Abstract
Mitochondrial dysfunction plays a key role in doxorubicin-induced cardiotoxicity (DIC). In this proof-of-principle study, we investigated whether PET mapping of cardiac membrane potential, an indicator of mitochondrial function, could detect an acute cardiotoxic effect of doxorubicin (DOX) in a large animal model. Eight Yucatan pigs were imaged dynamically with [18F](4-Fluorophenyl)triphenylphosphonium ([18F]FTPP+) PET/CT. Our experimental protocol included a control saline infusion into the left anterior descending coronary artery (LAD) followed by a DOX test infusion of either 1 mg/kg or 2 mg/kg during PET. We measured the change in total cardiac membrane potential (ΔΨT), a proxy for the mitochondrial membrane potential, ΔΨm, after the saline and DOX infusions. We observed a partial depolarization of the mitochondria following the DOX infusions, which occurred only in myocardial areas distal to the intracoronary catheter, thereby demonstrating a direct association between the exposure of the mitochondria to DOX and a change in ΔΨT. Furthermore, doubling the DOX dose caused a more severe depolarization of myocardium in the LAD territory distal to the infusion catheter. In conclusion, [18F]FTPP+ PET-based ΔΨT mapping can measure partial depolarization of myocardial mitochondria following intracoronary DOX infusion in a large animal model.
Similar content being viewed by others
Introduction
Doxorubicin (DOX) is a widely used chemotherapeutic for treatment of a variety of cancers, either as a primary intervention or in combined therapy. While DOX therapy often results in increased cancer survival, it can also be accompanied by cardiotoxic effects, resulting in myocardial injury. Current methods for detection of cardiotoxicity rely on serial evaluation of a patient’s left ventricular ejection fraction (LVEF). However, LVEF decline is a lagging index of cardiotoxic injury1,2 and by the time left ventricular function is impaired, damage is often irreversible. Therefore, new imaging approaches for detecting subclinical cardiotoxicity are greatly needed3,4.
The mechanisms underlying doxorubicin-induced cardiotoxicity (DIC) are complex and incompletely understood. Nevertheless, a key role has been attributed to mitochondrial damage and dysfunction5. Mitochondria are indeed the most extensively injured intracellular organelles upon exposure to DOX and mitochondrial injury is believed to represent one of the first events of cardiotoxicity5,6. Hence, monitoring cardiac mitochondrial function might allow early detection of DIC7,8.
The mitochondrial membrane potential (ΔΨm) is a comprehensive index of mitochondrial function. It provides the energy for mitochondrial adenosine triphosphate (ATP) production via oxidative phosphorylation, which is essential for normal cardiac function. In normal physiologic function, ΔΨm lies within a narrow range; dysregulation of ΔΨm results in increased reactive oxygen species production, leading to oxidative damage to DNA, proteins, and lipids, mitochondrial dysfunction, and ultimately cell death9,10. Importantly, most known mechanisms underpinning mitochondrial dysfunction in DIC, particularly inhibition of the electron transport chain, are associated with depolarization of ΔΨm. Notably, depolarization of ΔΨm following exposure to DOX has been observed in-vitro in various cell lines, such as cardiomyocytes11 and carcinoma cells12 as well as ex-vivo in Langendorff-perfused rat hearts13.
Lipophilic cations like tritiated tetraphenylphosphonium (TPP+) have been used for decades to measure ΔΨm in-vitro14. More recently, lipophilic cationic PET tracers, such as [18F](4-Fluorophenyl)triphenylphosphonium ([18F]FTPP+)15,16, [18F]fluorobenzyl-triphenylphosphonium17 and [18F]MitoPhos7 have been introduced, enabling in-vivo assessment of ΔΨm. Our group has recently developed an approach to quantify cardiac tissue membrane potential (ΔΨT), a proxy of ΔΨm, in units of millivolts with [18F]FTPP+ PET16. We have further shown that cardiac ΔΨT is sensitive to changes induced by a proton gradient uncoupler causing partial depolarization of ΔΨm18. We have also demonstrated that ΔΨT mapping with [18F]FTPP+ PET is feasible in human subjects, resulting in measured values in excellent agreement with those in the literature19.
Given the established role of mitochondrial dysfunction in DIC, we hypothesized that PET-based ΔΨT mapping could provide an early biomarker of DIC. As a first step in testing this hypothesis, we evaluated whether our approach could detect a cardiotoxic effect of DOX in an acute setting. In this proof-of-principle study, we used a porcine model in which DOX was infused into the left anterior descending (LAD) coronary artery during [18F]FTPP+ PET imaging. Through this localized administration, the experiments were designed such that ΔΨT could be measured before and during intracoronary infusion of DOX. While the DOX administration was thus different from a clinical IV infusion, with our protocol, each animal served as its own control, thereby allowing more sensitive detection of mitochondrial depolarization.
Materials and methods
Eleven male Yucatan pigs (45–56 kg, 9–11 months, Sinclair Bio-Resources, Columbia, MO), of which three were later excluded from data analysis as explained below, were studied. All animals were housed and maintained under the supervision of the veterinary staff of the Center for Comparative Medicine at Massachusetts General Hospital (MGH). Experiments were performed in accordance with the US. Department of Agriculture (USDA) Animal Welfare Act and Animal Welfare Regulations (Animal Care Blue Book), Code of Federal Regulations (CFR), Title 9, Chapter 1, Subchapter A, Part 2, Subpart C, §2.31. 2017. The study protocol was approved by the MGH Institutional Animal Care and Use Committee in accordance with The Guide for the Care and Use of Laboratory Animals20 and all methods were carried out following relevant guidelines and regulations. For this proof-of-principle study, a small sample size was used and no blinding was performed for the data analysis. The methods are reported in accordance with ARRIVE guidelines21.
Animal preparation
The animals were premedicated for three days with amiodarone (150–200 mg) prior to the study, fasted overnight, and anesthetized via intramuscular administration of a mixture of Telazol (4.4. mg/kg) and Xylazine (2.2 mg/kg). Anesthesia was maintained during the entire experiment with a mixture of isoflurane (1.25–3%) and oxygen. A constant infusion of amiodarone (50–150 mg over 2–3 h) was administered during the surgery.
For [18F]FTPP+ injection, a 22G catheter was placed into an auricular vein. To administer fluids, medications, and CT contrast, another 4–6 Fr catheter was inserted into a femoral vein, and a femoral artery was cannulated for arterial blood sampling. A left thoracotomy was performed via a skin incision; the pericardium was opened, and a side branch of the mid left anterior descending artery (LAD) was isolated. A 22G angiocatheter was inserted into the lumen of the LAD via the side branch, then exteriorized through the chest wall, and secured on the skin for saline/DOX infusions.
Experimental protocol
Figure 1 provides an overview of the experimental protocol. The animals were divided into two groups, A and B, as discussed below. Each animal underwent dynamic [18F]FTPP+ PET imaging for up to three hours on a hybrid PET/CT (GE Discovery MI) using a bolus-plus-infusion protocol22. When the tracer concentrations reached secular equilibrium—typically after 90 min–0.5 ml/kg of saline was infused into the LAD over 10 min to serve as a negative control. Ten minutes after the end of the saline infusion, a DOX infusion (doxorubicin hydrochloride, Millipore Sigma, Saint Louis, MO) was likewise administered over 10 min into the LAD. Group A (resp. B) included 5 (resp. 6) animals, each of which received a DOX infusion of 1 mg/kg (resp. 2 mg/kg) dissolved in 0.5 ml/kg saline. At the end of the procedures, the animals were euthanized with 100 mg/kg Euthasol (Virbac Animal Health, Westlake, TX) administered IV consistent with AVMA guidelines23.
Three animals were excluded from the study due to technical problems during surgical preparation and/or difficulties with LAD catheter placement, resulting in four animals in Group A (P1, …, P4) and four animals for Group B (P5, …, P8), for a total of eight pigs.
Quantification of myocardial membrane potential
The kinetic and biophysical basis of our PET-based membrane potential mapping technique has been detailed elsewhere16,24. Briefly, the total myocardial membrane potential ΔΨT, which represents the sum of the cellular and mitochondrial membrane potentials, can be approximated as \(\Delta {\Psi }_{T}\approx \frac{1}{\beta }ln\left[\frac{\left(1-{f}_{ECV}\right){\cdot f}_{mito}}{{V}_{T}}\right]\) 16, where β is a physical constant and fmito and fECV refer to the mitochondrial and extracellular volume fractions. The total volume of distribution, VT, can be measured at secular equilibrium by taking the ratio of the tracer concentration in tissue over that in plasma. The value of fmito was set to 0.2616,25. The extracellular volume fraction, fECV, was calculated based on pre- and late post-contrast CT images as outlined below.
PET/CT imaging and blood sampling
[18F]FTPP+ (molar activity 90.5 ± 38.1 GBq/μmol end of synthesis) was synthesized and purified on a GE Tracerlab FX2N synthesis unit using [18F]fluoride produced onsite by a GE PETtrace cyclotron bombarding > 98% enriched [18O]water as previously reported15. [18F]FTPP+ was formulated in sterile saline for injection (0.9% sodium chloride solution) and EtOH (6% bolus; 0.8% infusion). PET data were acquired dynamically in list mode for up to three hours beginning from bolus administration of 601.1 ± 83.9 MBq [18F]FTPP+ and start of an infusion of 183.1 ± 27.3 MBq over 180 min (3 mL/min). Arterial blood samples were manually drawn every five minutes, starting from the beginning of the saline infusion.. Tracer concentration in plasma was measured with a calibrated well counter and was expressed as Bq/cc. Following PET, diagnostic-quality CT images were acquired for quantification of myocardial fECV26. First, a pre-contrast scan was acquired. A post-contrast image was acquired ten to fifteen minutes after an IV contrast injection (1.8 mL/kg Iodine 300 (Ultravist, Bayer Healthcare, Pittsburgh, PA) or 1.45 mL/kg Iodine 370 (Bracco Diagnostic, Monroe Township, NJ)) into the femoral vein using a power injector. Pre- and post-contrast images had a voxel size of 0.65 × 0.65 × 2.5 mm3 and were acquired with a tube voltage of 100–120 kVp. Details of the imaging parameters for each animal are shown in Suppl. Table 1.
Data processing
List mode PET data were reconstructed dynamically (frames: 12 × 5, 6 × 10, 6 × 30, and 175 × 60 s) using OSEM with CT-based attenuation correction, resulting in radioactivity concentration maps in units of Bq/cc, with 89 slices and a voxel size of 2.73 × 2.73 × 2.8 mm3. The dynamic PET images were transformed into short axis. Next, defined frames of the experiment (see below) were summed and non-rigidly registered using ITK27 to an in-house developed cardiac [18F]FTPP+ atlas constructed using data acquired in four healthy pigs. The inverse deformation fields were then applied to the masks of the standard 17 myocardial segments28, which had been defined in atlas space, to obtain subject-specific masks for all segments. Time activity curves (TACs) for the 17 segments were extracted for each animal from the average segmental concentration for each frame.
fECV was calculated based on the pre- and post-contrast CT images26 and the average value over the entire myocardium (excluding the apex, segment 17) was used for subsequent quantification of ΔΨT. VT before and after the saline and DOX infusions was calculated from the average [18F]FTPP+ concentration in tissue and arterial plasma for the different phases of the experiment, as defined in Suppl. Table 2. Different data analysis periods were used to calculate the average tracer concentration before and after the 1 and 2 mg/kg DOX infusions because of apparent differences in their temporal responses. The tracer concentration in plasma was assumed to be constant starting from the time of the saline infusion and was calculated as the average concentration over time. Next, ΔΨT was quantified based on VT for each phase using the equation introduced above.
Parametric images of ΔΨT were also computed from the dynamic PET images for the different phases of the experiment using the same approach.
To help visualize experimental results, we present segmental concentrations averaged over the cohorts of pigs for all measurement times. Tissue tracer concentrations were normalized by animal weight and the cumulative tracer activity injected by bolus-plus-infusion until time t, i.e., \(TAC_{{norm}} (t) = \frac{{TAC(t)}}{{\int_{0}^{t} {C(u)_{{inj}} du/W} }}\), where \({\int }_{0}^{t}C{(u)}_{inj}du\) refers to the administrated tracer activity up to time t (MBq) and W is the animal weight (g). This normalization is an extension of the well-known standard uptake value (SUV) index, taking time dependence into account.
Statistical analysis
For the purpose of hypothesis testing we used the change in ΔΨT (δΔΨT) for each of the infusions (e.g. δΔΨT,saline = ΔΨT,after_saline—ΔΨT,before_saline) as the dependent variable in a mixed effects statistical model with “myocardial segment” (1,2, …,16) and “treatment type” (saline vs. DOX of 1 mg/kg vs. DOX of 2 mg/kg) as categorical variables. We first tested whether a model with “treatment type” and “myocardial segment” was able to explain the observed data (ANOVA omnibus test). Next, we specifically tested three hypotheses: whether δΔΨT was significantly different (1) for segments 7, 8, 13, and 14 (all part of the LAD territory) compared to all other segments, (2) for the saline infusion compared to the DOX infusion, and (3) for the DOX infusion with a dose of 1 mg/kg compared to the 2 mg/kg infusion. These a-priori hypotheses were tested as planned contrasts within a linear mixed effects model, taking account of repeated measures in the same animal.
Due to the localized administration of the infusions, the effects of DOX and saline were expected to differ between segments. Therefore, we also included two interaction terms between the contrasts of hypothesis (1) and (2) and between (1) and (3), to test whether the change in ΔΨT was significantly different between the saline and DOX infusion as well as for the different DOX doses when taking differences between segments (i.e., LAD vs. non-LAD segments) into account.
The tested contrasts were considered significant for p < 0.05. Segment 17 was excluded from the analysis due to difficulties in accurate segmentation of the apex.
All statistical analyses were performed in R29, the linear mixed effects model was applied using the lme4 package30. P-values from approximate F- and t-tests were calculated using Satterthwaite’s method for approximating the degrees of freedom31, implemented in lme4.
Results
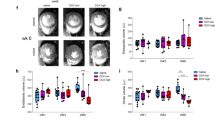
Figure 2 provides an empirical summary of the results of the experiment, showing the mean normalized tracer concentrations in tissue over time for the eight animals. For segments of the LAD territory (e.g., 13 and 14, Fig. 2, top), a clearly visualized decrease in [18F]FTPP+ concentration occurred, starting from the beginning of the DOX infusion. No such change was observed for the control segments (e.g., RCA segments 4 and 10, Fig. 2, bottom), nor for the control saline infusion. This localized reduction in tracer concentration signifies partial mitochondrial depolarization and clearly indicates an effect due to DOX that may be appreciated without a formal statistical analysis. Representative examples of normalized TACs for group A and B are shown in Suppl. Figure S1. For group A (1 mg/kg DOX infusion), the tissue tracer concentration tended to recover after the DOX infusion to a value close to the baseline value, whereas for group B (2 mg/kg DOX infusion), the observed effect was generally less reversible.
Mean normalized TACs for two segments of the LAD (top) and two segments of the RCA (bottom) for the eight animals. The grey shaded area indicates the mean + /-standard error. The blue dotted lines indicate the beginning and end of the control saline infusion (C), whereas the orange dotted lines mark the beginning and end of the DOX infusion (D).
The localized reduction in myocardial [18F]FTPP+ concentration can also be appreciated on the parametric images of the apparent ΔΨT presented for two example animals in Fig. 3 and Suppl. Figs. S2 and S3. They show clear evidence for a partial depolarization of ΔΨT after the DOX infusion in the apical septal area of the myocardium, i.e., the area distal to the LAD catheter, but not after the saline infusion. Overall, a partial depolarization of ΔΨT after the DOX infusion was observed for seven of the eight animals. For those animals, the maximum change in apparent ΔΨT over all segments ranged between 2.5 mV (P6) and 10.5 mV (P3, see Table 1). For all animals, the maximum ΔΨT change occurred in the LAD territory, particularly segment 13 or 14, i.e., the apical anterior or apical septal area of the myocardium.
The results of the statistical analysis are summarized in Figs. 4 and 5 as well as Suppl. Table S3. The change in ΔΨT was significantly associated with the categorical variables “segment” and ”treatment type” (p < 0.0001 and p = 0.04, respectively). More specifically, δΔΨT was significantly different for the segments directly exposed to DOX (a-priori defined as segments 7, 8, 13, and 14) compared to the other segments (p < 0.0001). The change in ΔΨT after the DOX infusion was significantly different from the change after the control saline infusion when taking different effects for LAD vs. non-LAD segments into account (p < 0.0001): For the segments directly exposed to DOX, ΔΨT was partially depolarized. Much smaller changes in ΔΨT—often in the opposite direction (i.e., more negative ΔΨT)—occurred for the other segments as well as for the control saline infusion. Furthermore, δΔΨT was significantly larger for a DOX infusion of 2 mg/kg compared to 1 mg/kg (p = 0.0013).
Mean (bars) + /- standard error of change in ΔΨT for each of the 16 segments and the three types of “interventions” (saline – “SAL”, DOX 1 mg/kg – “DOX1”, and DOX 2 mg/kg – “DOX2”) averaged over the eight studies. The four segments that had a significantly different change in ΔΨT compared to the other segments are indicated with asterisks.
Mean (bars) + /- standard error of change in ΔΨT for the segments directly exposed to the saline and DOX infusions (a priori defined as segments 7, 8, 13, and 14) as well as the control segments after the saline and DOX infusion (left) and comparison of change in ΔΨT for a dose of 1 mg/kg vs 2 mg/kg (right).
The more negative ΔΨT observed after the DOX infusion for some of the segments not part of the LAD territory (which can be seen in Figs. 4 and 5) can be explained by the fact that perfect secular equilibrium was not reached in all the experiments. Therefore, the [18F]FTPP+ concentration in tissue continued to increase moderately during the data acquisition for those segments not affected by the DOX infusions, resulting in a more negative apparent ΔΨT.
Overall, these results demonstrate that DOX infusion caused a significant change in ΔΨT, indicating a partial depolarization of cardiac mitochondria. These changes occurred only in myocardial areas distal to the intracoronary catheter, demonstrating a causal association between the direct exposure of the mitochondria to DOX and partial depolarization of ΔΨT as measured using our proposed technique. Furthermore, doubling DOX dose caused a more severe depolarization of the myocardial mitochondria.
Discussion
This study is the first to non-invasively measure a partial depolarization of myocardial mitochondria after acute exposure to DOX in a large animal model. Mitochondrial damage plays a central role in DIC with direct effects of DOX on mitochondrial function5. Consequently, imaging of mitochondrial function may enable early detection of DIC. Voltage sensitive tracers such as [18F]FTPP+ accumulate in the mitochondria according to the Nernst equation, relating the tracer concentration on each side of the inner mitochondrial membrane to ΔΨm, and thus enabling imaging of mitochondrial function. We have previously developed an approach for quantifying ΔΨT, a proxy of ΔΨm, in mV with [18F]FTPP+ PET16,19. The current study extends these results, clearly establishing that this technique can measure a depolarization of myocardial mitochondria following an acute DOX challenge in-vivo in a porcine model. While DOX-induced cardiotoxicity often manifests after several months of treatment5, this proof-of-principle study is an important first step providing the rationale for more detailed studies mimicking clinical therapeutic protocols.
New approaches for early diagnosis of cardiotoxicity are greatly needed3. Methods under investigation include echocardiography-based assessment of LV global longitudinal strain (GLS)32,33 and cardiac MRI for measurement of fECV34 or T2 mapping for detection of edema35. While GLS decline was associated with a later drop of LVEF32,36, a recent prospective randomized study did not identify a significant benefit of GLS-guidance over LVEF-based strategy for initiation of cardioprotective therapy33. This finding suggests a need for even earlier detection of cardiotoxicity. Similarly, cardiac MRI-based assessment relies on initial myocardial damage to detect an increase in fECV or edema37.
With our imaging approach, we were able to measure a localized decrease of myocardial [18F]FTPP+ concentration following acute exposure to DOX. An important strength of our study design pertains to the intracoronary infusion of DOX, directly into the LAD, resulting in exposure of specific segments of the myocardium to this drug. That is, we only observed a significant change in ΔΨT for LAD segments distal to the intracoronary catheter. This setting allows us to relate the observed drop in myocardial tracer concentration directly to the exposure of the corresponding segments to DOX. We observed an immediate effect of DOX on cardiac membrane potential. This aspect is an important difference compared to LVEF measurement, where a change can only be identified once—often irreversible—damage to the myocardium has occurred. Furthermore, the maximum change in ΔΨT was (for the studies with a detectable effect of DOX) in the range of 2.5–10.5 mV; for none of the studies was ΔΨT fully depolarized. Based on this observation, we speculate that DOX injury may affect subsets of mitochondria differently, resulting overall in partial depolarization of ΔΨT for a segment: some mitochondria might not be affected by DOX and remain fully functional, others might recover function after initial injury, and a third group of mitochondria might become completely dysfunctional. We expect the number of mitochondria falling into the last group to increase over the course of chemotherapy. In other words, mitochondrial damage—measurable in terms of drop in ΔΨT—may add up as the cumulative DOX dose increases, allowing, when detected early, for implementation of cardioprotective interventions before ultimate mitochondrial dysfunction and irreversible myocardial damage.
We measured ΔΨT, the sum of the cellular membrane potential (ΔΨc) and ΔΨm, as a proxy of ΔΨm. ΔΨc, which represents the average cellular membrane potential over a cardiac cycle, is correlated with the heart rate. For our studies, we observed a largely constant heart rate during and after the DOX infusions. However, DOX is known to affect ion currents across the cellular membrane, thus, potentially altering ΔΨc38. Nevertheless, ΔΨm represents the major part of ΔΨT so that the observed changes in ΔΨT are most likely related to a partial depolarization of ΔΨm.
Study design and comparison to clinical treatment with DOX
For our study, we administered between 1 and 2 mg/kg of DOX, which is comparable to the dose given clinically to treat patients39. However, there is an important difference. In our study, the entire dose of 1–2 mg/kg of DOX was delivered directly to the myocardium, and more specifically the vascular territory perfused by the LAD. The direct infusion of DOX into the LAD cannot be directly translated to human investigation; in clinical treatment DOX is administered IV and thus permeates the entire myocardium, as well as other organs. Using the reported area under the curve of 4.2 μmol*hour/L for an IV infusion of 1.62 mg/kg of DOX over 15 minutes40, we computed the estimated exposure of the LAD territory from an IV administration of DOX as 0.7647 mg * hour. In contrast, assuming no clearance during the intracoronary DOX infusion as well as full clearance after the end of the infusion, the LAD territory was exposed to 4.167 mg * hour of DOX for an intracoronary infusion of 1 mg/kg. Based on this estimate, the DOX exposure in our setting was approximately five times higher compared to an IV infusion. This finding indicates a potentially smaller change of ΔΨT after one cycle of clinical DOX treatment than observed in our study. At the same time, as described above, the partial depolarization of ΔΨT is likely to increase over several cycles of DOX administration. Identifying a threshold of depolarization in ΔΨT for definition of subclinical cardiotoxicity will require clinical studies. Moreover, the partial depolarization of ΔΨT that we observed likely resulted from acute cardiotoxic effects of DOX, such as DOX binding to cardiolipin, a phospholipid present in the mitochondrial membrane involved in electron transport chain activity and thus required for mitochondrial ATP production5,41,42. On the other hand, chronic effects of DOX involve binding to topoisomerase 2β, which is largely present in mitochondria, resulting in DNA double-strand breaks and transcriptome changes leading to defective mitochondrial biogenesis and formation of reactive oxygen species5,43,44,45. The relation between the immediate change in ΔΨT that was observed in our acute study and more chronic cardiotoxicity will need to be elucidated in further studies.
In our study, anesthesia was initiated with a mixture of Telazol and Xylazine and maintained with isoflurane. Isoflurane has a known depolarizing effect on ΔΨm and the observed magnitude of change in ΔΨT could also be influenced by isoflurane46. However, it is unlikely that the observed changes in ΔΨT were caused by anesthesia since isoflurane levels, which were monitored with a gas analyzer, remained constant during imaging. Furthermore, the combination of Xylazine with Telazol/Zolazepam has been associated in pigs with cardiovascular effects, such as a lowered heart rate47 so that a similar effect might be caused by Xylazine-Telazol. Hence, these two drugs could have affected the baseline cellular membrane potential ΔΨc and thus ΔΨT, but unlikely influenced the observed change in ΔΨT.
Effect of different DOX doses
For the higher dose of 2 mg/kg, a significantly different change in ΔΨT compared to the 1 mg/kg dose occurred. We further observed that for a dose of 1 mg/kg, the DOX infusion often had a transient effect on the [18F]FTPP+ concentration in the affected segments, whereas, for the higher dose, the concentration did not recover to its value prior to the infusion (see Suppl. Fig. S1 for example TACs). While the mechanisms behind these different effects are not clear, we speculate that some mitochondria can recover from initial, temporary exposure to DOX for lower doses, whereas this ability is limited for higher doses. Furthermore, the maximum decrease in ΔΨT overall occurred later when administering a dose of 2 mg/kg. For this reason, we computed the change in ΔΨT for a dose of 2 mg/kg based on different frames compared to the 1 mg/kg dose.
Other approaches for imaging of mitochondrial function
Other groups have recently evaluated the use of other lipophilic cations for PET imaging such as [18F]MitoPhos7 or [68 Ga]Galmydar48 to detect signs of cardiotoxicity in an acute setting, where rodents received DOX intravenously 2–5 days before PET imaging. Compared to the control animals, a significantly lower myocardial tracer uptake was observed, indicating a decrease in ΔΨm; however, absolute quantification of membrane potential was not performed. Furthermore, unlike [18F]FTPP+, these tracers have not yet been translated to humans. SPECT imaging with 99mTc-sestamibi, a routinely used cardiac perfusion tracer whose distribution in myocardium is based both on perfusion and ΔΨm, has also been proposed for detection of DIC49. Besides the poorer spatial resolution and quantification of SPECT compared to PET, the dependence of 99mTc-sestamibi distribution in myocardium on perfusion poses another complication, requiring an additional SPECT scan with a “pure” perfusion tracer to correct for the effect of perfusion in order to measure ΔΨm49.
Study limitations
For quantification of VT as the ratio of tracer concentration in tissue over tracer concentration in plasma and subsequently relating it to ΔΨT, the concentrations should be in secular equilibrium. For our data analysis and statistical comparison, we made the simplifying assumption that after each of the two intracoronary infusions, a new secular equilibrium was reached, allowing us to compute ΔΨT before and after each of the infusions. However, irrespective of this underlying assumption, a clear decrease in [18F]FTPP+ concentration in tissue following the DOX infusions could be observed, which is ultimately related to an underlying change in ΔΨT.
Perfect secular equilibrium was not reached for some of the experiments. For those studies, the tracer concentration in tissue continued to increase slightly in the segments not exposed to DOX (non-LAD segments), resulting in an apparent hyperpolarization of the mitochondria for those segments after DOX infusion.
We assumed the [18F]FTPP+ concentration in plasma (Cpl) to be constant starting from the first intracoronary infusion. This aspect is an additional approximation. However, importantly, when calculating δΔΨT, as δΔΨT = ΔΨT,after − ΔΨT,before and setting Cpl,before = Cpl,after for calculating VT as well as the corresponding ΔΨT, then δΔΨT is independent of fmito and fECV. Hence, our results in terms of δΔΨT were not influenced by the assumed or measured values of fmito and fECV.
The administered [18F]FTPP+ dose of 601.1 ± 83.9 MBq was larger than a clinically feasible dose due to imaging of over three hours with our experimental protocol. We have previously demonstrated that [18F]FTPP+ PET-based membrane potential mapping is feasible in human subjects with lower doses of ~ 400 MBq19.
The ΔΨT values measured with PET were not compared to ex-vivo measurements in excised tissue after animal sacrifice. However, we have previously validated the dependence of the measured imaging parameters on ΔΨm with the proton uncoupler BAM1518. Furthermore, we have evaluated [18F]FTPP+ PET-based quantification of ΔΨT in humans, with measured values in very good agreement with the literature19.
Conclusion
[18F]FTPP+ PET mapping of cardiac membrane potential can measure acute partial depolarization of myocardial mitochondria following intracoronary DOX infusion in a large animal model. Future studies will assess the potential of this technique for early detection of DIC in a chronic setting over several cycles of chemotherapy treatment first in animals and subsequently, in a clinical study in patients.
References
Sawaya, H. et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 107, 1375–1380 (2011).
Čelutkienė, J. et al. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the heart failure association of the European society of cardiology. Eur. J. Heart Fail. 20, 1615–1633 (2018).
Cardinale, D. & Cipolla, C. M. Chemotherapy-induced cardiotoxicity: importance of early detection. Exp. Rev. Cardiovasc. Ther. 14, 1297–1299 (2016).
Menna, P. & Salvatorelli, E. Primary prevention strategies for anthracycline cardiotoxicity: a brief overview. Chemotherapy 62, 159–168 (2017).
Wenningmann, N., Knapp, M., Ande, A., Vaidya, T. R. & Ait-Oudhia, S. Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol. Pharmacol. 96, 219 (2019).
Sacks, B. et al. Mitochondrial targeted antioxidants, mitoquinone and SKQ1, not vitamin C, mitigate doxorubicin-induced damage in H9c2 myoblast: pretreatment vs. co-treatment. BMC Pharmacol. Toxicol. 22, 49 (2021).
McCluskey, S. P. et al. Imaging of chemotherapy-induced acute cardiotoxicity with 18 F-labeled lipophilic cations. J. Nucl. Med. 60, 1750–1756 (2019).
Sivapackiam, J., Sharma, M., Schindler, T. H. & Sharma, V. PET radiopharmaceuticals for imaging chemotherapy-induced cardiotoxicity. Curr. Cardiol. Rep. 22, 62 (2020).
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T. & Kwong, J. Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13 (2019).
Richardson, A. G. & Schadt, E. E. The role of macromolecular damage in aging and age-related disease. J. Gerontol. A. Biol. Sci. Med. Sci. 69, S28–S32 (2014).
Dhingra, R. et al. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc. Natl. Acad. Sci. 111, E5537–E5544 (2014).
Kuznetsov, A. V., Margreiter, R., Amberger, A., Saks, V. & Grimm, M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim. Biophys. Acta BBA Mol. Cell Res. 1813, 1144–1152 (2011).
Montaigne, D. et al. Stabilization of mitochondrial membrane potential prevents doxorubicin-induced cardiotoxicity in isolated rat heart. Toxicol. Appl. Pharmacol. 244, 300–307 (2010).
Kamo, N., Muratsugu, M., Hongoh, R. & Kobatake, Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 49, 105–121 (1979).
Shoup, T. M. et al. Evaluation of (4-[18F]Fluorophenyl)triphenylphosphonium Ion. A potential myocardial blood flow agent for PET. Mol. Imag. Biol. 13, 511–517 (2011).
Alpert, N. M. et al. Quantitative in vivo mapping of myocardial mitochondrial membrane potential. PLoS ONE 13, e0190968 (2018).
Madar, I. et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur. J. Nucl. Med. Mol. Imag. 34, 2057–2065 (2007).
Alpert, N. M. et al. In-vivo imaging of mitochondrial depolarization of myocardium with positron emission tomography and a proton gradient uncoupler. Front. Physiol. 11, 491 (2020).
Pelletier-Galarneau, M. et al. In vivo quantitative mapping of human mitochondrial cardiac membrane potential: a feasibility study. Eur. J. Nucl. Med. Mol. Imag. 48, 414–420 (2021).
National Research Council. Guide for the care and use of laboratory animals. (National Academies Press, 2011).
Percie du Sert, N. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 20. PLOS Biol. 18, e3000411 (2020).
Carson, R. E. et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18 F]cyclofoxy and positron emission tomography. J. Cereb. Blood Flow Metab. 13, 24–42 (1993).
Leary, S. L. & American Veterinary Medical Association. AVMA guidelines for the euthanasia of animals: 2013 edition. (2013).
Pelletier-Galarneau, M. et al. Quantification of myocardial mitochondrial membrane potential using PET. Curr. Cardiol. Rep. 23, 70 (2021).
Barth, E., Stämmler, G., Speiser, B. & Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 24, 669–681 (1992).
Treibel, T. A. et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J. Cardiovasc. Comput. Tomogr. 9, 585–592 (2015).
Johnson H.J., McCormick M.M., Ibanez, I. The ITK software guide: design and functionality; updated for ITK version 4.7, 4th. Edition. Kitware Inc. (2015).
Cerqueira Manuel, D. et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 105, 539–542 (2002).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing Version 3.6.2. https://www.R-project.org/ (2016).
Bates, D., Mächler, M., Bolker, B., & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, (2015).
Satterthwaite, F. E. Synthesis of variance. Psychometrika 6, 309–316 (1941).
Thavendiranathan, P. et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy. J. Am. Coll. Cardiol. 63, 2751–2768 (2014).
Thavendiranathan, P. et al. Strain-guided management of potentially cardiotoxic cancer therapy. J. Am. Coll. Cardiol. 77, 392–401 (2021).
Hong, Y. J. et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using T1-mapping cardiac magnetic resonance imaging: an animal study. Sci. Rep. 7, 2663 (2017).
Thavendiranathan, P., et al. Regional myocardial edema detected by T2 mapping is a feature of cardiotoxicity in breast cancer patients receiving sequential therapy with anthracyclines and trastuzumab. J. Cardiovasc. Magn. Reson. 16, P273 (2014).
Charbonnel, C. et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Heart J. Cardiovasc. Imag. 18, 392–401 (2017).
Galán-Arriola, C. et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 73, 779–791 (2019).
Wang, G.-X., Wang, Y.-X., Zhou, X.-B. & Korth, M. Effects of doxorubicinol on excitation–contraction coupling in guinea pig ventricular myocytes. Eur. J. Pharmacol. 423, 99–107 (2001).
Tap, W. D. et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 388, 488–497 (2016).
Joerger, M. et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European Organization for Research and Treatment of Cancer-Pharmacology and Molecular Mechanisms Group and New Drug Development Group. Clin. Pharmacokinet. 46, 1051–1068 (2007).
Nicolay, K. & de Kruijff, B. Effects of adriamycin on respiratory chain activities in mitochondria from rat liver, rat heart and bovine heart. Evidence for a preferential inhibition of complex III and IV. Biochim. Biophys. Acta BBA Bioenerg. 892, 320–330 (1987).
Pointon, A. V. et al. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 5, e12733 (2010).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Lyu, Y. L. et al. Topoisomerase II mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 67, 8839–8846 (2007).
Zhu, H. et al. Doxorubicin redox biology: redox cycling, topoisomerase inhibition, and oxidative stress. React. Oxyg. Species Apex NC 1, 189–198 (2016).
Agarwal, B., Camara, A. K. S., Stowe, D. F., Bosnjak, Z. J. & Dash, R. K. Enhanced charge-independent mitochondrial free Ca2+ and attenuated ADP-induced NADH oxidation by isoflurane: implications for cardioprotection. Biochim. Biophys. Acta BBA Bioenerg. 1817, 453–465 (2012).
Lee, J. Y., Jee, H. C., Jeong, S. M., Park, C. S. & Kim, M. C. Comparison of anaesthetic and cardiorespiratory effects of xylazine or medetomidine in combination with tiletamine/zolazepam in pigs. Vet. Rec. 167, 245–249 (2010).
Sivapackiam, J. et al. 68Ga-Galmydar: a PET imaging tracer for noninvasive detection of Doxorubicin-induced cardiotoxicity. PLoS ONE 14, e0215579 (2019).
Safee, Z. M. et al. Detection of anthracycline-induced cardiotoxicity using perfusion-corrected 99mTc sestamibi SPECT. Sci. Rep. 9, 216 (2019).
Acknowledgements
We thank Marina T. Macdonald-Soccorso, Helen Deng, Koushiar Moshe Yaghoubian, Eric J. McDonald, Brittan A. Morris, Zakhar Levin, and Julia-Ann Kaiser for their essential contributions to this project.
Funding
This work was supported in part by NIH grants P41EB022544, R01HL137230, and S10OD018035.
Author information
Authors and Affiliations
Contributions
N.M.A., Y.P., G.E.F., M.P.-G., M.D.N., and J.L.G. designed the study. F.J.D., Y.P., M.D., J.L.G., and M.P.-G performed the experiments. N.J.G., F.X., and P.B. contributed materials and analytical tools. S.M. and T.M.S. synthesized the radiotracer. F.J.D., Y.P., and N.M.A. performed the data processing and analysis and wrote the first version of the manuscript, all other authors contributed to the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
Patent US 11,109,819 B2, ‘System and method for quantitatively mapping mitochondrial membrane potential’ (status: granted) is assigned to The General Hospital Corporation and lists Drs. Alpert, El Fakhri, Guehl and Normandin as inventors. The other authors declare that they have no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Detmer, F.J., Alpert, N.M., Moon, SH. et al. PET imaging of mitochondrial function in acute doxorubicin-induced cardiotoxicity: a proof-of-principle study. Sci Rep 12, 6122 (2022). https://doi.org/10.1038/s41598-022-10004-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10004-6
This article is cited by
-
FUNDC1 protects against doxorubicin-induced cardiomyocyte PANoptosis through stabilizing mtDNA via interaction with TUFM
Cell Death & Disease (2022)
-
Modulation of Apoptosis and Oxidative Stress with Nesfatin-1 in Doxorubicin Induced Cardiotoxicity in Male Rat
International Journal of Peptide Research and Therapeutics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.