Abstract
In spite of the fact that rock weathering performs an essential task in the evolution of the Earth’s surface, the quantitative assessment between pH and rates of chemical weathering remain unclear. This study aims to characterize the chemical weathering rate of purple rocks and then develops a model to calculate the release rates of cations (K+, Na+, Ca2+ and Mg2+) under various pH conditions. Two types of purple rock were sampled from the Shaximiao Group (J2s) and Penglaizhen Group (J3p), and a series of laboratory experiments were performed by soaking the purple rocks in solutions with pHs from 2.5 to 7.0, over 24 treatment cycles. The results showed that the release rates of cations apparently increased as the pH decreased. The release of Ca2+ was the dominant process of chemical weathering in J3p under various pH treatments, while K+ and Na+ were remarkably high in J2s (with the exception of the pH 2.5 treatment). Quantitative analysis revealed that the rate of cation release was significantly related to the H+ concentration (p < 0.001) and the air temperature (p < 0.001). The relationship between cation release and acidity was found to be an exponential function. Our results suggested that solution acidity serves as an important driving force for cation release rates from purple rocks and that environmental acidification would enhance rock weathering.
Similar content being viewed by others
Introduction
Weathering is one of the drivers of landform and soil development. The chemical weathering of rocks provides the cations that determine the long-term availability of plant nutrients1,2. Weathering changes drastically the physical properties of bedrock transforming the topography and influencing the relief evolution in different tectonic settings2,3,4. Additionally, the mineral weathering of bedrock is usually considered to be a crucial stage in the geochemical cycle because it directly affects all terrestrial life5. For example, if the balance between cation release via chemical weathering and loss through plant uptake is upset, then soil acidification occurs6,7. Therefore, the rate of cations that are released from parent materials through chemical weathering is a critical concern when investigating soil fertility, plant nutrient supply, buffering capacities of soils and surface water quality8,9.
The rate of chemical weathering is controlled by rock composition, climatic conditions (e.g., temperature and precipitation), topography, and biological activity10,11,12,13,14,15. Although a number of investigations have revealed the pH dependence of the slake-durability of rocks16,17, the influences of acids on the chemical weathering characteristics during purple rock disintegration have not been fully investigated. Recently, the combination of acid precipitation (known as ‘acid rain’) and secondary pollutants (formed through SO2, NOx and NH3 emissions) has become of an increasing global environmental concern. China has become the third largest global source of acid deposition, following North America and Europe18. Further, acidic rain in both small mountain villages and great cities has been observed, especially in the regions to the south of the Yangtze River, in the basin of Sichuan, and to the east of the Qinghai-Tibet Plateau19. Acid deposition may accelerate leaching losses of important nutrient cations such as Ca2+ and Mg2+ and mobilise toxic Al3+ in the soil solution, which is detrimental to the fertility of soils20,21. Related studies have shown that acidic precipitation not only directly accelerates chemical weathering rates through the input of H+ ions14, but also indirectly drives cation release through the proton production from nitrogen transformation22. The rate of chemical weathering is therefore crucial to determining whether soils are sensitive to the effects of acid, which is usually quantified by measuring solute concentrations and flux23,24. Past research has focused on the effects of changing temperature and reactive fluid composition on the chemical rates of individual minerals in the laboratory25,26. However, rocks consist of many different minerals with distinct reactive and surface areas; thus, different have distinct responses to pH and temperature. Therefore, the rate at which cations are released through rock weathering under acidic precipitation has always been difficult to measure and quantify because of a variety of environmental factors.
Purple soils (Regosols as classified by the Food and Agriculture Organization [FAO] Taxonomy or Entisols as classified by the United States Department of Agriculture [USDA])27 are formed from purple rocks (mudstones and/or sandstones) or their weathering products. These cover about 18 million ha in China and more than 75% of soil type in the upper Yangtze River, most commonly in the Sichuan basin of southwestern China by maintaining current topography of high in the northwest and low in the southeast, and where it is surrounded by the highlands of the Plateau of Tibet on the west and the Yunnan-Guizhou Plateau on the south and the Wu Mountains on the east and the Daba Mountains on the north, all of which protect the interior from temperature extremes (Fig. 1). The main characteristic of purple rocks is that they are easily decayed and very soft, and readily develops structural, diagenetic and decayed cracks. With rapid weathering, the purple rocks are easily broken up into rock fragments or gravel through anthropogenic activities, and crops can be planted directly in these. The physical weathering rate of purple rocks is much higher than other types of parent rocks under the same condition; He et al.27 found that the soil erosion modulus of purple rock was 15,800 t km−2 year−1, whereas it was only 55 t km−2 year−1 for limestone in the same area. Previous studies mainly focused on the effects of temperature and moisture on the disintegration characteristics of purple parent rock28,29. There’s little focus on the mineral release during the weathering process and the impact of acid solution, leading to it difficult to predict the effects of acid precipitation or environment acidification on the weathering rate of purple rock. The main distribution area of purple mudstone in southwestern China is a subtropical monsoon climate zone, and it is also sensitive to acid deposition. With the recent rapid development of industrial and agricultural activities, the problem of acidification is likely to worsen. Therefore, in this study, we aim to determine the quantitative effects of varying acidic solutions on rock decay when it is applied to purple rock by using controlled laboratory experiments.
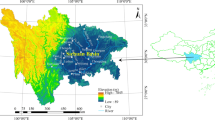
Location of the sampling sites. The map created by QGIS3.16, https://www.qgis.org/en/site/.
Materials and methods
Materials
The samples of fresh purple rocks utilized in the experimental measurements were chosen from purple and red-brown mudstones of the group of the Shaximiao Group (J2s) (E104° 51′, N29° 44′), and the purple and brown sandy mudstone of the Penglaizhen Group (J3p) (E105° 23′, N30° 6′) in Sichuan Basin, all laid down in the Jurassic period, which accounts for 26.23% and 35.02% of the purple parent rock distribution area in Sichuan Basin. Sichuan Basin possesses a subtropical, monsoon climate with a yearly mean temperature between 14 and 19 °C and average yearly precipitation of around 850 mm. Most of the precipitation occurs between May and October. To better reflect the authenticity of weathering characteristics in an acidic environment, the fresh purple rock samples without acid effects were selected. Then, all of the samples were taken and treated at the Field Observation and Science Research National Station of Farmland Ecosystem in Yanting, Sichuan of China (N31° 16′, E105° 27′) where the temperature ranged from 7.0 and 29.7 °C and showed a fluctuating downward trend throughout the trial period (Fig. 2). The clay mineral content of the tested samples (Table 1) was determined using the X-ray diffraction method30. The elements K, Na, Ca and Mg (Table 2) were determined by atomic absorption spectrophotometry. Si, Al, Fe and Ti were using the gravimetric-molybdenum blue photometric method, the fluoride replacement method, the dichromate volumetric method, and the hydrogen peroxide photometric method, respectively31.
Average air temperature during each soaking treatment. The figure created by OriginPro 2021, http://www.Originlab.com/OriginProLearning.aspx (the same of Figs. 3, 4 and 5).
About 58% of precipitation in southwestern China has a pH value between 4.5 and 5.632. The outcomes of acidic rain surveyed by the Sichuan Meteorological Bureau of Dazhou, Xichang, Chengdu, Panzhihua, E’meishan, and Anyue from 2006 to 2013 demonstrated that in Sichuan province, the average frequencies of stronger acids (pH ≤ 4.0) are about 17% and the average pH of precipitation is about 4.7433. Moreover, it has been detected that the pH value of precipitation in some regions of the Sichuan Basin was very low and ranged from 3.0 to 3.534,35, and the nitric acid and sulfuric acid were the fundamental resources of acidity within the precipitation. The molar ratio of SO42− to NO3− is around 4:136,37. On the basis of this range, a mixture of synthetic acid solutions comprising H2SO4 and HNO3 with a ratio of the molar concentrations of around 4:1 was prepared. However, a pH lower than 3.0 is unusual in natural environments, the outcomes of acidity on chemical weathering are relatively long procedures, and in the simulation assessment, a lower pH is frequently utilized to simulate the long-lasting impacts of an acidic media within the natural environments for diminishing the involvement of humidity, temperature, and other external parameters38. Hence, the preparation of acidic solutions with values of pH of 5.6, 4.5, 3.5, and 2.5 was carried out through dilution of the stock solution by utilizing deionized water. In these experiments, the deionized water (with a pH of 7.0) was utilized as a control (CK).
Experimental procedure
To ensure homogeneity, the samples for each treatment were selected from the same block of purple rock. Cut the parent rock sample into small pieces, and each cube was then shaped into a rough block with a mass of 660 ± 3 g. All of the tests were conducted in triplicate with the following detailed procedure.
All the prepared experimental samples were randomly placed into 500 ml glass beaker. Then, add 300 ml of acid solution to each beaker, and it was ensured that all the samples were submerged. After soaking for 72 h, each solution was filtered and collected to determine the ion (K+, Na+, Ca2+, Mg2+) concentration in solution. Put the residual rock cuttings on the filter paper back into the beaker and dried for more than 24 h at 105 °C to a constant weight. All of these decayed products were prepared for the next acid solution treatment after cooling, this procedure represented one treatment cycle. Particles of weathered products less than 2 mm in diameter are commonly regarded as soil particles27,39. Therefore, a total of 24 cycles were carried out for all of the treatments because the disintegration rate of the purple rock treated with each acid solution were exceed 90%, which indicated that the disintegration process has basically stopped. Herein, the pH of the collected filtered solutions was determined by a pHS-3E acidimeter, and ion chromatography was used to determine Na+, K+, Ca2+ and Mg2+ in the filtered solution.
Calculation of cation release in the treatment cycle
Based on the solution volume and the ions collected in each soaking test, the amount of K+, Na+, Ca2+ and Mg2+ in the filtered solution were calculated in accordance with the following Eq. (1):
where \({S}_{i}\) represents the released amounts of K+, Na+, Ca2+ and Mg2+ during each treatment cycle (mmol kg−1); \(i\) is K+, Na+, Ca2+ and Mg2+; \({C}_{i}\) is the concentration of each cation in the filtered solution (ppm); \({V}_{i}\) represents the volume of filtered solution collected after each soaking (ml); \({M}_{i}\) is the relative atomic mass of each atom (K-39, Na-23, Ca-40 and Mg-24) and \(m\) indicates the weight of the soaked rock (kg).
As a result, the release rate of cations is expressed by Eq. (2):
where \({V}_{T}\) is the total release rate of the cations K+, Na+, Ca2+ and Mg2+ in each treatment cycle (mmol kg−1 h−1), which was used as an index to measure the weathering rate40 and \(t\) is the duration time of each soaking (h).
Data analysis and statistics
Multiple comparisons are used to analyze the effects of different treatments and parent rock types on the amounts of cation release. Analyses of the least significant difference (LSD) tests were performed using the SPSS v.12.0 software to determine whether the amounts of cations released were significantly different for the various acid treatments. After regression analysis, the models of the relationship between the variables were set up via the regressive determination parameters compared by the OriginPro 2021 software. The statistically significant level was set at p < 0.05, and the statistical parameters for all of the data were then analyzed using SPSS v.12.0.
To effectively describe the reaction of rock to acidic experimental environments in the laboratory, a total of 120 sets (24 cycles of five treatments) of data were analyzed, including chemical weathering rates, H+ concentration of the soaking solution and natural temperature. Using nonlinear, multivariate regression, Eqs. (3) and (4) were formulated:
where \({V}_{T}\) is the same as in Eq. (2), \({c}_{({H}^{+})}\) is the concentration of H+ (mmol L−1), T is temperature (K) (Fig. 2), R is the gas constant (kJ mol−1 K−1) and \(a\), \(\beta\) and \(A\) are constants to be determined by regression analysis and are dependent on rock material properties.
where y is the released amount of cations, x is the pH of soaking solution, k, \(\gamma\), \(\delta\) are constants to be determined by regression analysis and are dependent on rock and were able to correctly.
Result
Release of cations in the soaking solution
The variation trend of cations in the soaking solution of parent rocks under different acid treatments is similar, that was, the stronger the acid treatment accompanied with the higher the release amount of each cation (Fig. 3). During the whole soaking process, the release amount of each cation under pH 2.5 treatment was significantly greater than that under other acid treatments. The K+ release process of the two groups of parent rocks under pH 2.5 immersion showed a single peak curve, and obvious peaks appeared in the middle stage of immersion, with the release amounts of 0.037 mmol kg−1 and 0.038 mmol kg−1 from J2s and J3p respectively, and then showed a fluctuating downward trend to tend to be stable. The released K+ of J2s under pH 3.5, pH 4.5, pH 5.6 and CK treatments were relatively gentle, with little fluctuation and unobvious peak value. However, the K+ release from J3p showed two obvious peaks during soaking between pH 3.5 and pH 7.0, and the release showed a slow downward trend after the second peak change. The release laws of Na+ between two parent rocks were basically similar, that the overall trend was fluctuating and declining with the soaking process. Na+ release of J3p under different pH acid solution treatment had obvious fluctuation peak in the middle stage of immersion, while it decreased continuously from the beginning of soaking in the J2s. Na+ increased slightly under pH 2.5 treatment in the later stage of soaking, while under other pH treatment Na+ release decreased slowly and the curve changed gently. The Ca2+ release curves of J2s and J3p in strong acid (pH = 2.5) solution were similar, which had a single peak curve trend, and arrived the peak value of 0.912 mmol kg−1 and 1.417 mmol kg−1 respectively at the 10th immersion. The Ca2+ release laws of J2s under pH 3.5 to pH 7.0 were similar to that under the treatment of strong acid solution with pH 2.5, and a relative high peak appears in the middle stage of soaking, and then had a fluctuating downward trend. However, the Ca2+ release curves of J3p under the treatment of acid solution with pH value of 3.5–7.0 were consistent, and the Ca2+ release amount were relatively high at the beginning of soaking. With the soaking cycle, the Ca2+ release curve fluctuated and the decline range was large at the beginning stage, and it decreased slowly or tends to be gentle at the later stage. The release law of Mg2+ under different acid treatments were similar to that of Na+. With the progress of soaking process, the overall trend of Mg2+ were fluctuating and declining, and the release of Mg2+ rapidly decreases to a relatively low value during the middle stage of soaking. From the overall trend, it is easy to find that leaching of the cations from the purple rock were affected most strongly by pH. With the pH of soaking solution decreases, the H+ input increased as well, which not only provide more protons to exchange with the active and adsorbed cations, but also promote minerals weathering. Add to all that its results caused more cations release and leaching from minerals.
The effect of pH on the amount of cations released
The amounts of elements released by an acid attack in laboratory experiment (the unit charge of elements released from 1 kg of soil during the weathering process) were utilized as a criterion to assess the rate of weathering40. In this study, the amounts of K+, Na+, Ca2+ and Mg2+ released from J3p and J2s under the effects acid solutions were obvious after 24 treatment cycles. As Table 3 showed, the acid solutions caused more cations to be released than the deionized water for the two rock types. The whole amount of cations released from all of the studied types of rocks enhanced as pH diminished, particularly when pH was less than 3.5, and a significant difference (p < 0.05) was observed after the acid treatments. Compared with the control treatment of pH 7.0 (CK), the amount of cations released from sample J3p after treatment with solutions of pH 2.5, 3.5, 4.5 and 5.6 increased by multiples of 6.50, 1.55, 1.25 and 1.14 times, respectively. Comparable values were 8.23, 1.74, 1.41 and 1.22 times for J2s. Compared to the CK, the Ca2+ released from the pH 2.5 solution apparently increased by 19.59 and 9.59 mmol kg−1 for J3p and J2s, respectively, and Mg2+ increased by 1.20 and 5.82 mmol kg−1. Ca2+ was the predominant cation released under the various acid solutions, with the average Ca2+ amount accounting for 49.80% for the two rock types, while Na+ represented 33.14%, Mg2+ 10.96% and K+ 6.09%.
Relationship between the amount of cations released and pH
As expected, the stronger the acid treatment, the higher the amount of K+, Na+, Ca2+, and Mg2+ in the leachates of the two rock groups. Regression analysis showed that the amount of released individual cations appeared to exponentially decrease as pH increased in all two of the rock types tested. The exponential correlations between pH and cation release were significant (p < 0.001), especially for the amount of Ca2+ and Mg2+ released, with correlation coefficients (R2) above 0.999 (Fig. 4). This also illustrated that the sensitivity of cation release to pH variation for the various cation types was high for all two rock types and that Ca2+ and Mg2+ were more apparent than K+ and Na+. On the whole, these data fit the exponential Eq. (4) and quantitatively predict the cation amounts released from purple rock under varying acidic conditions in the laboratory.
Determination of the quantitative relationship between cation release rates and H+ concentrations
Intuitively, it can be expected that acid would exert a strong influence on chemical weathering; however, this effect may vary with environmental changes such as air temperature. In general, the net total output of cations (K+, Na+, Ca2+ and Mg2+) is used to represent ‘chemical weathering’41; therefore, to provide credible estimates, the chemical weathering rate in acidic environments should be considered and incorporated into future models. In this study, the chemical weathering rate of cations was calculated and combined with other variables in Eq. (3) using SPSS v.12.0, and the results showed that the data fit the model to a significant degree (p < 0.001). The correlation coefficients of the cation release rates related to the concentrations of H+ were 0.897 and 0.767 for J3p and J2s, respectively, and they were apparently greater than those related to temperature, which were 0.045 and 0.116 for J3p and J2s, respectively. The multiple correlation coefficients (\({R}^{2}\)) were above 0.883 and were higher than the partial correlation coefficients (Table 4). Thus, our results showed that the model of chemical weathering rates was not satisfactory when the rate was determined only based on solution acidity (pH) without consideration of the temperature effects.
Discussion
A remarkable number of laboratory investigations have illustrated the essential role of pH in the processes of chemical weathering42,43. In this study, our results indicates that the concentration of H+ in the reaction environment is an important impact factor for weathering kinetics, consistent with those of previous studies14,44,45,46. The rate of the release of cations from the purple rocks obviously enhanced as the solution pH diminished, especially when the value of pH was less than 3.5 (Table 3). Similarly, the effect of acidification on chemical weathering is low47, therefore, long-term evaluation is necessary to reveal the effects of acid deposition on chemical weathering46. In the current research, the performed analysis of cation release divulged substantial impacts of pH on chemical weathering among the two purple rocks, particularly when the amount of Mg2+ and Ca2+ released during the pH 2.5 processing was considered. It indicated that with the increasing of the acidity of the solution promoted the amount of leaching of divalent cations, but has less influence on the leaching of univalent cations, which suggests that the differences of univalent cations leached from purple rock between the acid- and water-eluted became small. These effects were attributed to the fact that compared to univalent cations, the divalent cations released were more affected by the pH of the environment. The effects were significantly greater than those caused by the other treatments, which was attributed to the fact that compared to univalent cations, the divalent cations released were more affected by the pH of the environment48,49. Calcium in the rock mainly exists in inorganic form, and inorganic calcium can be further divided into mineral and exchange state calcium50. Of these, mineral calcium mainly exists in the crystal lattice of the rock in its solid phase and can seldom be released through hydrolysis. In contrast, the calcium-bearing minerals in purple rock are mainly silicate minerals and carbonate minerals. The acidities of silicic acid and carbonic acid are weaker than those of the simulated solutions due to the action of mixed acids of SO42− and NO3−. In this way, the calcium within silicate and carbonate minerals can be easily dissolved by acid. In contrast, the released quantity of potassium is evidently lower than that of calcium, which is mainly because most of the calcium-bearing primary minerals in the rock are easily weathered. In particular, the weathering rate of calcium-bearing plagioclase is much higher than that of potassium feldspar or sodic feldspar50. Meanwhile, the lattice energy of calcium-bearing minerals is much smaller than those of sodium- or potassium-containing minerals of the same type50. Additionally, the major clay minerals of the purple block (including illite, montmorillonite, and chlorite) are all phyllosilicate-based minerals with a ratio of 1:2 in illite and montmorillonite and in chlorite a ratio of 2:1:151. The reactions of these minerals in acidic solutions may represented as follows:
Montmorillonite52:
Illite53:
Chlorite54:
These reactions promoted the replacement of intercrystalline Ca2+, Mg2+, Fe3+ and Na+ by H+ in the solution. However, the crystal structure of illite, which accounts for the vast majority of the clay mineral composition of the rock, constitutes two tetrahedral sheets with face-to-face top oxygen molecules. Moreover, the layers are tightly stacked in a staggered arrangement, and a coordinate, co-edge, octahedral combination is produced by the superposition, forming an octahedral sheet (O). Thus, a basic structural layer is formed, the interlayer space of which is filled with K+ with an equilibrium electrovalence. The K+ exposed between the layers is directly absorbed onto the silica tetrahedra and is not easily replaced by H+ in the solution.
On the basis of transition state theory, chemical weathering processes may be influenced by the proton or aqueous activities of elements55. The distribution of cations (K+, Na+, Ca2+ and Mg2+) on the ternary diagrams presented in Fig. 5 can be used to indicate the relative contributions of major ions under various acidic environments56. Silicate- and carbonate-rich minerals are major components of the purple rock lithologies; therefore, considering that they can be weathered easily (especially carbonates), they are expected to contribute significantly to the major cation budget of the chemical weathering process55. Therefore, Ca2+ was the dominant species in J3p under various acid treatments, while K+ and Na+ were remarkably high in J2s except for the pH 2.5 treatments, because of the difference in mineral composition of the two purple rocks, In particular calcite in J3p is significantly higher than that in J2S. Notably, the relative contributions of major cations to chemical weathering varied with pH and time. The proportion of Ca2+ released by pH ≥ 3.5 treatments was substantially decreased when compared with the Ca2+ released by the treatment at pH 2.5. The proportions of Na+ and K+ behaved in the opposite manner, and the contribution rate of Mg2+ changed only slightly with changing pH. On average, the release of Mg2+ only accounted for a small fraction of the major cations, and its contribution in J3p approached 0 with an increase in time; it should also be noted that the contributions of Na+ and K+ increased.
Many researchers57,58,59 have studied the effects of pH and temperature on mineral dissolution kinetics. Previous studies indicated that the rate of water exchange around an octahedral cation can be correlated to the mineral dissolution rates in orthosilicates60. Regression analysis of our results revealed that the relationships between cation release rate, H+ concentration and natural temperature were significant for the two purple rock samples (p < 0.001). According to partial correlation coefficients (Table 4), both the H+ concentration and temperature significantly influenced the cation release rates (p < 0.001). That were related to the constituents of the parent rock materials, which include a considerable percentage of clay minerals, namely, illite, montmorillonite, chlorite, and previous studies have suggested that the clay mineral dissolution rates increase with temperature strongly depends on pH61,62 In addition, more studies have shown that the more quickly dissolution could be achieved of clay minerals by a departure from neutral pH circumstances, and at greater temperatures, the rate of solution is greater63,64. A thorough study on phyllosilicate weathering indicates that the rate of weathering is lesser at low temperatures and moderately acidic to neutral pH, and it is greater at higher temperatures and lower pH, which are divided through a transition zone65. Obviously, the rate of cation release was significantly related to the pH and the air temperature. Therefore, any attempts to model cation release rates based on the independent effect of pH alone and without consideration of temperature effects will be unsatisfactory, and our developed model (Eq. 3) would be more accurate for the prediction of cation release rates under environments with variable acidity.
Conclusion
Our results show that the total amount of cations (K+, Na+, Ca2+, and Mg2+) released from purple rock during the chemical weathering process significantly (P < 0.001) increases with an increase in H+ concentration. The release of Ca2+ is the main contribution to cation release from the J3p under various acid treatments, while K+ and Na+ are the main cations released from the J2s, with the exception of the pH 2.5 treatment. The models for cation release rates fit the observed data well when the two variables are introduced (i.e., H+ concentration and air temperature), and there is a significant (P < 0.01) exponential relationship between the amount of cations released and the pH. Therefore, these models can be considered to be a baseline that will allow the cation release rates of purple rock to be quantitatively predicted under varying environmental acidification conditions. In conclusion, our results suggest that acidic condition will accelerate the chemical weathering of purple rock.
Data availability
All the data generated and provided in the study is included under the main article and supplementary material file.
References
Duan, L., Hao, J. M., Xie, S. D., Zhou, Z. P. & Ye, X. M. Determining weathering rates of soils in China. Geoderma 110, 205–225 (2002).
Velde, B. & Meunier, A. The Origin of Clay Minerals in Soils and Weathered Rocks (Springer, 2008).
Eppes, M. C., McFadden, L. D., Matti, J. & Powell, R. Influence of soil development on the geomorphic evolution of landscapes: An example from the transverse ranges of California. Geology 30(3), 195–198 (2002).
Bauer, A. & Velde, B. D. Geochemistry at the Earth’s Surface (Springer, 2014).
Goldsmith, S. T. et al. Stream geochemistry, chemical weathering and CO2 consumption potential of andesitic terrains, Dominica, Lesser Antilles. Geochim. Cosmochim. Acta. 74, 85–103 (2010).
Langan, S. J., Reynolds, B. & Bain, D. C. The calculation of base cations release from mineral weathering in soils from Palaeozoic greywackes and shales in upland UK. Geoderma 69, 275–285 (1996).
Sverdrup, H. & Warfvinge, P. Weathering of primary silicate minerals in the natural soil environment in relation to a chemical weathering model. Water Air Soil Pollut. 38, 387–408 (1998).
Hornung, M., Le-Grice, S., Brown, N. & Norris, D. The role of geology and soils in controlling surface water acidity in Wales. In Acid Waters in Wales (eds Edwards, R. W. et al.) 55–66 (Kluwer Academic Publishing, 1990).
Langan, S. J., Sverdrup, H. U. & Coull, M. The calculation of base cation release from the chemical weathering of Scottish soils using the profile model. Water Air Soil Pollut. 85, 2497–2502 (1995).
Nesbitt, H. W., Markovics, G. & Price, R. C. Chemical processes affecting alkalis and alkaline earths during continental weathering. Geochim. Cosmochim. Acta. 44, 1659–1666 (1980).
Chen, P. Y., Lin, M. L. & Zheng, Z. On the origin of the name kaolin and the kaolin deposits of the Kauling and Dazhou areas, Kiangsi, China. Appl. Clay Sci. 12, 1–25 (1997).
Kirschbaum, A., Martínez, E., Pettinari, G. & Herrero,. Weathering profiles in granites, Sierra Norte (Cordoba, Argentina). J. S. Am. Earth Sci. 19, 479–493 (2005).
Green, E. G., Dietrich, W. E. & Banfiel, J. F. Quantification of chemical weathering rates across an actively eroding hillslope. Earth Planet Sci. Lett. 242, 155–169 (2006).
Huang, L. M., Zhang, G. M. & Yang, J. L. Weathering and soil formation rates based on geochemical mass balances in a small forested watershed under acid precipitation in subtropical China. CATENA 105, 11–20 (2013).
Giardino, J. R. & Houser, C. Principles and Dynamics of the Critical Zone (Elsevier, 2015).
Gupta, V. & Ahmed, I. The effect of pH of water and mineralogical properties on the slake durability (degradability) of different rocks from the Lesser Himalaya, India. Eng. Geol. 95, 79–87 (2007).
Zhou, C. Y., Tan, X. S., Deng, Y. M., Zhang, L. M. & Wang, J. H. Research on softening micro-mechanism of special soft rocks. Chin. J. Rock Mech. Eng. 24, 394–400 (2005) (in Chinese).
Rodhe, H., Dentener, F. & Schulz, M. The global distribution of acidifying wet deposition. Environ. Sci. Technol. 3, 4382–4388 (2002).
Ling, D. J., Zhang, J. E., Ouyang, Y. & Huang, Q. C. Role of simulated acid rain on cations, phosphorus and organic matter dynamics in latosol. Arch. Environ. Contam Toxicol. 52, 16–21 (2007).
Likens, G. E., Driscoll, C. T. & Buso, D. C. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272, 244–246 (1996).
Tao, F., Hayashi, Y. & Lin, E. Soil vulnerability and sensitivity to acid deposition in China. Water Air Soil Pollut. 140, 247–260 (2002).
Huang, L. M. et al. Proton production from nitrogen transformation drives stream export of base cations in acid sensitive forested ecosystems. Ecol. Ind. 48, 348–357 (2015).
Drever, J. & Zobrist, J. Chemical weathering of silicate rocks as a function of elevation in the southern Swiss Alps. Geochim. Cosmochim. Acta. 56, 3209–3216 (1992).
Stonestrom, D. A., White, A. F. & Akstin, K. C. Determining rates of chemical weathering in soils-solute transport versus profile evolution. J. Hydrol. 209, 331–345 (1998).
Oelkers, E. H. & Schott, J. An experimental study of enstatite dissolution rates as a function of pH, temperature and aqueous Mg and Si concentration, and the mechanism of pyroxene/pyroxenoid dissolution. Geochim. Cosmochim. Acta. 65, 1219–1231 (2001).
Schott, J., Pokrovsky, O. S. & Oelkers, E. H. The link between mineral dissolution/precipitation kinetics and solution chemistry. Rev. Miner. Geochem. 70, 207–258 (2009).
He, Y. R. Purple Soils in China (Chinese Science Press, 2003).
Zhang, D., Chen, A. Q., Xiong, D. H. & Liu, G. C. Effect of moisture and temperature conditions on the decay rate of purple mudstone in southwestern China. Geomorphology 182, 125–132 (2013).
Zhang, D., Chen, A. Q., Wang, X. M. & Liu, G. C. Quantitative determination of the effect of temperature on mudstone decay during wet-dry cycles: A case study of “purple mudstone” from south-western China. Geomorphology 246, 1–6 (2015).
Zhang, Y. K. & Fan, G. Quantitative analytic method and experiments of X-ray diffration phase of clay minerals. Uranium Geol. 19(3), 180–185 (2003) (in Chinese).
Ministry of Geology, Resources, Mineral. 2002. GB/T 14506.1-28-93. Standards Press, Beijing (in Chinese).
Zhou, X. D. et al. Progress in the studies of precipitation chemistry in acid rain areas of southwest China. Environ. Sci. 38, 4438–4446 (2017) (in Chinese).
Zhao, X. L. et al. Variation characteristics analysis of acid rain in Sichuan from 2006 to 2013. Meteorol. Environ. Sci. 38, 54–59 (2015) (in Chinese).
Wang, H. & Han, G. Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos. Res. 99(2), 190–196 (2011).
Zhao, M. et al. Chemical composition and sources of rainwater collected at a semi-rural site in Ya’an, southwestern China. Atmos. Clim. Sci. 3(4), 486–496 (2013).
Zhang, X. M., Chai, F. H., Wang, S. L., Sun, X. Z. & Han, M. Research progress of acid precipitation in China. Res. Environ. Sci. 23, 527–532 (2010) (in Chinese).
Yang, X. H., Shu, L. & Chen, S. L. Study on changing characteristics of acid rain and chemistry composition of rain water in Nanchong in recent decades. Sichuan Environ. 33, 26–31 (2014) (in Chinese).
Liu, J. et al. Study of time scale and strength model of Yichang sandstone under different pH values of acidic solution immersion. Chin. J. Mech. Eng. 29, 2319–2327 (2010) (in Chinese).
Zhu, B., Gao, M. R., Liu, G. C., Liu, R. G. & Tsunekawa, A. Weathering erosion and environmental effects of purple shale. J. Soil Eros. Water Conserv. 5, 33–37 (1999) (in Chinese).
Qiu, R. L. & Yang, P. Study of sensitivity of soil to acid deposition in south China. Weathering characteristics of soil minerals under simulated acid rain. Acta Sci. Nat. Univ. Sunyatseni 37, 89–93 (1998) (in Chinese).
Paces, T. Weathering rates of gneiss and depletion of exchangeable cations in soils under environmental acidification. J. Geol. Soc. 143, 673–677 (1986).
Schnoor, J. L. Kinetics of chemical weathering: A comparison of laboratory and field weathering rates. In Aquatic Chemical Kinetics (ed. Stumm, W.) 475–504 (Wiley-Interscience, 1990).
Guicharnaud, R. & Paton, G. I. An evaluation of acid deposition on cation leaching and weathering rates of an Andosol and a Cambisol. J. Geochem. Explor. 88, 279–283 (2006).
Wu, Q., Qiu, R. L. & Lu, Y. N. Effects of simulated acid rain on cation releasing in soils of south China. J. Environ. Sci. 10, 309–315 (1998).
Zhang, J. E., Ouyang, Y. & Ling, D. J. Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67, 2131–2137 (2007).
Probst, A., Viville, D., Fritz, B., Ambroise, B. & Dambrine, E. Hydrochemical budgets of a small forested granitic catchment exposed to acid deposition: The Strenbach catchment case study (Vosges Massif, France). Water Air Soil Pollut. 62, 337–347 (1992).
Sverdrup, H., Warfvinge, P. Estimating field weathering rates using laboratory kinetics. In Chemical Weathering Rates of Silicate Minerals, vol. 31 (eds. White, A. F. & Brantley, S. L.) 485–539 (Mineralogical Society of America, 1995).
Liu, K. H., Mansell, R. S. & Rhue, R. D. Cation removal during application of acid solution into air dry soil columns. Soil Sci. Soc. Am. J. 54, 1747–1753 (1990).
Hartikainen, H. Soil response to acid percolation: Acid–base buffering and cation leaching. J. Environ. Qual. 25, 638–645 (1996).
Yuan, K. N. Soil Chemistry of Plant Nutrient Elements (Science Press, 1983).
Verburg, K. & Baveye, P. Hysteresis in the binary exchange of cations on 2:1 clay minerals: A critical review. Clays Clay Miner. 42, 207–220 (1994).
Amram, K. & Ganor, J. The combined effect of pH and temperature on smectite dissolution rate under acidic conditions. Geochim. Cosmochim. Acta. 66, 3913–3926 (2002).
Vieillard, P. A new method for the prediction of gibbs free energies of formation of hydrated clay minerals based on the electronegativity scale. Clays Clay Miner. 48, 459–473 (2000).
Tang, H. M., Zhao, F., Li, G., Wang, C. H. & Xie, X. Y. Experimental study on reactions of chlorite with mud and fluoboric acids. Oilfield Chem. 24, 307–309 (2007) (in Chinese).
Zhu, B. Q. et al. Identification of rock weathering and environmental control in arid catchments (northern Xinjiang) of Central Asia. J. Asian Earth Sci. 66, 277–294 (2013).
Huh, Y. Chemical weathering and climate—A global experiment: A review. Geosci. J. 7, 277–288 (2003).
Yang, C. & Brantley, S. L. Temperature- and pH-dependence of albite dissolution rate at acid pH. Chem. Geol. 135, 275–290 (1997).
Gislason, S. R. & Oelkers, E. H. Mechanism, rates and consequences of basaltic glass dissolution: II. An experimental study of the dissolution rates of basaltic glass as a function of pH and temperature. Geochim. Cosmochim. Acta. 67, 3817–3832 (2003).
Gudbrandsson, S., Wolff-Boenisch, D., Gislason, S. R. & Oelkers, E. H. An experimental study of crystalline basalt dissolution from 2 ≦ pH ≦ 11 and temperatures from 5 to 75 °C. Geochim. Cosmochim. Acta. 75, 5496–5509 (2011).
Casey, W. H. & Westrich, H. R. Control of dissolution rates of orthosilicate minerals by divalent metal-oxygen bonds. Nature 355, 157–159 (1992).
Huertas, F. J. et al. Kinetics of montmorillonite dissolution in granitic solutions. Appl. Geochem. 16, 397–407 (2001).
Köhler, S. J., Dufaud, F. & Oelkers, E. H. An experimental study of Illite dissolution kinetics as a function of pH from 1.4 to 12.4 and temperature from 5 to 50 °C. Geochim. Cosmochim. Acta. 67, 3583–3594 (2003).
Oelkers, E. H., Schott, J., Gauthier, J. M. & Herrero-Roncal, T. An experimental study of the dissolution mechanism and rates of muscovite. Geochim. Cosmochim. Acta. 72, 4948–4961 (2008).
Bray, A. W. et al. The effect of pH, grain size, and organic ligands on biotite weathering rates. Geochim. Cosmochim. Acta. 164, 127–145 (2015).
Lamarca-Irisarri, D., Driessche, A. E. S. V., Jordan, G., Cappelli, C. & Huertas, F. J. The role of pH, temperature, and NH4+ during mica weathering. ACS Earth Space Chem. 3, 2613–2622 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation Committee of China (No.42007002), Yunnan Basic Research Project-general projects (No. 202101AT070220).
Author information
Authors and Affiliations
Contributions
This study supervision by G.L., J.Z., C.L., L.D. performed sample preparation and simulation experiment, J.Z. writing-Original draft preparation, C.L. performed data curation, C.L. and L.D. prepared Figs. 1, 2, 3 and 4, G.L. reviewed the manuscript and M.F. edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Li, C., Lu, C. et al. Acidic condition accelerates cation release from purple rock in Southwestern China. Sci Rep 12, 11412 (2022). https://doi.org/10.1038/s41598-022-14851-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14851-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.