Abstract
The devastating effect of health system overload was observed after cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) peaked in April 2020 in Belém, a capital metropolis in the Brazilian Amazon. Our results showed a high seroprevalence (39.24%) of anti-SARS-CoV-2 IgG antibodies among the population of the capital of the state of Pará after the first wave of the pandemic. Old age, mixed race, a high school education level, low income and contact with infected individuals are risk factors and may be driving seropositivity and exposure to SARS-CoV-2 in this population. This study is one of the first to provide important information to understand the socioeconomic inequalities, behavioral characteristics and viral transmission dynamics associated with the risk of SARS-CoV-2 infection in the capital of the state of Pará, northern Brazil.
Similar content being viewed by others
Introduction
On December 8, 2019, in the city of Wuhan, Hubei Province, China, the occurrence of cases of an unusual respiratory syndrome (coronavirus disease 2019—COVID-19) associated with a novel Betacoronavirus (severe acute respiratory syndrome coronavirus 2—SARS-CoV-2) was reported, and a pandemic was decreed on March 11, 20201. Thus, China was considered the initial epicenter of the spread of SARS-CoV-2.
Outside the Asian continent, two new epicenters became prominent: Italy, with the first case identified at the end of February 2020 and cases peaking between the end of March and mid-April 20202, and the United States, where cases first peaked around April 2020; currently, the USA is the most affected country in the world3.
With the rapid spread of the virus on the American continent, Brazil became the leading country in terms of the number of SARS-CoV-2 infections in South America. The first report of SARS-CoV-2 infection in Brazil occurred on February 25, 2020, and as of August 18, 2021, the country had amassed 20,416,183 confirmed cases and was the third most affected country in the world4. The state of Pará, northern Brazil, has recorded 578,383 confirmed cases and approximately 16,280 deaths4. In the city of Belém, the capital of the state of Pará, 105,507 cases were confirmed, and 5077 deaths had occurred by August 17, 20215. The lethality rate in the city of Belém (4.81%) was higher than that in the state of Pará (2.81%)5.
To evaluate viral exposure at the population level, the commonly used serological assays aim to identify the presence of antibodies specific to the nucleocapsid (N) or spike (S) protein of SARS-CoV-2, with high sensitivity and specificity6,7,8,9. Seroprevalence data are essential for understanding the prevalence of subclinical infections, determining the susceptibility of the population to the virus, estimating the actual number of people previously exposed, generating information that provides support for the formulation of public health policies to reduce cases of infection, improving planning for future outbreaks and, finally, evaluating the effectiveness of restrictive measures for containing the spread of the virus.
In this context, the objectives of this study were to describe the seroprevalence of anti-SARS-CoV-2 IgG antibodies and the epidemiological aspects of the risk of exposure to the virus and to determine the proportion of asymptomatic or subclinical infections in residents of the city of Belém, Pará, Brazil, approximately 6 months after the first wave of COVID-19 in the capital city.
Methods
Study design and sampling
This was a cross-sectional study conducted with 736 volunteers living in the city of Belém, the capital of Pará, from October 2020 to February 2021, after the first wave of COVID-19. The study was submitted to and approved by the National Research Ethics Committee (CONEP, acronym in Portuguese) and the Human Research Ethics Committee of the Health Sciences Institute of Federal University of Pará (UFPA) under CAAE n. 31,800,720.1.0000.0018 in compliance with the guidelines and regulatory standards for research involving human beings, with all methods conducted in accordance with the Declaration of Helsinki. Individuals already vaccinated with one or two doses against SARS-CoV-2, those who did not complete the epidemiological questionnaire, those who did not sign the informed consent form and those younger than 7 years were excluded from the study.
Data collection
After obtaining a signed informed consent form for participation in the study, individuals were interviewed using a structured questionnaire addressing clinical, demographic and behavioral characteristics as possible risk factors for SARS-CoV-2. We obtained signed informed consent forms from individuals aged 18 years or older. Children aged 7–11 years and adolescents aged 12–17 years signed a free and informed assent form, and their parents or guardians signed an informed consent form.
The following individual data were collected: (1) sociodemographic data—age, sex, income, ethnicity and marital status; (2) comorbidities; (3) behavioral information on preventive measures, such as mask use, travel, hand hygiene, social distancing and contact with people infected with SARS-COV-2; and (4) information related to COVID-19—the presence of symptoms, previous diagnosis, self-medication and medication prescribed to treat symptoms, hospitalization and the need for mechanical ventilation. The participants’ data were recorded using EPI Info™ software version 7.2.4.10 and stored in the local server of the Virology Laboratory of UFPA.
Sample collection, processing and storage
After questionnaire completion and collection of signed informed consent forms, venous blood samples (10 ml) were collected from the volunteers by venipuncture into a vacuum tube containing EDTA. Subsequently, the samples were processed anonymously in the Virology Laboratory; plasma and leukocytes were isolated and then stored at − 70 °C.
Serological analysis
Anti-SARS-COV-2 IgG antibodies were detected by ELISA (Euroimmun, Lübeck, Germany), which uses the recombinant structural protein (S1 domain) of the S protein as an antigen, following the manufacturer's protocol. The samples were classified as nonreactive (ratio < 0.8), indeterminate (0.8 ≤ ratio ≤ 1.1) or reactive (ratio > 1.1) for IgG, as suggested by the manufacturer. The manufacturer reported a clinical sensitivity of 75–93.8% (> 10–20 days to ≥ 21 days after disease onset) and a specificity of 99.6% for IgG antibodies.
Statistical analysis
The seroprevalence estimated based on the sociodemographic characteristics of the population is presented as a count and a percentage with a 95% confidence interval (CI), which were calculated in the program BioEstat 5.0. Correlations between symptoms and positivity were estimated using BioEstat 5.0. Associations between the presence of anti-SARS-CoV-2 IgG antibodies and the study variables were estimated using univariate analysis with the chi-square test or G test. A significance level of 5% (p < 0.05) was established. Uni- and multivariate logistic regression analyses were used to explore associations between risk factors (sociodemographic and behavioral characteristics and symptoms) and SARS-CoV-2 infection and between symptomatic and asymptomatic cases and the presence of antibodies. The definitions established by the Brazilian Ministry of Health were used as a classification criterion for symptomatic individuals11. Cases that did not meet these criteria or individuals who did not show any symptoms were classified as asymptomatic. All univariate and multivariate analyses were performed using Minitab software, version 14.0. Graphs were generated in GraphPad version 7.0 and Excel 2010.
Ethics approval and consent to participate
The study was submitted to and approved by the National Research Ethics Committee (CONEP, acronym in Portuguese) and the Human Research Ethics Committee of the Health Sciences Institute of Federal University of Pará (UFPA) under CAAE n. 31800720.1.0000.0018 in compliance with the guidelines and regulatory standards for research involving human beings, with all methods conducted in accordance with the Declaration of Helsinki.
Results
Socioeconomic and behavioral characteristics and comorbidities associated with the risk of SARS-CoV-2 infection.
Between October 2020 and February 2021, 736 individuals were invited and agreed to participate in the study. Based on serological results, 275 (37.3%) individuals were reactive for anti-SARS-CoV-2 IgG antibodies, 429 (58.2%) were nonreactive, and 32 (4.3%) had indeterminate results. In total, only 704 (100%) individuals with a confirmed laboratory diagnosis were eligible for statistical analysis; those with indeterminate results were excluded from the analysis. The prevalence of anti-SARS-CoV-2 IgG antibodies was higher in women (F = 65.1%/M = 34.9%; p = 0.4445) than in men. Advanced age ≥ 70 years (odds ratio (OR) = 2.02; 95% CI 1.02–3.05; p = 0.044), a high school education level (OR = 2.41; 95% CI 1.08–2.16; p = 0.016), mixed race (self-report; OR = 3.24; 95% CI 1.23–2.35; p = 0.001) and income ≤ 2 minimum wages (OR = 2.39; 95% CI 1.08–2.12; p = 0.017) were associated with a higher risk of infection (Table 1). The most frequent comorbidities were hypertension (24.7%) and asthmtic (9.1%). No correlation was found between seropositivity for anti-SARS-CoV-2 antibodies and the presence of comorbidities (Table 1).
Behavioral patterns considered important for prevention, such as mask use, hand washing and lack of travel during the pandemic, were not associated with the risk of SARS-CoV-2 infection (p > 0.005) (Table 2). Interestingly, the prevalence of antibodies was higher among individuals who had been under lockdown (85.1%; OR = 2.11; 95% CI 1.04–2.67; p = 0.035), which was not considered a risk factor for infection. However, contact with infected individuals (OR = 3.45; 95% CI 1.30–2.59; p = 0.001) may be a risk factor for SARS-CoV-2 infection, in addition to other socioeconomic factors (Fig. 1).
Antibody seroprevalence among symptomatic and asymptomatic individuals
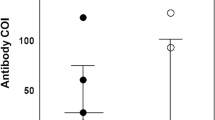
Approximately 62.5% of the individuals were classified as symptomatic, and 37.5% were classified as asymptomatic. No significant difference in seropositivity was noted between the groups (Fig. 2A). The presence of characteristic COVID-19 symptoms was higher in IgG + individuals. Symptoms such as fever (48.7%; p = 0.0001), cough (53.3%; p = 0.0206), body aches (58.9%; p = 0.0001), abdominal pain (26.5%; p = 0.0203), loss of smell (50.5%; p = 0.0001), loss of taste (48.4%; p = 0.0001), shortness of breath (24.0%; p = 0.0007) and fatigue (48.0%; p = 0.0001) were more prevalent among seropositive individuals (Fig. 2B) (Table 3). Among the symptoms, the correlation between loss of smell and taste was highly significant in symptomatic patients (Fig. 2C). Among the variables analyzed, only individuals in the age groups of 40–49 years (OR = 1.99; 95% CI 1.01–3.75; p = 0.047) and 50–59 years (OR = 2.62; 95% CI 1.28–5.62; p = 0.009) were more susceptible to the presence of clinical symptoms related to COVID-19. No other variable was correlated with the presence of symptoms (p > 0.005).
Prevalence of antibodies and symptoms among symptomatic and asymptomatic individuals. (A) Percentage of ± anti-SARS-CoV-2 IgG antibodies between symptomatic and asymptomatic individuals. (B) Prevalence of symptoms characteristic of COVID-19. (C) Frequency of the cooccurrence of symptom pairs in symptomatic individuals. Values with * correspond to p values < 0.05.
Discussion
We estimated the prevalence of anti-SARS-CoV-2 IgG antibodies for the S1 subunit of the spike glycoprotein and factors potentially associated with SARS-CoV-2 infection 6 months after the beginning of the first wave of COVID-19 in Belém, Pará, Brazil. To our knowledge, this is one of the first seroepidemiological studies describing the prevalence of anti-SARS-CoV-2 antibodies in the city of Belém.
The seroprevalence of anti-SARS-CoV-2 antibodies varies based on geographic region, study population, sampling time, laboratory method and COVID-19 incidence at the sampling site. In Brazil, the prevalence of antibodies was highly heterogeneous when considering the five regions of the country, with the North and Northeast regions clearly having the highest seroprevalence rates, which were strongly associated with low socioeconomic status12,13. Two of the highest seroprevalence estimates in Brazil were reported in Manaus at 29.10% and in the capital of Maranhão, with an antibody prevalence of approximately 40.4% in the population14,15,16. Our results indicate a seropositivity of 39.24% in the city of Belém. We believe that the seroprevalence in Belém may be even higher than that observed when we consider our previous results of a follow-up of a post-COVID-19 cohort in which we found a 30% loss of anti-SARS-CoV-2 IgG antibodies 3 months after COVID-19 diagnosis17. The choice of serological test was a determining factor for the prevalence rate of antibodies found in this study because due to good sensitivity and specificity, the test allowed the detection of asymptomatic seropositive individuals who usually do not seek medical care or undergo diagnostic tests and are therefore underreported in official numbers. According to the Brazilian Institute of Geography and Statistics, the population of the capital city of Belém is 1.5 million inhabitants18. Based on our seroprevalence results, which indicated that 39% of participants had been exposed to SARS-CoV-2, the estimated number of infected individuals in the capital would be 585,000, which is 10 times higher than the number reported by the Municipal Department of Health as of December 30, 202019, as these reports are based only on cases of symptomatic individuals seeking diagnosis and treatment services. We believe that the seroprevalence results observed in the present study are not influenced by possible false-positive or false-negative results, since a methodology with high sensitivity and specificity was used.
Our study failed to identify a significant difference between the sexes regarding the risk of SARS-CoV-2 infection, which is similar to findings reported in other seroprevalence studies20,21. The presence of comorbidities was not a predictor of positivity for anti-SARS-CoV-2 IgG antibodies, which is similar to observations in another study22. However, comorbidities may be associated with a high risk of serious complications and death due to COVID-1923,24. The age group most exposed to the virus and with the highest seroprevalence rates was adults aged over 70 years, which is similar to observations in other studies25,26. As home lockdown was voluntary, the recommendations for social lockdown may not have been adequately followed, suggesting exposure at the family level. The antibody rate by age can be a useful tool to track vaccine prioritization for the population, as defined by the National Plan for the Operationalization of Vaccination against COVID-19 of the Brazilian Ministry of Health27.
Individuals who self-reported being mixed race have a high prevalence of antibodies and a high risk of viral infection because they represent the majority of the Brazilian population. Our results demonstrate a robust association between socioeconomic conditions and the risk of SARS-CoV-2 infection, with a higher risk for individuals with a high school education and with a family income less than or equal to one or two minimum wages—socioeconomic characteristics that are prevalent in low- and middle-income countries such as Brazil. Family income was the most significant risk factor. Families with incomes between three and five times the minimum wage were less likely to be infected than were families with lower incomes, which is similar to the rest of the country12,13,28. Socioeconomic inequalities became even more evident with the pandemic. We assume that social health differences contributed to these initial observations and resulted in differential exposure to the virus and differential vulnerability to infection, indicating the need for preparedness plans for vulnerable communities to ensure a rapid and coordinated response to protect these groups from future pandemics or crises at an early stage.
Regarding infection prevention measures, interestingly, we found that individuals who regularly used a mask and frequently engaged in hand hygiene had the highest seroprevalence rates. Notably, our data suggest that the lack of information on proper handwashing techniques, proper mask use and mask efficiency explain the high prevalence of IgG anti-SARS-CoV-2 antibodies in this population. Our data indicate that direct contact with individuals infected with SARS-CoV-2 increases the risk of infection, which is consistent with results reported in other studies29,30.
Our results indicate a relatively high seroprevalence of asymptomatic SARS-CoV-2 infection (37.5%) in the city of Belém, corroborating other studies conducted in Brazil16,31, which complicates disruption of the transmission cycle, since a high percentage of individuals who claimed to have had no symptoms of COVID-19 were seropositive, thus highlighting the possibility of silent transmission of the virus by asymptomatic individuals.
Mass testing is important for rapid identification of asymptomatic cases and isolation of infected individuals, with testing and monitoring of their contacts, to prevent the spread of the virus32. Clinical symptoms such as fever, body aches, loss of smell, loss of taste, shortness of breath and fatigue are strongly associated with SARS-CoV-2 infection, with studies showing symptom profiles similar to those found in our population. In March 2020, the first reports relating the loss of smell and/or taste with COVID-19 were published33,34. The available data suggest a prevalence of loss of smell and/or taste ranging from 31 to 85% among patients with COVID-1933,34,35,36,37. Our results support that otorhinolaryngological symptoms are prevalent among patients with mild to moderate COVID-19 infection because we observed a high prevalence of these symptoms in participants with mild or moderate infection and positive results for anti-SARS-CoV-2 IgG antibodies.
In Belém, we observed that individuals aged 40–59 years had a higher risk of developing symptoms than younger people. A retrospective cohort study found that most hospitalized patients with COVID-19 symptoms had a mean age of 54 years and that advanced age was a risk factor for death among adults38. The effects of age on immune system function, the activity of T and B lymphocytes and excess production of pro-inflammatory cytokines influence the onset of symptoms owing to poor control of viral replication39. Thus, the correlation between age and the risk of SARS-CoV-2 infection or the risk of developing critical illness observed in this study may be essential information for planning health resources to ensure the sustainability of the system.
All countries are urged to make increasingly rapid decisions regarding socioeconomic issues and public health policies to contain the pandemic. High-quality data, as presented here, are necessary to facilitate decision making. This study provides important information about the high prevalence of SARS-CoV-2 in the city of Belém and indicates that individuals with low socioeconomic status and/or little education are more susceptible to infection—a pattern commonly found in low-income countries. We also discovered the impact of the proper use of prevention and control measures and suggest that preventive measures are essential to minimize symptomatic and asymptomatic transmission in the community. Seroprevalence surveys will be needed to identify dynamic trends and monitor disparities to profile future transmission dynamics and formulate protective responses for vulnerable groups.
We understand that the present study has some limitations. We encountered difficulties when obtaining samples due to the lockdown and social distancing decrees related to the pandemic, which may have led to a sample bias in the statistical analysis. Nevertheless, our analyses have strengths. The study population was representative of both sexes, all ages and different economic groups and races. The serological test based on the S protein can detect past infections, including asymptomatic and recovered cases, providing better information on the prevalence of the disease in the population. To date, this is one of the first epidemiological studies to investigate risk factors for SARS-CoV-2 infection in the city of Belém, northern Brazil.
All countries are urged to make increasingly rapid decisions regarding socioeconomic issues and public health policies to contain the epidemic. We were able to estimate the high seroprevalence of SARS-CoV-2 in the first six months after the first wave of COVID-19 in the city of Belém and identify the factors that may be driving seropositivity and exposure to SARS-CoV-2 in this population. These findings highlight the importance of serosurveillance for estimating the real impact of the COVID-19 pandemic, identifying trends in viral transmission and monitoring population disparities to profile future transmission dynamics and formulate protective responses for vulnerable groups. In addition, these results may be essential for monitoring the evolution of the pandemic after easing social distancing measures and the implementation of a vaccination schedule in Brazil as a basis for other countries.
Data availability
All data supporting the findings of this study are available in the article.
References
Lu, H., Stratton, C. W. & Tang, Y. W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 92, 401–402 (2020).
Porcheddu, R., Serra, C., Kelvin, D., Kelvin, N. & Rubino, S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J. Infect. Dev. Ctries. 14, 125–128 (2020).
Gonzalez-Reiche, A. S. et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 369, 297–301 (2020).
Johns Hopkins. Brazil - COVID-19 Overview. https://coronavirus.jhu.edu/region/brazil (2021).
Secretaria de Saúde Pública do estado do Pará (SESPA). http://www.saude.pa.gov.br/coronavirus/ (2021).
Okba, N. M. A. et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 26, 1478–1488 (2020).
Liu, W. et al. Evaluation of nucleocapsid and spike protein-based enzymelinked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microb. 58, e00461-e520 (2020).
Grzelak, L et al. A comparison of four serological assays for detecting anti–SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 12, eabc3103 (2020).
Gededzha, M. P. et al. Performance of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies in South Africa. PLoS ONE 16, e0252317 (2021).
EPI InfoTM. Version 7.2.4. Atlanta: Centers for Disease Control and Prevention. http://www.cdc.gov/epiinfo, (2020).
Ministério da Saúde. Protocolo de Manejo Clínico para o Novo Coronavírus (2019-nCoV). (2020).
Hallal, P. C. et al. SARS-CoV-2 antibody prevalence in Brazil: Results from two successive nationwide serological household surveys. Lancet Glob. Health. 8, e1390–e1398 (2020).
Horta, B. L. et al. Prevalence of antibodies against SARS-CoV-2 according to socioeconomic and ethnic status in a nationwide Brazilian survey. Rev. Panam. Salud Publica. 44, e135 (2020).
Lalwani, P. et al. SARS-CoV-2 seroprevalence and associated factors in Manaus, Brazil: Baseline results from the DETECTCoV-19 cohort study. Int. J. Infect. Dis. 110, 141–150 (2021).
Buss, L. F. et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 371, 288–292 (2021).
Silva, A. A. M. et al. Population-based seroprevalence of SARS-CoV-2 and the herd immunity threshold in Maranhão. Rev. Saude Publica. 54, 131 (2020).
Bichara, C. D. A. et al. Dynamics of anti-SARS-CoV-2 IgG antibodies post-COVID-19 in a Brazilian Amazon population. BMC Infect. Dis. 21, 443 (2021).
Instituto Brasileiro de Geografia e Estatística (IBGE). https://www.ibge.gov.br/cidades-e-estados/pa/belem.html (2021).
Secretaria de Saúde Pública do estado do Pará (SESPA). http://contratoemergencial.belem.pa.gov.br/painel-covid-19/ (2021).
Murhekar, M. V. et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int. J. Infect. Dis. 108, 145–155 (2021).
Nah, E. et al. Nationwide seroprevalence of antibodies to SARS-CoV-2 in asymptomatic population in South Korea: a cross-sectional study. BMJ Open 11, e049837 (2021).
Napolitano, E., Cho, S., Park, H., Hwang, I. & Cho, H. Seroprevalence of SARS-CoV-2 antibodies in adults and healthcare workers in Southern Italy. Int. J. Environ. Res. Public Health. 18, 4761 (2021).
Adams, M. L., Katz, D. L. & Grandpre, J. Population-based estimates of chronic conditions affecting risk for complications from coronavirus disease United States. Emerg. Infect. Dis. 26, 1831–1833 (2020).
Singh, A. K. et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes. Metab. 22, 1915–1924 (2020).
Bogogiannidou, Z. et al. Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April 2020. Euro Surveill. 25, 2001369 (2020).
Reichberg, S. B. et al. Rapid emergence of SARS-CoV-2 in the Greater New York metropolitan area: Geolocation, demographics, positivity rates, and hospitalization for 46 793 persons tested by Northwell Health. Clin. Infect. Dis. 71, 3204–3213 (2020).
Ministério da Saúde. https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/plano-nacional-de-vacinacao-covid-19/view (2021).
Baqui, P., Bica, I., Marra, V., Ercole, A. & van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: A cross-sectional observational study. Lancet Glob. Health. 8, e1018–e1026 (2020).
To, K. K. et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes. Infect. 10, 507–535 (2020).
Varona, J. F. et al. Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int. J. Epidemiol. 50, 400–409 (2021).
Castro, M. C. et al. Spatiotemporal pattern of COVID-19 spread in Brazil. Science 372, 821–826 (2020).
Borges, L. P. et al. Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe Brazil. Rev Panam. Salud. Publica 44, e108 (2020).
He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020).
Spinato, G. et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 323, 2089–2090 (2020).
Hopkins, C., Surda, P. & Kumar, N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology 58, 295–298 (2020).
Lee, Y., Min, P., Lee, S. & Kim, S. W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Kor. Med. Sci. 35, e174 (2020).
Lechien, J. R. et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 277, 2251–2261 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1038 (2020).
Opal, S. M., Girard, T. D. & Ely, E. W. The immunopathogenesis of sepsis in elderly patients. Clin. Infect. Dis. 41, S504–S512 (2005).
Acknowledgements
We thank all subjects who participated in the study.
Funding
This study was funded by the National Council for Scientific and Technological Development (CNPq, #301869/2017-0 and #401235/2020-3), the Paraense Amazon Research Support Foundation (FAPESPA-005/2020), the Coordination for Improvement of Higher Education Personnel (CAPES) and the Dean of Research and Graduate Studies at the Federal University of Pará (PROPESP/UFPA).
Author information
Authors and Affiliations
Contributions
A.C.R.V. conceived the idea for the study. M.K.S.T. performed the experimental analyses and wrote the manuscript. The experimental analyses were conducted by F.T.L., C.N.C.L., A.C.R.L., W.R.S.B., B.C.S., B.J.S.B. and A.C.A.C. The epidemiological data were input in the database by R.S.S. and J.L.C.G. The statistical analyses were performed by S.S.L. and M.K.S.T. Intellectual content was added and the submitted version was approved by L.F.A.M., R.N.M.F, I.M.V.C.V. and A.C.R.V.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Silva Torres, M.K., Lopes, F.T., de Lima, A.C.R. et al. Seroprevalence and risk factors for COVID-19 in the metropolis of the Brazilian Amazon. Sci Rep 12, 8571 (2022). https://doi.org/10.1038/s41598-022-12629-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12629-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.