Abstract
This study investigated the 3-year clinical outcomes in relation to the severity of encephalopathy in high-survival infants who underwent therapeutic hypothermia. This retrospective observational study was conducted in level II/III neonatal intensive care units in Japan. The nationwide cohort included 474 infants registered in the Baby Cooling Registry of Japan between January 2012 and December 2016. Clinical characteristics, mortality rate and severe neurological impairment at age 3 years were evaluated. Of the infants, 48 (10.4%), 291 (63.1%) and 122 (26.5%) had mild, moderate and severe encephalopathy, respectively, upon admission. By age 3, 53 (11.2%) infants died, whereas 110 (26.1%) developed major disabilities. The mild group survived up to age 3. In the moderate group, 13 (4.5%) died and 44 (15.8%) developed major disabilities. In the severe group, 39 (32.0%) died by age 3. Adverse outcomes were observed in 100 (82.0%) infants. Mortality was relatively low in all subgroups, but the incidence of major disabilities was relatively high in the severe group. The relatively low mortality and high morbidity may be due to Japanese social and ethical norms, which rarely encourage the withdrawal of intensive life support. Cultural and ethical backgrounds may need to be considered when assessing the effect of therapeutic interventions.
Similar content being viewed by others
Introduction
Hypoxic-ischaemic encephalopathy (HIE) contributes to both infant mortality and long-term morbidity in childhood1. The prevalence of moderate or severe HIE is 0.4–2.0 cases per 1000 births in high-income countries, including Japan, United States and European countries2,3,4. Therapeutic hypothermia is the only neuroprotective treatment that has been proven to reduce death and neurological impairments in infants with moderate or severe HIE5. However, the outcomes of HIE in infants are diverse, even after therapeutic hypothermia. In early large-scale randomised controlled trials of therapeutic hypothermia, the mortality rate and composite outcome of death or moderate to severe neurological impairments among cooled infants by 2 years of age were 23.1–33.3% and 39.2–56.0%, respectively5. A more recent randomised controlled trial, which provided therapeutic hypothermia (32.0 °C or 33.5 °C for either 72 or 120 h) to encephalopathic infants, reported relatively lower mortality rates (8.7–19.4%) and adverse composite outcomes (29.3–34.5%)6. A lower mortality rate (7.5%) and similar composite adverse outcome rate (29.5%) were reported in a study assessing 18-month follow-up data from the Japanese national registry7. Furthermore, a subgroup analysis of infants with a 10-min Apgar score of zero from the same registry indicated a relatively low mortality rate (32% versus 48–54% in previous studies), as well as a high incidence of moderate to severe neurological impairments among survivors (84% versus 42–55% in previous studies)8,9,10,11. The relatively low mortality and high morbidity rates observed in the survivors were attributed to the cultural and ethical norms in Japan, where physicians rarely propose the withdrawal of intensive life support, even for infants with the most severe degrees of HIE11.
By contrast, mild HIE has rarely been a focus of study, as its prognosis is generally considered favourable5,12,13,14. However, a recent study of 43 infants found that 16% of uncooled infants with mild HIE had neurological impairments by age 18–22 months15. Despite the lack of clinical evidence to support the benefit of therapeutic hypothermia for infants with mild HIE, a considerable proportion of these infants have received such treatment in Western countries16,17. However, such a ‘therapeutic creep’ has not been as apparent in Japan, possibly due to a nationwide dissemination campaign encouraging physicians to adhere to evidence-based therapeutic hypothermia regimens18.
Thus, for an appraisal of preferred neuroprotective regimens, domestic backgrounds, such as cultural and ethical backgrounds and local adherence to evidence-based guidelines, may need to be considered. To facilitate comparisons of the benefits of cooling among studies conducted in different countries, clinical outcomes need to be assessed in conjunction with the level of HIE severity.
Thus, this study aimed to review the 3-year mortality rate and incidence of severe neurodevelopmental impairments according to the initial HIE severity in a Japanese cohort of infants with a high survival rate following therapeutic hypothermia.
Results
Final study cohort
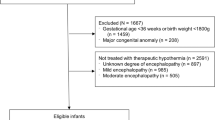
Clinical data were obtained from 756 infants, who were registered at 110 intensive care units over the 5-year window period. Among these infants, 474 (62.7%, final cohort) had outcome data at 3 years of age; 282 were lost to follow-up (Fig. 1). The baseline characteristics were similar between those with and without follow-up data; an exception to this was that the encephalopathy stage was more severe among infants in the final study cohort (Table 1).
Encephalopathy stages and clinical course during hospitalisation
Among 474 infants treated with therapeutic hypothermia, encephalopathy at admission was mild in 48 (10.4%) infants, moderate in 291 (63.1%) and severe in 122 (26.5%); data were unavailable for 13 infants. The rate of emergency delivery, gestational age and body weight at birth were similar by the severity of HIE. In contrast, there was a trend that greater HIE severities were associated with being outborn, low Apgar scores at 10 min, need for resuscitation beyond 10 min and more severe metabolic acidosis represented by lower blood pH and greater base deficit (see online Supplementary Table for details and other clinical backgrounds, including the Thompson encephalopathy scores, presence of seizures and requirement for tube feeding and mechanical ventilation).
Outcomes at 3 years of age
Among the 474 infants in the final cohort, 53 (11.2%) died by the time of the 3-year follow-up, whereas 110 (26.1%) survivors developed major disabilities; 163 (34.4%) cases of death or major disability were recorded (Table 2). Among the surviving children, 67 (15.9%) and 44 (10.5%) were dependent on tube feeding and respiratory support, respectively. Hearing loss, blindness, epilepsy, Gross Motor Function Classification System19 (GMFCS) > 2 and Manual Ability Classification System20 (MACS) > 2 were noted in 28 (6.7%), 12 (2.9%), 14 (3.3%), 89 (21.1%) and 97 (23.0%) children, respectively.
Encephalopathy stages and outcomes
All 48 infants diagnosed with mild encephalopathy upon admission survived to 3 years of age without hearing loss, blindness or requirement for chronic healthcare. Of the infants with mild encephalopathy, 4.2% (2/48) had a major disability; one infant was diagnosed with cerebral palsy (GMFCS level 3), while another infant was diagnosed with both cerebral palsy (GMFCS level 5) and epilepsy (Table 2).
Of the 291 infants diagnosed with moderate encephalopathy upon admission, 13 (4.5%) died, while 44 (15.8%) survivors developed a major disability; 57 (19.6%) cases of composite adverse outcomes were recorded. Among survivors, 21 (7.6%) were dependent on respiratory support or tube feeding. At 3 years of age, 10 (3.6%), 5 (1.8%), 5 (1.8%), 30 (10.8%) and 35 (12.6%) children exhibited hearing loss, blindness, epilepsy, GMFCS > 2 and MACS > 2, respectively (Table 2).
Of the 122 infants with severe encephalopathy, 39 (32.0%) died by 3 years of age; 100 (82.0%) cases of death or major disability were noted. Among the survivors, 46 (55.4%) children required respiratory support or tube feeding. Hearing loss, blindness, epilepsy, GMFCS > 2 and MACS > 2 were noted in 17 (20.5%), 7 (8.4%), 8 (9.6%), 55 (66.3%) and 58 (69.9%) children, respectively, at 3 years of age (Table 2).
Discussion
In a cohort of infants with high-survival rate from the Japanese national registry, the relationship between the severity of encephalopathy and outcomes following therapeutic hypothermia differed from that reported in other developed countries5,21. The 3-year outcomes of Japanese HIE infants were characterised by consistently low mortality rates for those diagnosed with mild, moderate and severe encephalopathy (0.0%, 4.5% and 32.0%, respectively), as well as a relatively high rate of major disabilities in survivors of severe encephalopathy (73.5%). The social and ethical norms in Japan, which do not allow the withdrawal of intensive life support (despite severe neurological impairment) may have accounted for the low mortality and high morbidity rates observed among infants with severe encephalopathy.
A meta-analysis based on clinical studies from Western countries reported mortality rates of 13.5% and 52.4% in infants with moderate and severe neonatal encephalopathy, respectively, following therapeutic hypothermia5; these rates were considerably higher than the mortality rates documented among Japanese infants in the present study (4.5% and 32.0%, respectively). Another study utilising an international registry in The Children’s Hospitals Neonatal Database (n = 945) reported hospital mortality rates of 3.5% and 46.8% for infants with moderate and severe encephalopathy, respectively. The majority of deaths (82.4% and 86.1% of deaths following moderate and severe encephalopathy, respectively) occurred after the withdrawal of life-sustaining support21. Although our current study did not collect information regarding withdrawal of intensive life support, our prior study showed that even among infants with most severe encephalopathy (with 10-min Apgar scores of zero), only 2 of the 9 (22.2%) died following withdrawal11. In the current study, the rate of major disability among survivors of severe encephalopathy was 73.5%, which was relatively higher than the morbidity rate (36.8%) reported in a previous study5. The association of severe encephalopathy with the combination of low mortality (32%) and high morbidity (57%, severe disabilities) was also observed in a previous study conducted among severely asphyxiated infants (10-min Apgar scores of zero) in Japan11. These findings may be attributed to the reluctance among physicians in Japan to withdraw intensive life support, even in the most severe cases of encephalopathy. Future studies of neuroprotective treatments may need to consider the influence of local social and ethical backgrounds to more accurately assess the effect of therapeutic hypothermia on long-term morbidity and mortality rates.
To date, only a few studies have addressed the outcomes of infants with mild HIE at or beyond 18 months of age22,23. However, recent studies have reported that these infants are at a risk of adverse outcomes, such as behavioural problems and neurodevelopmental impairments24,25. For example, a multicentre cohort study demonstrated that 16% of infants with mild encephalopathy who did not undergo therapeutic hypothermia had cognitive developmental quotients of < 85, while approximately 40% had at least one cognitive, motor or language score of < 8515. In the present study, only 4.2% of infants with mild encephalopathy developed adverse outcomes at 3 years of age. In this study, the majority of the registered infants had moderate to severe HIE; this is perhaps due to the Baby Cooling Registry of Japan specifically recruiting infants who underwent therapeutic hypothermia and the strict adherence to international guidelines in Japan, where therapeutic hypothermia is considered only in cases with moderate to severe HIE18. It is possible that the HIE severity in the 48 patients with mild HIE in this study was different from that in previous studies. Although both the mortality rate and long-term outcome of these infants were optimal, the influence of therapeutic hypothermia remains unclear. Further studies are required to determine whether therapeutic hypothermia is beneficial in this cohort of infants.
We were able to elucidate the relationship between the severity of encephalopathy, as assessed in infants upon admission, and long-term outcomes. This was achieved by assessing a high-survival cohort from a large registry in Japan. However, several limitations should be considered. First, 37.3% of the infants were lost to follow-up. The follow-up rate was lower in infants with mild encephalopathy, presumably reflecting the optimistic perspective of the neurological outcome and subsequent cessation of follow-up. Therefore, our current findings may predominantly reflect outcomes associated with infants with relatively more severe disease. Second, as the assessment of neurological outcomes using individualised batteries was only performed in 40% of the study population, the use of standard outcome measures was problematic. Similarly, we were unable to incorporate potential independent variables of outcomes within the analysis, such as the type of respiratory care, use of inotropic support, incidence of pulmonary hypertension and seizures and findings of amplitude-integrated electroencephalogram (aEEG) and MRI, because the type, dose and duration of supportive treatment and assessment of ultrasound sonography, aEEG and MRI significantly differ between participating units26. The evaluation of outcomes focused on chronic healthcare needs and motor function, but not on cognitive or language function, as reported in previous studies27,28,29. Third, although the Baby Cooling Registry of Japan disseminated tools and domestic guidelines for the evidence-based implementation of therapeutic hypothermia, including the precise assessment of encephalopathy using the Sarnat encephalopathy staging12 and the Thompson encephalopathy scoring30, the classification of the severity of encephalopathy was not standardised for this particular registry across centres. Recently, Chalak et al. demonstrated that the long-term outcomes of encephalopathic infants can be predicted using a relatively more objective categorisation of the modified Sarnat scoring criteria31. This may improve patient selection in future prospective studies.
In conclusion, the incidence of death and major disability following mild, moderate and severe HIE was reviewed for a large-scale national cohort in Japan. Mortality was relatively low in all subgroups (patients were stratified based on HIE severity), whereas the incidence of major disabilities was relatively high in infants with severe HIE. Cultural and ethical backgrounds, as well as the quality of medical care, may need to be considered when assessing the benefits of therapeutic interventions for HIE. Although the outcomes of infants with mild HIE appeared to be optimal following therapeutic hypothermia, the benefit of such treatment for this cohort of infants needs to be validated further. The natural outcomes associated with mild HIE and the neuroprotective effect of therapeutic interventions need to be addressed in future studies.
Methods
Population and data collection
The Baby Cooling Registry of Japan is an online case registry that was established in January 2012 and includes patient data from all registered Japanese level II/III neonatal intensive care centres. The details of this registry have been reported previously18. This observational study was based on 3-year outcome data from 474 infants registered between January 1, 2012, and December 31, 2016 (Fig. 1).
Assessment of outcomes
The outcomes of cooled infants were assessed at 3 years of age according to the protocol of the Baby Cooling Registry of Japan18. The infants’ parents were asked to assess the outcome of their cooled infants at 18 months postconceptional age and 36 months chronological age by consulting a neonatologist, paediatrician or child neurologist. Data pertaining to the following variables were collected: hearing loss; blindness; epilepsy (requiring anticonvulsant treatment); chronic healthcare needs (e.g., requirements for tube feeding, gastrostomy, tracheotomy or prolonged ventilator management upon reaching the age of 3 years old); and neuromotor function assessed using the GMFCS19 and MACS20. GMFCS and MACS scores range from 0 to 5, with higher scores indicating greater impairment. Major disability was defined as survival with at least one of the following conditions: requirement for tube feeding or respiratory support, hearing loss, blindness, epilepsy, GMFCS > 2 or MACS > 2.
Statistical analysis
The primary outcome was death or major disability upon the 3rd year of follow-up. To assess potential bias due to follow-up loss, baseline characteristics were compared between children with and without follow-up data at 3 years of age using Student’s t-test, Mann–Whitney U test or Chi-squared test, where appropriate. Statistical findings were not corrected for multiple comparisons. For all analyses, the level of significance was set at P < 0.05. Values are shown as a number (proportion, %) for categorical variables or mean ± standard deviation (or median and interquartile range) for quantitative variables.
Ethics approval and consent
This study was conducted in accordance with the principles of the Declaration of Helsinki. The registry protocols were approved by the Ethics Committees of the Kurume University School of Medicine and Saitama Medical University, Japan. The Ethics Committee of Kurume University School of Medicine approved that informed consent was not required because only anonymised data obtained for clinical reasons were used in the study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lawn, J. E. et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 384, 189–205. https://doi.org/10.1016/s0140-6736(14)60496-7 (2014).
Hayakawa, M. et al. Incidence and prediction of outcome in hypoxic–ischemic encephalopathy in Japan. Pediatr. Int. 56, 215–221. https://doi.org/10.1111/ped.12233 (2014).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 86, 329–338. https://doi.org/10.1016/j.earlhumdev.2010.05.010 (2010).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74(Suppl 1), 50–72. https://doi.org/10.1038/pr.2013.206 (2013).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, CD003311. https://doi.org/10.1002/14651858.CD003311.pub3 (2013).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic–ischemic encephalopathy: A randomized clinical trial. JAMA 318, 57–67. https://doi.org/10.1001/jama.2017.7218 (2017).
Tsuda, K. et al. Body temperature, heart rate and long-term outcome of cooled infants: An observational study. Pediatr. Res. https://doi.org/10.1038/s41390-021-01502-w (2021).
Kasdorf, E., Laptook, A., Azzopardi, D., Jacobs, S. & Perlman, J. M. Improving infant outcome with a 10 min Apgar of 0. Arch. Dis. Child Fetal Neonatal Ed. 100, F102-105. https://doi.org/10.1136/archdischild-2014-306687 (2015).
Natarajan, G. et al. Apgar scores at 10 min and outcomes at 6–7 years following hypoxic–ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 98, F473-479. https://doi.org/10.1136/archdischild-2013-303692 (2013).
Laptook, A. R. et al. Outcome of term infants using apgar scores at 10 minutes following hypoxic–ischemic encephalopathy. Pediatrics 124, 1619–1626. https://doi.org/10.1542/peds.2009-0934 (2009).
Shibasaki, J. et al. Outcomes related to 10-min Apgar scores of zero in Japan. Arch. Dis. Child Fetal Neonatal Ed. 105, 64–68. https://doi.org/10.1136/archdischild-2019-316793 (2020).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Robertson, C. M., Finer, N. N. & Grace, M. G. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J. Pediatr. 114, 753–760. https://doi.org/10.1016/s0022-3476(89)80132-5 (1989).
Handley-Derry, M. et al. Intrapartum fetal asphyxia and the occurrence of minor deficits in 4- to 8-year-old children. Dev. Med. Child Neurol. 39, 508–514. https://doi.org/10.1111/j.1469-8749.1997.tb07478.x (1997).
Chalak, L. F. et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: Neurodevelopmental outcomes at 18–22 months. Pediatr. Res. 84, 861–868. https://doi.org/10.1038/s41390-018-0174-x (2018).
Mehta, S. et al. Eligibility criteria for therapeutic hypothermia: From trials to clinical practice. J. Paediatr. Child Health 53, 295–300. https://doi.org/10.1111/jpc.13378 (2017).
Oliveira, V. et al. Therapeutic hypothermia in mild neonatal encephalopathy: A national survey of practice in the UK. Arch. Dis. Child Fetal Neonatal Ed. 103, F388–F390. https://doi.org/10.1136/archdischild-2017-313320 (2018).
Tsuda, K. et al. Therapeutic hypothermia for neonatal encephalopathy: A report from the first 3 years of the Baby Cooling Registry of Japan. Sci. Rep. 7, 39508–39508. https://doi.org/10.1038/srep39508 (2017).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 39, 214–223 (1997).
Eliasson, A. C. et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 48, 549–554. https://doi.org/10.1017/s0012162206001162 (2006).
Massaro, A. N. et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: A report from the Children’s Hospitals Neonatal Consortium HIE focus group. J. Perinatol. 35, 290–296. https://doi.org/10.1038/jp.2014.190 (2015).
Murray, D. M., O’Connor, C. M., Ryan, C. A., Korotchikova, I. & Boylan, G. B. Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics https://doi.org/10.1542/peds.2016-0659 (2016).
van Kooij, B. J. et al. Serial MRI and neurodevelopmental outcome in 9- to 10-year-old children with neonatal encephalopathy. J. Pediatr. 157, 221-227 e222. https://doi.org/10.1016/j.jpeds.2010.02.016 (2010).
Finder, M. et al. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. 174, 48–55. https://doi.org/10.1001/jamapediatrics.2019.4011 (2020).
Conway, J. M., Walsh, B. H., Boylan, G. B. & Murray, D. M. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome—A systematic review. Early Hum. Dev. 120, 80–87. https://doi.org/10.1016/j.earlhumdev.2018.02.007 (2018).
Iwata, O. et al. Hypothermia for neonatal encephalopathy: Nationwide Survey of Clinical Practice in Japan as of August 2010. Acta Paediatr. 101, e197-202. https://doi.org/10.1111/j.1651-2227.2011.02562.x (2012).
Guillet, R. et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr. Res. 71, 205–209. https://doi.org/10.1038/pr.2011.30 (2012).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 371, 140–149. https://doi.org/10.1056/NEJMoa1315788 (2014).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092. https://doi.org/10.1056/NEJMoa1112066 (2012).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Chalak, L. F., Adams-Huet, B. & Sant’Anna, G. A total sarnat score in mild hypoxic–ischemic encephalopathy can detect infants at higher risk of disability. J. Pediatr. 214, 217-221 e211. https://doi.org/10.1016/j.jpeds.2019.06.026 (2019).
Acknowledgements
The authors are grateful to the staff of participating centres for their contribution to the data collection, the infants and their parents for sharing the clinical information. The authors also thank Baby Cooling Registry of Japan Collaboration Team members: Drs Hiroyuki Adachi (Akita University Hospital, Akita, Japan), Satoru Aiba (Yamagata Prefectural Central Hospital, Yamagata, Japan), Shinnosuke Akiyoshi (Ehime Prefectural Central Hospital, Ehime, Japan), Toshifumi Ikeda (Aomori Prefectural Central Hospital, Aomori, Japan), Mikihiro Aoki (National Hospital Nagasaki Medical Center, Nagasaki, Japan), Hirokazu Arai (Japanese Red Cross Akita Hospital, Akita, Japan), Junichi Arai (Ibaraki Children’s Hospital, Ibaraki, Japan), Hideomi Asanuma (Hokkaido Medical Center for Child Health and Rehabilitation, Hokkaido, Japan), Hiromasa Uchizono (National Hospital Organization Mie Chuo Medical Center, Mie, Japan), Yusuke Daimon (Tomakomai City Hospital, Hokkaido, Japan), Tomoko Egashira (National Hospital Organization Saga Hospital, Saga, Japan), Rie Fukuhara (Hiroshima Prefectural Hospital, Hiroshima, Japan), Naoki Fukushima (Almeida Memorial Hospital, Oita, Japan), Yasumasa Yamada (Aichi Medical University Hospital, Aichi, Japan), Sayaka Harada (Osaka City General Hospital, Osaka, Japan), Masato Mizushima (Sapporo City General Hospital, Hokkaido, Japan), Yosuke Yamada (Tokyo Women's Medical University Medical Center East, Tokyo, Japan), Takehiko Hiroma (Nagano Children's Hospital, Nagano, Japan), Mitsuaki Umino (St.Mary's Hospital, Fukuoka, Japan), Kuniko Ieda (Tosei General Hospital, Aichi, Japan), Koichi Iida (Oita Prefectural Hospital, Oita, Japan), Shigeo Iijima (Hamamatsu University School of Medicine, Shizuoka, Japan), Ken Imai (Tokyo Women’s Medical University, Tokyo, Japan), Hiromichi Ariga (Takeda General Hospital, Fukushima, Japan), Masatoshi Nakamura (Fukuoka University Hospital, Fukuoka, Japan), Akio Ishiguro (Saitama Medical Center, Saitama Medical University, Saitama, Japan), Masanori Iwai (Kumamoto University Hospital, Kumamoto, Japan), Shinnichiro Iwataki (Onomichi Hospital, Hiroshima, Japan), Mashiro Sugino (Shikoku Medical Center for Children and Adults, Kagawa, Japan), Akihiko Kai (Aizenbashi Hospital, Osaka, Japan), Muneichiro Sumi (Sasebo City General Hospital, Nagasaki, Japan), Masahiro Kinoshita (Kurume University School of Medicine, Fukuoka, Japan), Mina Chishiki (Fukushima Medical University, Fukushima, Japan), Shin Kato (Nagoya City University Graduate School of Medical Sciences, Aichi, Japan), Hitoshi Kawato (Narita Red Cross Hospital, Chiba, Japan), Yuiko Sonoda (Matsudo City Hospital, Chiba, Japan), Koichi Tanda (Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan), Hiroyuki Kitano (Ishikawa Prefectural Central Hospital, Ishikawa, Japan), Tomoyuki Shimokaze (Kanagawa Children's Medical Center, Kanagawa, Japan), Osamu Kito (Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Aichi, Japan), Akira Kobayashi (Nagaoka Red Cross Hospital, Niigata, Japan), Yoshinori Kohno (Gifu Prefectural General Medical Center, Gifu, Japan), Minoru Kokubo (Kainan Hospital, Aichi, Japan), Masatoshi Kondo (Tokyo Metropolitan Children's Medical Center, Tokyo, Japan), Takahiro Sugiura (Toyohashi Municipal Hospital, Aichi, Japan), Masaaki Kugo (Himeji Red Cross Hospital, Hyogo, Japan), Takashi Kusaka (Kagawa University Hospital, Kagawa, Japan), Kazumasa Takahashi (Yamaguchi University Hospital, Yamaguchi, Japan), Naoki Kamada (Japanese Red Cross Ise Hospital, Mie, Japan), Tomoko Makiya (Okinawa Prefectural Chubu Hospital, Okinawa, Japan), Kenichi Maruyama (Gunma Children’s Medical Center, Gunma, Japan), Ken Masunaga (Tokyo Metropolitan Ohtsuka hospital, Tokyo, Japan), Atsushi Matsumoto (Iwate Medical University, Iwate, Japan), Hiroshi Matsumoto (Asahi General Hospital, Chiba, Japan), Aya Mima (Yodogawa Christian Hospital, Osaka, Japan), Mazumi Miura (Tottori University Faculty of Medicine, Tottori, Japan), Masafumi Miyata (Fujita Health University School of Medicine, Aichi, Japan), Yayoi Miyazono (University of Tsukuba, Ibaraki, Japan), Hiroshi Mizumoto (Kitano Hospital, Osaka, Japan), Kazumichi Fujioka (Kobe University Hospital, Hyogo, Japan), Takeshi Morisawa (Kakogawa Central City Hospital, Hyogo, Japan), Ken Nagaya (Asahikawa Medical University Hospital, Hokkaido, Japan), Yoshihisa Nagayama (Niigata City General Hospital, Niigata, Japan), Kenji Nakamura (Japanese Red Cross Otsu Hospital, Shiga, Japan), Makoto Nakamura (National Hospital Organization Okayama Medical Center, Okayama, Japan), Atsushi Nakao (Japanese Red Cross Medical Center, Tokyo, Japan), Hideto Nakao (Hyogo Prefectural Kobe Children's Hospital, Hyogo, Japan), Yusuke Nakazawa (Shizuoka Children's Hospital, Shizuoka, Japan), Yutaka Nishimura (Hiroshima City Hiroshima Citizens Hospital, Hiroshima, Japan), Naoto Nishizaki (Juntendo University Urayasu Hospital, Chiba, Japan), Masatoshi Nozaki (Osaka Women’s and Children’s Hospital, Osaka, Japan), Shigeru Ohki (Seirei Hamamatsu General Hospital, Shizuoka, Japan), Isaku Omori (Tokyo Metropolitan Bokutoh Hospital, Tokyo, Japan), Mika Tomita (Kimitsu Chuo Hospital, Chiba, Japan), Junko Saito (Miyagi Children's Hospital, Miyagi, Japan), Masahiro Hayakawa (Nagoya University Hospital, Aichi, Japan), Kazuo Seki (Yokohama City University Medical Center, Kanagawa, Japan), Yoshitsugu Shirakawa (Fukuoka Shin Mizumaki Hospital, Fukuoka, Japan), Hiroyuki Shiro (Yokohama Rosai Hospital, Kanagawa, Japan), Yoshifumi Murayama (Tokai University Hospital, Kanagawa, Japan), Hiroshi Suzumura (Dokkyo Medical University Hospital, Tochigi, Japan), Masatoshi Sanjo (Japanese Red Cross Sendai Hospital, Miyagi, Japan), Yasushi Takahata (Fukuoka Children’s Hospital, Fukuoka, Japan), Atsuko Taki (Tokyo Medical and Dental University, University Hospital of Medicine, Tokyo, Japan), Taihei Tanaka (Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Aichi, Japan), Kuriko Nakamura (Saiseikai Yokohamashi Tobu Hospital, Kanagawa, Japan), Mikio Tsunei (Tottori Prefectural Central Hospital, Tottori, Japan), Touhei Usuda (Niigata University Medical & Dental Hospital, Niigata, Japan), Yukari Yada (Jichi Medical University Hospital, Tochigi, Japan), Junko Yamamoto (Japan Community Healthcare Organization Kyushu Hospital, Fukuoka, Japan), Masahito Yamamoto (Nagahama Red Cross Hospital, Shiga, Japan), Hitoshi Yoda (Toho University Omori Medical Center, Tokyo, Japan), Kyoko Yokoi (Nagoya City University West Medical Center, Aichi, Japan), Shinobu Yoshida (Omihachiman Community Medical Center, Shiga, Japan), Taketoshi Yoshida (Toyama University Hospital, Toyama, Japan), Kayo Yoshikawa (Nihon University Itabashi Hospital, Tokyo, Japan), Yuichi Kato (Anjo Kosei Hospital, Aichi, Japan), Masako Nakamura (Kurashiki Central Hospital, Okayama, Japan), Kenichiro Hosoi (Kyorin University School of Medicine, Tokyo, Japan), Fumiko Kinoshita (Nagasaki University Hospital, Nagasaki, Japan), Kengo Miyashita (National Hospital Organization Kanazawa Medical Center, Ishikawa, Japan), Masashi Zuiki (National Hospital Organization Maizuru Medical Center, Kyoto, Japan), Yutaka Uzuki (Obihiro Kosei General Hospital, Hokkaido, Japan), Misao Yoshii (Osaka Red Cross Hospital, Osaka, Japan), Tetsuya Kunikata (Saitama Medical University Hospital, Saitama, Japan), Masaaki Ueda (Toyooka Public Hospital, Hyogo, Japan), Manabu Sugie (Tsuchiura Kyodo General Hospital, Ibaraki, Japan) and Shunsuke Araki (University of Occupational and Environmental Health, Fukuoka, Japan).
Funding
This work was supported by the Japan Society of Perinatal and Neonatal Medicine, and the Ministry of Health, Labour and Welfare, Japan (H27-001, Special research in perinatal medicine). Dr Tsuda is funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research 18K15722). Dr Shibasaki was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C20K08247). Dr Iwata S was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C18K09955). Dr Iwata O was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research A20H00102).
Author information
Authors and Affiliations
Contributions
K.T., S.I., M.N. and O.I. designed the study and the survey items. All authors participated in data collection. K.T., T.I., S.I. and O.I. performed the statistical analyses. K.T., J.S., T.I., A.T., T.M., Y.S. and O.I. contributed to the interpretation of the findings. K.T. and O.I. drafted the manuscript. All authors critically reviewed and revised the manuscript and the final approval of the published version. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsuda, K., Shibasaki, J., Isayama, T. et al. Three-year outcome following neonatal encephalopathy in a high-survival cohort. Sci Rep 12, 7945 (2022). https://doi.org/10.1038/s41598-022-12091-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12091-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.