with Egner's soil sampler. At each site, the samples were collected from three spots, the samples being subsequently combined into one to be used in laboratory assays. The soil samples were dried at room temperature and then rubbed through a sieve to remove fractions larger than 1 mm. The soil material prepared this way was used to determine the organic matter content (as a loss on ignition at 550 °C), pH (potentiometrically, in 1 M KCl), contents of assimilable nutrients: phosphorus (P) and potassium (K) using the Egner-Riehm method, magnesium (Mg) using Schachtschabel’s method, calcium (Ca) using atomic absorption spectrophotometry, and total nitrogen (N) using the Kjeldahl method (follwing the American Society of Agronomy17).
Data processing
Significance of differences between the data distribution and the theoretical normal distribution was examined using the Shapiro–Wilk test. As the distributions of most data sets deviated from normal, the non-parametric Mann–Whitney U test, Kruskal–Wallis test and Dunn’s multiple comparisons test were used to test for significance of differences between C. buekii populations. Relationships between the morphological traits and soil properties were examined with Spearman’s rank association test.
A preliminary sorting of the specimens was carried out using the Euclidean distance-based Ward’s minimum variance. Subsequently, the principal component analysis (PCA, on the correlation matrix) based on the complete data set, comprising all morphological traits, was carried out to quantify C. buekii’s morphological variability. The data used in the multivariate analyses were standardised so that each variable would have a mean of 0 and a standard deviation of 1. All the analyses were run in Statistica v. 13.1 for Windows18.
Sample distribution patterns and morphological traits in relation to soil variables were analysed by the redundancy analysis (RDA) using CANOCO v. 4.5119. The Monte Carlo permutation test was applied to determine statistical significance of soil properties applicable to explaining the C. buekii variability.
Results
Variability of morphological traits and its relationship with soil properties
Biometric analyses showed the most variable traits in C. buekii to include the peduncle length, PL (V = 63%); the culm height, CH; the bract length, BL and the number of male spikes, NMS (V ≥ 30%); the utricle beak length, UBL; the female spike length, FSL; and the inflorescence length, IL (V ≥ 25%) (Table 2). The mean plant height of 117.20 cm was associated with a standard deviation of 23.84 cm, indicating a relatively high degree of data dispersion. A high data variability was also observed in the cauline leaf length (13.08 cm and 40.04 cm standard deviation and mean, respectively), bract length (5.23 cm and 16.31 cm standard deviation and mean, respectively), and inflorescence length (4.67 cm and 18.57 cm standard deviation and mean, respectively). The variation amplitudes of the utricle length, UL and glume length, GL were narrow (coefficients of variation lower than 15%), indicating a low phenotypic plasticity with respect to these relatively homogeneous characters (Table 2).
Variability of the C. buekii morphological traits was found to be associated with soil conditions. Spearman’s rank association test showed significant (p ≤ 0.05) positive correlations between (1) the soil contents of potassium and the culm height, CH (rs = 0.635); the cauline leaf length, CLL (rs = 0.447); the cauline leaf width, CLW (rs = 0.643); and the bract length, BL (rs = 0.448); (2) the soil pH and the cauline leaf width, CLW (rs = 0.433) and the male spike length, MSL (rs = 0.465); (3) the soil contents of phosphorus and the cauline leaf width, CLW (rs = 0.432) and the bract length BL (rs = 0.448). Significant negative correlations were observed between (1) the soil contents of magnesium and the number of female spikes, NFS (rs = −0.401) and the peduncle length, PL (rs = −0.394); (2) the soil contents of calcium and the peduncle length, PL (rs = −0.418) (Table S2).
Between-populations variability and its relationship with soil properties
The non-parametric Kruskal–Wallis test detected significant differences between C. buekii populations (p ≤ 0.05) in all the morphological traits analysed. The post-hoc Dunn's multiple comparisons test identified the largest differences in morphological traits between the following population pairs: 2 vs 18, 3 vs 12, and 3 vs 21, with significant differences in 10 traits out of 16, and 1 vs 23, 2 vs 12, 2 vs 15, 2 vs 23, 19 vs 23, and 20 vs 23, with significant differences in 9 traits (Table S3).
In most traits, the ranges of values were overlapping between the populations (Fig. 2). Generally, the most distinct and significant differences were observed in the culm height (CH) of populations 4 and 15; the leaf length (CLL) of populations 12 and 2; the leaf width (CLW) of populations 2, 3 and 20; the male spike length (MSL) of populations 3 and 10; the female spike length (FSL) of populations 1 and 9; the female spike width (FSW) of populations 1 and 16; the utricle length (UL) of populations 1, 2 and 8, 12, 23; the beak length (UBL) of populations 1, 2, 4, 5 and 23; and the glume length (GL) of populations 1, 2, 7 and 15 (Fig. 2). The mean value of the beak length to utricle length ratio (UBL/UL) was at its lowest and highest in populations 5 and 23, respectively.
Ranges of morphological traits of Carex buekii populations. Large boxes indicate 25–75% of the interquartile ranges; small boxes represent medians; 1, 2, 3 ….26 are population numbers. The populations are numbered as in Table S1.
Based on the cluster analysis involving 16 morphological traits, the C. buekii specimens were assigned to two different groups (Fig. 3). This division reflects the distribution of the samples (populations) in the phenetic space (Fig. 4). Populations representing group I (8, 10, 12, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26) occupy the space on the left-hand side of the first (horizontal) axis, whereas populations making up group II (1, 2, 3, 4, 5, 6, 7, 9, 11, 13, 14, 16) are placed in the right-hand side of the plot (Fig. 4). Along the first axis, populations 2, 3 and 4 (right-hand side) as well as population 12 (left-hand side) are farthest away from the plot centre. The strongest effect on the first PCA axis was exerted by the culm height (CH), the cauline leaf length and width (CLL and CLW), the inflorescence length (IL), the male spike length (MSL), the bract length (BL), and the glume length (GL). The second axis was determined mostly by the female spike width (FSW), the utricle length (UL), the utricle beak length (UBL), and the glume length (GL) (Fig. 4A). The traits associated with the third axis included primarily those related to the female spike length (FSL), the peduncle length (PL), and the beak length to utricle length ratio (UBL/UL) (Fig. 4B). The first three principal component axes, taken together, explained 61% of the variance (31%, 18% and 12%, respectively). The fourth principal component axis explained 10% of the variance, other axes explaining a still lower percentage (Table S4).
Results of Euclidean distance-based cluster analysis (Ward’s minimum variance) for Carex buekii populations. The populations are numbered as in Table S1.
Distribution of the Carex buekii populations studied in two-dimensional space (Principal Component Analysis, PCA). Blue and pink circles represent group I and group II populations, respectively (cf. Fig. 3). The populations are numbered as in Table S1. Loadings for the first axis (PC1) (only absolute values ≥ 0.5): CH (culm height) = − 0.82; CLL (cauline leaf length) = −0.73; CLW (cauline leaf width) = −0.72; IL (inflorescence length) = −0.83; MSL (male spike length) = −0.61; BL (bract length) = − 0.85; and GL (glume length) = −0.51. Loadings for the second axis (PC2) (only absolute values ≥ 0.5): FSW (female spike width) = −0.80; UL (utricle length) = −0.75; UBL (utricle beak length) = −0.69; and GL (glume length) = −0.59. Loadings for the third axis (PC3) (only absolute values ≥ 0.5): FSL (female spike length) = 0.51; PL (peduncle length) = 0.50; and UBL/UL (beak length to utricle length ratio) = 0.70.
The Mann–Whitney U test showed the two C. buekii groups described above to differ significantly in 13 morphological traits (Table S5). The group I specimens are generally smaller, show shorter and narrower leaves, have fewer female spikes, their inflorescences are shorter, their male spikes are shorter and wider, their female spikes are narrower, their bracts are shorter, their utricles are smaller, and the utricle beaks are shorter than those in group II. In addition, the beak length to utricle length ratio in group I is lower than that in group II (Fig. S1). Group I contains Hungarian and some Polish populations, whereas group II includes Slovak populations and the remaining Polish ones (Table S1).
Results of the redundancy analysis (RDA) showed all the variables used to account for 48.2% of the total variance in the data (Table 3). Results of the step-wise forward selection of variables demonstrated the soil potassium content to be the only significant variable (Table 4). The C. buekii populations were scattered in the ordination space. The location of populations 15, 19, 21, 22 and 26 was associated with a relatively high soil potassium content as well as low nitrogen and organic matter contents (Fig. 5). Those populations were composed of relatively tall individuals with long and wide leaves. In contrast, populations 1, 2 and 4, which grew in relatively potassium-poor soils, consisted of specimens usually showing lower values of many morphological traits, including the taxonomically significant generative ones such as the utricle and beak lengths (Figs. 2 and 5; Table S1).
Ordination plot of populations (triangle), morphological traits and soil properties along the first two RDA axes; org. mat., organic matter content; pH, soil pH; N, nitrogen; P, phosphorus; K, potassium; Ca, calcium; *variables statistically significant (p ≤ 0.05). For trait codes see Table 2. The populations are numbered as in Table S1.
Discussion
Effects of potassium on plant growth and morphology
The potassium content was significantly correlated with the magnitude of some morphological traits of C. buekii. It was the only soil-related variable, among those analysed, to be significant, as determined by the RDA analysis. Doubtless, potassium—in addition to nitrogen and phosphorus—is one of the basic elements necessary for plant growth and development20,21. Potassium is the most frequent inorganic cation in plants, and accounts for up to 10% of the plant dry weight22,23. Potassium contents in Carex tissues are relatively high24, but are highly species-specific and habitat-dependent, as they are influenced mainly by the soil water content, soil type, and potassium availability25,26.
Changes in water level in flooding-prone areas usually constitute a major stressor which affects marsh vegetation growth and physiological processes27. As a rule, soil flooding limits plant growth by reducing oxygen penetration to the roots28. Some marsh plants are capable of diminishing damage due to oxygen deficiency and can increase their ability to tolerate flooding by biomass relocation (more biomass in the above-ground part to increase oxygen availability and less biomass in the below-ground part to diminish oxygen utilisation) and adaptation of shoot morphology, e.g. formation of elongated shoot organs such as internodes and petioles29,30. C. buekii seems to be relatively tolerant of disturbance due to flooding and increased water level11. However, similarly to other marsh species, its response to long-lasting flooding involves growth restriction (as shown by observations not used in the analyses presented in this work).
The plant growth on floodplains of large rivers, the usual C. buekii habitat, is practically not nutrient-limited. The nutrient supply there is mainly associated with water quality, flooding regime, and potential effects of agricultural use13. Flooding has profound impacts on the soil nutrient stoichiometry. Cao et al.31 found flooding to have significantly increased the contents of C and N in the terrestrial ecosystems they studied. However, the potassium form available to plants is easily leached out by the water, hence the amount of available potassium is frequently lower than that of nitrogen or phosphorus32,33.
C. buekii may grow on inundated meadows as well as on the overdried soil of dykes and embankments11. As shown by Sardans et al.32, consequences of overdrying are more important for the content of bioavailable potassium than phosphorus in the soil, most probably because potassium is more mobile in the soil and its absorption depends strongly on water transpiration and is associated with the osmotic control exerted by plants. Reduction of the soil moisture implied a decline in the soil diffusion capacity and a decreased amount of potassium available to plants32.
Generally, the potassium-poorer sites supported smaller C. buekii specimens, that is numerous morphological traits attained lower values compared to those recorded at potassium-richer sites. The utricle and beak lengths, traits important in sedge taxonomy, were also shorter. Effects of available potassium deficiency on plant morphology has been studied so far in cultivated species. For example, soil potassium deficiency was a cause of reduced growth of maize34, cotton35 and white clover36. Moreover, at a potassium deficiency, the leaf emergence rate was observed to be lower in rice37, tomatoes38 and maize34.
The potential of potassium to stimulate plant growth is directly related to its role in maintenance of the cellular turgor39 and indirectly to its role in controlling the osmotic potential of the stomata guard cells40; it is also associated with interactions and feedbacks between cellular potassium contents and the synthesis of abscisic acid (ABA) and auxin39.
In the present study, the soil potassium content was the only soil property significant for the morphological variability of C. buekii. It could have been important for the availability of other elements to the sedge. Interactions between potassium and other elements have been described in the literature; for example, high potassium concentrations in soil solutions inhibit magnesium uptake41; potassium deficiency may hamper absorption of calcium and sodium42 as well as nitrogen43. Reisch et al.44 observed effects of soil nutrient conditions on clonal diversity and genetic variation in C. nigra; both increased with the phosphorous concentration and decreased with that of potassium. Such interactions at the C. buekii sites we studied cannot be ruled out. Plant morphology is likely to be dependent on a number of factors and their interactions, and—as a rule—conforms to the primary limiting factor45.
Phenotypic plasticity within the genus Carex and its taxonomic implications
Variability of C. buekii specimens allowed to distinguish between two morphologically different groups. However, despite the differences in generative traits important for taxonomy (mainly the utricle size and utricle beak length), we think it is not appropriate to establish new taxa at the level of species, subspecies or even a variety. The morphological variability observed is most likely a result of C. buekii adaptation to the variable habitat conditions on floodplains and at sites altered by human activities such as construction of levees and canals.
As shown by studies on the C. flava agg., periodic flooding, local desiccation, trampling, sun exposure, and local edaphic conditions may lead to the emergence of different morphotypes5. The type of land use (grazing, mowing) may affect plant morphology as well46. Sedges growing at sites with poorer light conditions employ a shade avoidance strategy and develop higher culms as an advantage in the relatively strong competition for light47. Abnormally developed spikes are frequently observed in dry areas, e.g. in the section Racemosae taxa48. Lower temperatures in the mountains limit cell divisions and result in a smaller size of the plant (dwarf morphotypes). Some isolated montane Carex populations support morphotypes so different as to merit a separate taxonomic status, e.g. C. lepidocarpa subsp. nevadensis in the Sierra Nevada and C. lepidocarpa subsp. ferraria in the High Atlas49.
The botanical literature contains several reports on taxa within the genus Carex which have lost their previous taxonomic status after their wide phenotypic plasticity, resulting from adaptation to local habitat conditions, was examined. Such taxa include C. viridula Michx. var. pulchella (Lönnr.) B. Schmid from the section Ceratocystis Dumort., known also as the subspecies C. viridula Michx. subsp. pulchella (Lönnr.) Malyschev or, earlier, as the species C. pulchella (Lönnr.) Lindm.5,50. Another example of a controversial taxon is C. norvegica subsp. pusteriana (Kalela) Á.Löve & D.Löve (sect. Racemosae G.Don) described from the eastern Alps (Pustertal). According to Kalela51, the taxon differs from C. norvegica Retz. subsp. norvegica mainly in having longer utricles, narrower and shorter bracts, and wider leaves. Like in C. buekii, these are important taxonomic characters that proved variable and insufficient for supporting the validity of the taxon52, which has been also confirmed by molecular analyses53.
In our opinion, taxonomic studies on the genus Carex, based on both morphological and molecular analyses, should be carried out with reference to habitat conditions as well as biology and ecology of populations in the field; subsequently, the research can be complemented by herbarium data. Unfortunately, numerous taxonomist use only the latter and distinguish new species based on morphological and/or genetic variability of dried plants. Morphological examination of herbarium-held specimens of the section Phaestoglochin Dumort. have resulted in the description of 10 new taxa54,55. Due to the phenotypic plasticity observed, those descriptions raise controversies and are treated by some authors as synonyms of the already known species56. Molecular and morphological analyses within the section Rhynchocystis Dumort. revealed C. agastachys L.f., a neglected species from C. pendula agg.57. However, some of the morphological traits listed by Míguez et al.57 are hardly applicable to the identification of the species. Examination of the Czech specimens of C. pendula agg. showed the presence of individuals with a combination of vegetative traits of one species and the achene (nut) shape typical of another58.
Knowledge on the range of phenotypic plasticity in field populations seems to be of a key importance in taxonomic research. In the case of C. buekii, analysing herbarium-held specimens without considering the relationship between a trait and soil conditions could result in creating new entities which, on account of their morphological variability, would be difficult and/or impossible to identify. Taxonomic research should focus on an entity identifiable in the field (with a due consideration to its phenotypic plasticity), with specific ecological preferences, characterized by distinct biology and a specific distribution range.
Conclusions
The central-European populations of C. buekii studied differed significantly in their morphology. Despite the differences in generative traits, important for the taxonomy of the genus Carex (mainly the size of the utricle and its beak), we do not think it appropriate to distinguish new taxa at the level of species, subspecies, or even variety. The morphological variability observed is most likely an effect of the species’ adaptation to the variable edaphic conditions on floodplains and at sites changed by anthropogenic activities, such as levees and channels. In this study, the soil potassium content was the only edaphic factor that significantly affected the morphological variability of C. buekii; nevertheless, interactions between potassium and other elements (e.g. nitrogen, magnesium, calcium) cannot be ruled out. Future studies should examine hydrological conditions and explore their effects on both C. buekii morphology and nutrient concentrations in the soil. In light of the results obtained, we posit that knowledge on the range of phenotypic plasticity in natural populations seems to be of a key importance in taxonomic studies.
Data availability
The datasets analysed during the current study available from the corresponding author on reasonable request.
References
Mal, T. K. & Lovett-Doust, J. Phenotypic plasticity in vegetative and reproductive traits in an invasive weed, Lythrum salicaria (Lythraceae), in response to soil moisture. Am. J. Bot. 92, 819–825 (2005).
Wang, S., Li, L. & Zhou, D.-W. Morphological plasticity in response to population density varies with soil conditions and growth stage in Abutilon theophrasti (Malvaceae). Plant Ecol. 218, 785–797 (2017).
Eid, E. M., Shaltout, K. H., Al-Sodany, Y. M., Haroun, S. A. & Jensen, K. A comparison of the functional traits of Phragmites australis in Lake Burullus (a Ramsar site in Egypt): Young vs. old populations over the nutrient availability gradient. Ecol. Eng. 166, 106244 (2021).
Hassan, M. O. et al. Habitat variations affect morphological, reproductive and some metabolic traits of Mediterranean Centaurea glomerata Vahl populations. Heliyon 6, e04173 (2020).
Więcław, H. Within-species variation among populations of the Carex flava complex as a function of habitat conditions. Plant Ecol. Divers. 10, 443–451 (2017).
MacLeod, N. Phylogenetic signals in morphometric data. In Morphology, Shape and Phylogeny (eds MacLeod, N. & Forey, P.) 100–138 (Taylor & Francis, Routledge, 2002).
Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 208747 (2014).
Koopman, J. et al. Global distribution of Carex buekii (Cyperaceae) reappraised. Phytotaxa 358, 139–161 (2018).
Egorova, T. V. The Sedges (Carex L.) of Russia and Adjacent States (Within the Limits of the Former USSR) (St.-Petersburg State Chemical-Pharmaceutical Academy, St.-Petersburg, 1999).
Burkart, M. River corridor plants (Stromtalpflanzen) in Central European lowland: A review of a poorly understood plant distribution pattern: River corridor plants. Glob. Ecol. Biogeogr. 10, 449–468 (2001).
Więcław, H. et al. Ecology, threats and conservation status of Carex buekii (Cyperaceae) in Central Europe. Sci. Rep. 9, 11162 (2019).
Nobis, A. & Skórka, P. River corridor plants revisited: What drives their unique distribution patterns?. Plant Biosyst. 150, 244–253 (2016).
Spink, A., Sparks, R. E., Van Oorschot, M. & Verhoeven, J. T. A. Nutrient dynamics of large river floodplains. Regul. Rivers. Res. Manag. 14, 203–216 (1998).
Myśliwy, M. Diversity and environmental variability of riparian tall herb fringe communities of the order Convolvuletalia sepium in Polish river valleys. Monographiae Botaniceae 108, 1–129 (2019).
Fischer, W. Die Stromtalpflanzen Brandenburgs. Unter Havel. Naturkundliche Berichte 5, 4–13 (1996).
Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Available from: http://sweetgum.nybg.org/science/ih/ (assessed: 08 March 2022).
Methods of Soil Analysis: Part 3 Chemical Methods. (Soil Science Society of America, American Society of Agronomy, 1996). https://doi.org/10.2136/sssabookser5.3.
StatSoft Inc. Electronic Statistics Textbook. (Tulsa, OK: StatSoft, 2013).
ter Braak, C. J. F. & Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 4.5). (Ithaca NY, 2002).
Xu, X. et al. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 11, 904 (2020).
Sardans, J. & Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 10, 419 (2021).
Broadley, M. R. et al. Phylogenetic variation in the shoot mineral concentration of angiosperms. J. Exp. Bot. 55, 321–336 (2004).
Watanabe, T. et al. Evolutionary control of leaf element composition in plants. New Phytol. 174, 516–523 (2007).
Konings, H., Koot, E. & Wolf, A. T. Growth characteristics, nutrient allocation and photosynthesis of Carex species from floating fens. Oecologia 80, 111–121 (1989).
Busch, J. Characteristic values of key ecophysiological parameters in the genus Carex. Flora 196, 405–430 (2001).
Zhang, D. et al. Effect of hydrological fluctuation on nutrient stoichiometry and trade-offs of Carex schmidtii. Ecol. Ind. 120, 106924 (2021).
Zhang, D. et al. Growth and physiological responses of Carex schmidtii to water-level fluctuation. Hydrobiologia 847(3), 967–981 (2020).
Yan, H. et al. Growth and physiological responses to water depths in Carex schmidtii Meinsh. PLoS ONE 10(5), e0128176 (2015).
Luo, W. & Xie, Y. Growth and morphological responses to water level and nutrient supply in three emergent macrophyte species. Hydrobiologia 624(1), 151–160 (2009).
Lu, Y. Growth and morphological responses to water level variations in two Carex species from Sanjiang Plain, China. Afr. J. Agric. Res. 6, 28–34 (2011).
Cao, Y. et al. Flooding influences on the C, N and P stoichiometry in terrestrial ecosystems: A meta-analysis. CATENA 215, 106287 (2022).
Sardans, T., Peñuelas, T., Prieto, P. & Estiarte, M. Drought and warming induced changes in P and K concentration and accumulation in plant biomass and soil in a Mediterranean shrubland. Plant Soil 306, 261–271 (2007).
Flórez-Flórez, C. P., León-Peláez, J. D., Osorio-Vega, N. W. & Restrepo-Llano, M. F. Nutrient dynamics in forest plantations of Azadirachta indica (Meliaceae) established for restoration of degraded lands in Colombia. Rev. Biol. Trop. 61, 515–529 (2013).
Jordan-Meille, L. & Pellerin, S. Leaf area establishment of a maize (Zea mays L.) field crop under potassium deficiency. Plant Soil 265, 75–92 (2004).
Gerardeaux, E., Jordan-Meille, L., Constantin, J., Pellerin, S. & Dingkuhn, M. Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum L. Environ. Exp. Bot. 67, 451–459 (2010).
Bailey, J. S. & Laidlaw, A. S. Growth and development of white clover (Trifolium repens L.) as influenced by P and K nutrition. Ann. Bot. 81, 783–786 (1998).
White, P. Relationship between the development and growth of rye (Secale cereale L.) and the potassium concentration in solution. Ann. Bot. 72, 349–358 (1993).
Pujos, A. & Morard, P. Effects of potassium deficiency on tomato growth and mineral nutrition at the early production stage. Plant Soil 189, 189–196 (1997).
Osakabe, Y. et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25, 609–624 (2013).
Lebaudy, A. et al. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. 105, 5271–5276 (2008).
Tränkner, M., Tavakol, E. & Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plantarum 163, 414–431 (2018).
Du, Q. et al. Effect of potassium deficiency on root growth and nutrient uptake in maize (Zea mays L.). Agric. Sci. 8, 1263–1277 (2017).
Hu, W., Coomer, T. D., Loka, D. A., Oosterhuis, D. M. & Zhou, Z. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 115, 408–417 (2017).
Reisch, C., Meier, S., Schmid, C. & Bartelheimer, M. Clonal diversity and genetic variation of the sedge Carex nigra in an alpine fen depend on soil nutrients. PeerJ 8, e8887 (2020).
Lenssen, J. P. M., Menting, F. B. J. & Van der Putten, W. H. Plant responses to simultaneous stress of waterlogging and shade: Amplified or hierarchical effects?. New Phytol. 157, 281–290 (2003).
Liu, Z. G. & Li, Z. Q. Effects of different grazing regimes on the morphological traits of Carex duriuscula on the Inner Mongolia steppe, China. N. Z. J. Agric. Res. 53(1), 5–12 (2010).
Więcław, H. et al. Morphological variability and genetic diversity in Carex buxbaumii and Carex hartmaniorum (Cyperaceae) populations. PeerJ 9, e11372 (2021).
Więcław, H., Kurnicki, B., Bihun, M., Białecka, B. & Koopman, J. Carex section Racemosae (Cyperaceae) in Europe: Morphological diversity, taxonomy and phylogenetic relationships. Bot. J. Linn. Soc. 183, 124–145 (2017).
Jiménez-Mejías, P., Benítez-Benítez, C., Fernández-Mazuecos, M. & Martín-Bravo, S. Cut from the same cloth: The convergent evolution of dwarf morphotypes of the Carex flava group (Cyperaceae) in Circum-Mediterranean mountains. PLoS ONE 12(12), e0189769 (2017).
Więcław, H. Carex flava agg. (section Ceratocystis, Cyperaceae) in Poland: taxonomy, morphological variation, and soil conditions. Biodivers. Res. Conserv. 33, 3–51 (2014).
Kalela, A. Systematische und Pflanzengeographische Studien an der Carex-Subsektion Alpinae Kalela. Annales Botanici Societatis Zoologicae-Botanicae Fennicae 19, 1–218 (1944).
Wallnöfer, B. Uber Carex melanostachya, C. norvegica, C. cespitosa und C. hartmanii in Südtirol. Gredleriana 4, 413–418 (2004).
Gebauer, S., Röser, M. & Hoffmann, M. H. Molecular phylogeny of the species-rich Carex sect. Racemosae (Cyperaceae) based on four nuclear and chloroplast markers. Syst. Bot. 40, 433–447 (2015).
Molina, A., Acedo, C. & Llamas, F. Taxonomy and new taxa in Eurasian Carex (Section Phaestoglochin, Cyperaceae). Syst. Bot. 33, 237–250 (2008).
Molina, A., Acedo, C. & Llamas, F. Taxonomy and new taxa of the Carex divulsa aggregate in Eurasia (section Phaestoglochin, Cyperaceae). Botan. J. Linn. Soc. 156, 385–409 (2008).
Jiménez-Mejías, P. & Luceño, M. Cyperaceae. in Euro+Med. etc. Plantbase – the information resource for Euro-Mediterranean plant diversity. Available from: http://www.emplantbase.org/home.html (accessed 07 January 2022). (eds Greuter, W. & Raab-Straube, E. von) (2011).
Míguez, M., Martín-Bravo, S. & Jiménez-Mejías, P. Reconciling morphology and phylogeny allows an integrative taxonomic revision of the giant sedges of Carex section Rhynchocystis (Cyperaceae). Botan. J. Linn. Soc. 188, 34–58 (2018).
Kaplan, Z. et al. Distributions of vascular plants in the Czech Republic. Preslia 93, 255–304 (2021).
Acknowledgements
We thank two anonymous reviewers for their helpful comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Contributions
H.W. and J.K. identified the studied species; H.W., Z.D. nad J.K. conducted fieldwork in Poland; R.H. conducted fieldwork in Slovakia; A.M. conducted fieldwork in Hungary; H.W. and B.B. taking measurements of morphological traits; H.W. analysed the data, wrote the first version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Więcław, H., Bosiacka, B., Hrivnák, R. et al. Morphological variability of Carex buekii (Cyperaceae) as a function of soil conditions: a case study of the Central European populations. Sci Rep 12, 11761 (2022). https://doi.org/10.1038/s41598-022-15894-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15894-0
This article is cited by
-
Environmental adaptability of the genus Carex-A case study of Carex heterostachya and Carex breviculmis in northwest China
Plant Ecology (2023)
-
Response Patterns of Fen Sedges to a Nutrient Gradient Indicate both Geographic Origin-Specific Genotypic Differences and Phenotypic Plasticity
Wetlands (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.