Abstract
Maternal tobacco smoke exposure during pregnancy impairs fetal body size, including head circumference (HC) at birth; however, the mechanism still remains unclear. This analysis using a large prospective cohort study evaluated the impact of maternal tobacco exposure on their offspring’s HC and the relationship with placental weight ratio (PWR) and placental abnormalities. Parents-children pairs (n = 84,856) were included from the 104,065 records of the Japan Environmental and Children’s Study. Maternal perinatal clinical and social information by self-administered questionnaires, offspring’s body size, and placental information were collected. Data were analyzed with binominal logistic regression analysis and path analysis. Logistic regression showed significantly elevated adjusted odds ratio (aOR) (1.653, 95% CI 1.387–1.969) for the impact of maternal smoking during pregnancy on their offspring’s smaller HC at birth. Maternal exposure to environmental tobacco smoke in the non-smoking group did not increase aOR for the smaller HC. Path analysis showed that maternal smoking during pregnancy decreased the offspring’s HC directly, but not indirectly via PWR or placental abnormalities. The quitting smoking during pregnancy group did not increase aOR for the smaller HC than the non-smoking group, suggesting that quitting smoking may reduce their offspring’s neurological impairment even after pregnancy.
Similar content being viewed by others
Introduction
Tobacco smoking contains an estimated 5,000 chemicals, including nicotine, and 97 other hazardous components1,2. Smoking is the leading cause of preventable deaths (7 million deaths by direct tobacco use and 1.2 million deaths by second-hand tobacco exposure)3. Nevertheless, tobacco is consumed worldwide with a worldwide mean smoking prevalence of 22.18%, despite of global efforts to control the epidemic of tobacco use4. Tobacco exposure during pregnancy harms maternal health and impairs fetal growth leading to a decreased body size at birth and reduced head circumference (HC)5,6,7,8,9,10. As well as maternal smoking, maternal exposure to environmental tobacco smoke (ETS), which is estimated prevalence of 11.1%6, is reported to be independently associated with smaller HC6,10.
Maternal smoking and second-hand smoke exposure are associated with offspring cognitive and behavioral impairments. There is increasing evidence of attentional deficits, impaired learning and memory, lowered intelligence quotient, cognitive dysfunction, and later childhood conduct problems, although not all studies have reported a significant negative relationship between maternal tobacco exposure and offspring outcomes11,12. Birth HC is an important physical measurement, which is easily accessed and associated with intellectual development13,14,15,16,17. Tobacco exposure during pregnancy may impair cognitive ability18,19,20,21 and increase the risk of attention-deficit hyperactivity disorder22,23, even though the results obtained so far are controversial21.
Maternal smoking is recognized as an unfavorable factor that causes oxidative stress in placental tissue24, and impairs placental development due to reducing blood flow25, leading to a decrease in placental weight26,27 and histological changes28,29 as well as an increased risk of placenta previa30, placenta abruption31, and miscarriage32. These findings suggest that maternal tobacco exposure during pregnancy causes decreased brain size at birth due to placental impairment. However, the evidence on the relationship of prenatal smoking exposure with placental impairment and decreased HC remains unclear in prospective birth cohort studies5,7 and retrospective birth cohort studies6,8. We assessed the hypothesis that prenatal smoking leads to a smaller HC due to placental dysfunctions using a prospective large birth cohort. In addition, we analyzed the association of maternal tobacco exposure during pregnancy and HC at birth with potential covariants, including placental information.
Materials and methods
Study design and participants
The data set in this study was adopted from the Japan Environmental and Children’s Study (JECS). JECS is an ongoing prospective birth cohort study, which was a national project funded directly by the Ministry of Environment to elucidate the influence of environmental factors during the fetal period and early childhood with follow-up until 13 years old. The protocol and baseline data of the JECS were published elsewhere33,34,35. The JECS protocol was approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the ethics committees of all participating institutions; the Medical Support Centre (National Centre for Child Health and Development), and 15 Regional Centers (Hokkaido University, Tohoku University, Fukushima Medical University, Chiba University, Yokohama City University, University of Yamanashi, University of Toyama, Nagoya City University, Kyoto University, Osaka University, Hyogo College of Medicine, Tottori University, Kochi University, University of Occupational and Environmental Health, and Kumamoto University). The JECS was conducted in accordance with the Declaration of Helsinki and other internationally valid regulations and guidelines, and with written informed consent from all participants.
Pregnant women were enrolled between January 2011 and March 2014 under the following inclusion criteria: (1) being resident in any of the 15 Study Areas at the time of recruitment and enrolled with Co-operating health care providers; (2) having an expected delivery date after August 1, 2011; and (3) being capable of comprehending the Japanese language and completing the self-administered questionnaire.
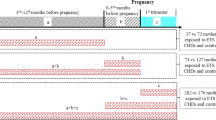
The current study employed the jecs-an-20180131 data set, which was released in March 2018. This data set included 104,065 fetal records. From these 104,065 records, we excluded cases with abortion or stillborn babies (n = 3,921), with multiple births (n = 1,889), with a gestational age before 37 weeks or over 41 weeks of pregnancy (n = 4,656), with newborns physical abnormalities (n = 7,293), with markedly abnormal body measurements at birth (n = 70), and with missing information about sex and body measurements at birth (n = 323) or maternal tobacco exposure (n = 857). Newborns with physical measurements outside the following ranges were excluded from statistical analyses: HC of 20–50 cm, body weight over 1000 g, body height over 30 cm, and chest circumference 20–40 cm. In total, 84,856 parents-children pairs were included in our analyses (Fig. 1).
Data collections
The maternal age, maternal height, maternal body mass index (BMI) (body weight [kg]/height [m]2), maternal parity, gestational hypertension, diabetes mellitus/gestational diabetes mellitus, and placental information were collected from medical records transcripts. Maternal education status, household income, maternal smoking, maternal exposure to ETS, and maternal alcohol exposure were collected by self-administered questionnaires at mid-late pregnancy. The information on maternal smoking exposure was categorized to 1 = never smoked (Never), 2 = quit before pregnancy (Previously did, but quit before recognizing current pregnancy), 3 = quit during pregnancy (Previously did, but quit after finding out current pregnancy), and 4 = smoking during pregnancy (Yes, I still smoke). The information on maternal exposure to ETS was categorized to 1 = seldom, 2 = 1–3 times a week, 3 = 4–7 times a week.
Offspring’s body size at birth was assessed according to the Japanese neonatal anthropometric charts for gestational age at birth36. According to a previous epidemiological study37, the point of smaller HC was determined as HC < 3rd percentile calculated by sex and gestational age in the Japanese standard reference36.
Statistical analysis
Clinical and social characteristics of parent–child participants for each sex were compared using Mann–Whitney U test and chi-squared tests. The outcome variables were cases of smaller HC, and placental abnormalities (placenta previa, abruption placentae, placental calcification, placental infarction, and other placental abnormalities were identified using medical records transcripts). Other placental abnormalities included nuchal cord, low-lying placenta, velamentous insertion, hyper coiled cord, excessively long umbilical cord, multilobate placenta, succenturiate placenta and placenta accreta. The effects of parental smoking and maternal exposure to ETS on smaller HC or placental abnormalities were evaluated as an odds ratio (OR) or adjusted odds ratio (aOR), and 95% confidence interval (CI) using binomial logistic regression analysis with crude and adjusted models. We conducted adjustments for maternal age, height, pre-pregnancy BMI and parity, maternal alcohol drinking during the first to third trimesters, placental weight (g) to birth weight placental weight (g) ratio (placental weight ratio, PWR) which is used to indicate the adequacy of fetal nutrition38, and gestational age.
The relationship of maternal smoking exposure with the offspring’s HC, placental weight, PWR, and the presence of placental anomalies were analyzed with path analysis. For missing values estimation, we used full information maximum likelihood estimation. Model fit was assessed using the root mean square error of approximation (RMSEA) ≤ 0.05 and comparative fit index (CFI) > 0.95. P < 0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics version 23 (IBM Corp. Armonk, NY). Outliers of physical measurements were evaluated with Smirnov‐Grubbs test using R version 3.6.339.
Results
This study enrolled 84,856 parents-child pairs after selection (Fig. 1). The range of outliers generated using the offsprings’ physical measurements as exclusion criteria were similar to those calculated by Smirnov–Grubbs’ test (HC ≤ 24 cm or ≥ 43 cm, body weight ≤ 540 g or ≥ 4890 g, body height ≤ 39.5 cm, and chest circumference ≤ 24 cm or ≥ 39.5 cm).
Comparing the male group’s and the female group’s backgrounds showed no significant differences in maternal body size, parental smoking status, maternal educational status, and household income (Table 1). There were significant differences in offspring body size and placental information between the male and female offspring (Table 1).
Adjusted binominal logistic regression analysis of maternal tobacco exposure for predicting the offsprings’ smaller HC showed a significantly increased aOR for smaller HC only by maternal smoking during pregnancy in the offspring (aOR 1.653, CI 1.387–1.969) (Table 2). Maternal exposure to ETS did not significantly increase aOR for smaller HC in offsprings with “never smoked” mothers (Table 2). Additionally, number of daily cigarettes did not significantly increase aOR for smaller HC in offsprings with “smoking during pregnancy” mothers (Table 2).
We performed adjusted binomial logistic regression analysis of parental smoking for predicting placenta previa, abruption placentae, placental calcification, and placental infarction in total offsprings (Table 3). Maternal smoking during pregnancy increased the aOR of placental calcification and infarction. Especially, placental calcification showed a clear dose–response with maternal smoking. On the other hand, maternal exposure to ETS did not elevate the risk of these placental abnormalities (Table 3).
To evaluate the relationship among maternal smoking, PWR, placental abnormalities, and offspring’s HC, we performed path analysis using path analysis in total offsprings (Fig. 2, Tables 4 and 5). We found that maternal smoking during pregnancy had counteract effect on offspring’s HC at birth. Path analyses showed that maternal smoking directly decreased their offspring’s HC (patho = − 0.027) (Fig. 2A, Tables 4 and 5), but did not indirectly change their offspring’s HC due to placenta previa (Fig. 2B, Tables 4 and 5), abruption placentae (Fig. 2C, Tables 4 and 5), placental calcification (Fig. 2D, Tables 4 and 5) or placental infarction (Fig. 2E, Tables 4 and 5), though R2 for offspring’s HC is low (0.001–0.03). We also tried to include alcohole drinking during pregnancy in the path analysis; however, we could not find a significant model with the covariate.
Path analysis for the relationship between maternal smoking toward offspring’s HC, PWR and placental abnormalities (A, gestation age; B, placenta previa; C, abruption placentae; D, placental calcification; E, placental infarction) showing the standardized estimates with p-values and coefficient of determination (R2). *: p < 0.05, **: p < 0.01, ***: p < 0.001, (n.s.): not significant. ”e” represent the errors. HC, head circumference; PWR, placental weight ratio.
Discussion
We performed logistic regression analysis and path analysis on this cohort birth study to evaluate the impact of parental smoking on their offspring’s HC at birth. Our results showed that maternal smoking during pregnancy directly increased the risk of reduced HC, which was independent of PWR or placental abnormalities.
There have so far been four large cohort studies with over 10,000 participants to evaluate the impact of parental smoking on offspring’s HC5,6,7,8. Our study was the largest-scale study with over 80,000 participants enrolled. A birth cohort study on the Murmansk County Birth Registry in Russia5 reported that maternal smoking increased the risk of reduced HC with an aOR of 1.69 in the 1–5 cigarettes per day, 2.08 in the 5–10 cigarettes per day, and 5.19 in the over ten cigarettes per day groups. Single institutional birth cohorts reported that maternal smoking during pregnancy was associated with HC with an aOR of 1.066 and 1.587. Inoue et al. reported that in non-smoking pregnant women, environmental tobacco exposure was associated with a − 0.24 cm difference in HC8. Meta-analysis studies found maternal active smoking was associated to a smaller HC at birth9,10. The decreased HC by parental smoking exposure was also observed during the fetal period by echographic examination40,41,42,43.
Previous reports found that placental weight was positively associated with HC44,45,46, while some investigators reported that maternal smoking was associated with decreased placental weight26,27. The present study found that maternal smoking was associated with a smaller HC, and a study by Mitsuda et al. using the same dataset found that maternal smoking was associated with increased placental weight47. Using path analysis, we found that maternal smoking was directly associated with a smaller HC, but was not indirectly associated with a smaller HC via PWR or placental abnormalities.
The pathophysiological mechanism of how maternal smoking led to a smaller HC, not due to placental weight or placental abnormalities, remains unclear. The possible serious confounders of a negative effect of maternal smoking in infantile brain development in such an investigation are innumerable48. We speculated the specific negative effect of maternal smoking on offsprings’ HC could be related to a positive association between maternal smoking and premature closure of one or more of the cranial sutures49. There was a possibility that our cohort involved newborns with premature closure of the cranial sutures, even although we excluded offsprings with physical abnormalities.
From another viewpoint, maternal smoking in late pregnancy causes in utero hypoxia and placental insufficiency, which causes the placenta to grow relative to the growing fetus as a compensatory response to provide sufficient oxygen and nutrients to the fetus. Therefore, enhanced angiogenesis and increased development of new vessels are observed in the placentas of women who continued smoking throughout pregnancy50. Maternal smoking during pregnancy was higher BMI and gained more weight during pregnancy, and these factors are also associated with the heavy placenta51. These pathophysiological changes in the placentas may explain intrauterine growth restriction and compensatory normalization of PWR that occurred in women who smoked throughout pregnancy.
Importantly, the group that quit smoking during pregnancy did not have a significantly higher risk of their offspring having a small HC compared to the non-smoking group (Table 2), suggesting that quitting smoking even after pregnancy potentially reduces their offspring’s neurological impairment.
Shobeiri et al. reported that maternal smoking increased the risk of abruption placentae or placenta previa30,31. In our statistical analyses, maternal smoking increased aOR of placental calcification and infarction, while it did not increase aOR of placental previa or abruptio placentae (Table 3). Additionally, maternal exposure to ETS did not increase aOR of these placental abnormalities (Table 3).
MRI-based analyses showed that decreased HC was strongly associated to decreased brain volumes at least in young children52,53, and several studies reported the association between HC and risk of intellectual disability, autism spectrum disorder, and attention-deficit hyperactivity disorder13,14,15,16,17. HC at birth has a positive relationship with higher intelligence quotient13,15, lower risk of attention-deficit hyperactivity disorder14, and neurocognitive disorder16. Hence, HC at birth is a useful neurological biomarker.
Our birth cohort study secondly found the smoking prevalence among Japanese women of reproductive age. In our study, 57% of 85,059 mothers selected “never smoked” in the self-administered questionnaires. The smoking prevalence among Japanese women aged 20–39 in the 2010s has been reported 6.8–16.9%54 and 16.5%55. The smoking prevalence reported by previous studies reflected the current smoking status of women of reproductive age, while our study showed that the prevalence of experienced smoker among Japanese women of reproductive age was higher than 40%. The difference of prevalence values may suggest the young women have many smoking opportunities than expected.
This study has some limitations. First, our exclusion criteria for remarked abnormal in body size at birth was determined according to our original exclusion criteria, not to previous epidemiological methodological studies, because this study is aimed to evaluate risk factor for small HC. At least, the range of outliers generated using the offsprings’ physical measurements as exclusion criteria were similar to those calculated by Smirnov-Grubbs’ test.
Second, intrauterine tobacco exposure was assessed by self-administered questionnaires and not assessed with chemical biomarkers. We could not completely rule out the possibility that mothers respond negatively and incorrectly to answer to “did you smoke during pregnancy?” out of shame or knowing the harm to fetal health of smoking during pregnancy. Using self-reports may have introduced misclassification mainly due to underreporting of cigarette consumption, which could lead to underestimation of the effects. Although the concentration of carbon monoxide, thiocyanate, or nicotine metabolites as cotinine (especially in meconium)56 are potential biomarkers for tobacco exposure, these have a short half-life. The ideal chemical biomarker for parental tobacco exposure has not yet been established43,57.
Finally, our study did not include a neurodevelopmental test or neuroimaging examination. Quantitative brain morphometry using brain magnetic resonance imaging would contribute to a more detailed and quantitative analysis of the associations between intrauterine tobacco exposure and brain morphology. There are a few possibilities of the presence of undetected brain ischemic changes in infants.
In conclusion, our statistical analyses on a large birth cohort data revealed that maternal smoking during pregnancy decreased offspring’s HC independent of placental weight changes or placental abnormalities.
Abbreviations
- CI:
-
Confidence interval
- CFI:
-
Comparative fit index
- EST:
-
Environmental tobacco smoke
- HC:
-
Head circumference
- JECS:
-
The Japan Environment and Children’s Study
- OR:
-
Odds ratio
- PWR:
-
Placental weight ratio
- RMSEA:
-
Root mean square error of approximation
References
Talhout, R. et al. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 8, 613–628 (2011).
Clifford, A., Lang, L. & Chen, R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicol. Teratol. 34, 560–570 (2012).
World Health Organization. WHO report on the global tobacco epidemic 2019 (https://www.who.int/tobacco/global_report/en/).
Gravely, S. et al. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: An association study. Lancet Public Health. 2, e166-174 (2017).
Källén, K. Maternal smoking during pregnancy and infant head circumference at birth. Early Hum. Dev. 58, 197–204 (2000).
Crane, J. M., Keough, M., Murphy, P., Burrage, L. & Hutchens, D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG 118, 865–871 (2011).
Kharkova, O. A., Grjibovski, A. M., Krettek, A., Nieboer, E. & Odland, J. Ø. Effect of smoking behavior before and during pregnancy on selected birth outcomes among singleton full-term pregnancy: A murmansk county birth registry study. Int. J. Environ. Res. Public Health. 14, 867 (2017).
Inoue, S. et al. Impact of maternal and paternal smoking on birth outcomes. J. Public Health (Oxf). 39, 1–10 (2017).
Quelhas, D. et al. The association between active tobacco use during pregnancy and growth outcomes of children under five years of age: A systematic review and meta-analysis. BMC Public Health 18, 1372 (2018).
Salmasi, G., Grady, R., Jones, J., McDonald, S. D. & Knowledge Synthesis Group. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 89, 423–441 (2010).
Hall, B. J. et al. Cognitive and behavioral impairments evoked by low-level exposure to tobacco smoke components: Comparison with nicotine alone. Toxicol. Sci. 151, 236–244 (2016).
Knopik, V. S. Maternal smoking during pregnancy and child outcomes: Real or spurious effect?. Dev. Neuropsychol. 34, 1–36 (2009).
Bach, C. C., Henriksen, T. B., Larsen, R. T., Aagaard, K. & Matthiesen, N. B. Head circumference at birth and school performance: A nationwide cohort study of 536,921 children. Pediatr. Res. 87, 1112–1118 (2020).
Aagaard, K., Bach, C. C., Henriksen, T. B., Larsen, R. T. & Matthiesen, N. B. Head circumference at birth and childhood developmental disorders in a nationwide cohort in Denmark. Paediatr. Perinat. Epidemiol. 32, 458–466 (2018).
Aagaard, K., Matthiesen, N. B., Bach, C. C., Larsen, R. T. & Henriksen, T. B. Head circumference at birth and intellectual disability: A nationwide cohort study. Pediatr. Res. 87, 595–601 (2020).
Wright, C. M. & Emond, A. Head growth and neurocognitive outcomes. Pediatrics 135, e1393-1398 (2015).
Gale, C. R., O’Callaghan, F. J., Bredow, M. & Martyn, C. N. Avon Longitudinal Study of Parents and Children Study Team The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 118, 1486–1492 (2006).
Julvez, J. et al. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int. J. Epidemiol. 36, 825–832 (2007).
O’Callaghan, F. V. et al. Is smoking in pregnancy an independent predictor of academic difficulties at 14years of age? A birth cohort study. Early Hum. Dev. 86, 71–76 (2010).
Martin, R. P., Dombrowski, S. C., Mullis, C., Wisenbaker, J. & Huttunen, M. O. Smoking during pregnancy: Association with childhood temperament, behavior, and academic performance. J. Pediatr Psychol. 31, 490–500 (2006).
Polańska, K., Jurewicz, J. & Hanke, W. Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment—A review of epidemiological studies. Int. J. Occup. Med. Environ. Health. 28, 419–443 (2015).
Thakur, G. A. et al. Maternal smoking during pregnancy and ADHD: A comprehensive clinical and neurocognitive characterization. Nicotine Tob. Res. 15, 149–157 (2013).
Milberger, S., Biederman, J., Faraone, S. V. & Jones, J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: Findings from a high-risk sample of siblings. J. Clin. Child Psychol. 27, 352–358 (1998).
Aycicek, A., Varma, M., Ahmet, K., Abdurrahim, K. & Erel, O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur. J. Pediatr. 170, 645–651 (2011).
Zdravkovic, T., Genbacev, O., McMaster, M. T. & Fisher, S. J. The adverse effects of maternal smoking on the human placenta: A review. Placenta 26 (Suppl A), S81-86 (2005).
Abdullah, B. et al. Pregnancy outcome and cord blood cotinine level: A cross-sectional comparative study between secondhand smokers and non-secondhand smokers. Eur. J. Obstet. Gynecol. Reprod. Biol. 214, 86–90 (2017).
Wang, N. et al. The effect of maternal prenatal smoking and alcohol consumption on the placenta-to-birth weight ratio. Placenta 35, 437–441 (2014).
Heidari, Z., Mahmoudzadeh-Sagheb, H. & Sheibak, N. Placenta structural changes in heavy smoking mothers: A stereological aspect. Curr. Med. Res. Opin. 34, 1893–1897 (2018).
Ganer Herman, H. et al. The effects of maternal smoking on pregnancy outcome and placental histopathology lesions. Reprod. Toxicol. 65, 24–28 (2016).
Shobeiri, F. & Jenabi, E. Smoking and placenta previa: A meta-analysis. J. Matern. Fetal Neonatal. Med. 30, 2985–2990 (2017).
Shobeiri, F., Masoumi, S. Z. & Jenabi, E. The association between maternal smoking and placenta abruption: A meta-analysis. J. Matern. Fetal Neonatal. Med. 30, 1963–1967 (2017).
Mishra, G. D., Dobson, A. J. & Schofield, M. J. Cigarette smoking, menstrual symptoms and miscarriage among young women. Aust. N. Z. J. Public Health. 24, 413–420 (2000).
Japan Environment and Children’s Study (JECS) Study Protocol, ver.1.4, 2016 (http://www.env.go.jp/chemi/ceh/en/about/advanced/material/jecs-study_protocol_14_en.pdf).
Ishitsuka, K. et al. Japan Environment and Children’s Study: Backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med. 22, 61 (2017).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28, 99–104 (2018).
Itabashi, K., Miura, F., Uehara, R. & Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 56, 702–708 (2014).
Villar, J. et al. international standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384, 857–868 (2014).
Grandi, C., Cardoso, V., Veiga, A., Mazzitelli, N. & Cavalli, R. Measures of placental growth in relation to birthweight in a latin american population. Rev. Bras. Obst. Ginecol. 38, 373–380 (2016).
Ihaka, R., Gentleman, R. R: a language for data analysis and graphics. J Comp Graph Stat. 5, 299–314 (1996). (Available at http://www.R-project.org.)
Gale, C. R., Walton, S. & Martyn, C. N. Foetal and postnatal head growth and risk of cognitive decline in old age. Brain 126, 2273–2278 (2003).
Roza, S. J. et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur. J. Neurosci. 25, 611–617 (2007).
Jaddoe, V. W. et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: The generation R study. Am. J. Epidemiol. 165, 1207–1215 (2007).
Zhou, S. et al. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr. Probl. Pediatr. Adolesc. Health Care. 44, 219–241 (2014).
Soliman, A. T. et al. Placental weight: Relation to maternal weight and growth parameters of full-term babies at birth and during childhood. J. Trop. Pediatr. 59, 358–364 (2013).
Matthiesen, N. B. et al. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation 134, 1546–1556 (2016).
Sivarao, S. et al. Weight, volume and surface area of placenta of normal pregnant women and their relation to maternal and neonatal parameters in Malay. Chinese and Indian ethnic groups. Placenta 23, 691–696 (2002).
Mitsuda, N. et al. Japan Environment and Children’s Study (JECS) Group. Association between maternal active smoking during pregnancy and placental weight: The Japan environment and Children’s study. Placenta 94, 48–53 (2020).
Lassen, K. & Oei, T. P. Effects of maternal cigarette smoking during pregnancy on long-term physical and cognitive parameters of child development. Addict. Behav. 23, 635–653 (1998).
Källén, K. Maternal smoking and craniosynostosis. Teratology 60, 146–150 (1999).
Pfarrer, C., Macara, L., Leiser, R. & Kingdom, J. Adaptive angiogenesis in placentas of heavy smokers. Lancet 354, 303 (1999).
Roland, M. C. et al. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PLoS ONE 9, e87303 (2014).
Lange, N., Froimowitz, M. P., Bigler, E. D. & Lainhart, J. E. Brain Development Cooperative Group. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev. Neuropsychol. 35, 296–317 (2010).
Rivkin, M. J. et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics 121, 741–750 (2008).
Okui, T. An age-period-cohort analysis of the difference in smoking prevalence between urban and non-urban areas in Japan (2004–2019). Epidemiol. Health. 42, e2020072 (2020).
Tomioka, K., Kurumatani, N. & Saeki, K. The association between education and smoking prevalence, independent of occupation: A nationally representative survey in Japan. J. Epidemiol. 30, 136–142 (2020).
Ostrea, E. M., Knapp, D. K., Romero, A., Montes, M. & Ostrea, A. R. Meconium analysis to assess fetal exposure to nicotine by active and passive maternal smoking. J. Pediatr. 124, 471–476 (1994).
Di Franza Aligne, C. A. & Weitzman, M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics 113, 1007–1015 (2004).
Acknowledgements
We thank the study participants and the officers and staff of the participating institutions at the JECS. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese government.
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese government.
Author information
Authors and Affiliations
Consortia
Contributions
T.S., A.H., M.Y., and K.S. was responsible for study design. A.H., M.Y., and R.T. analyzed data. T.S., A.H., and K.F. wrote/edited the manuscript. N.S. and C.M. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author (s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shiohama, T., Hisada, A., Yamamoto, M. et al. Decreased head circumference at birth associated with maternal tobacco smoke exposure during pregnancy on the Japanese prospective birth cohort study. Sci Rep 11, 18949 (2021). https://doi.org/10.1038/s41598-021-98311-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98311-2
This article is cited by
-
Prenatal Exposure to Tobacco and Childhood Cognition and Behavior: Effect Modification by Maternal Folate Intake and Breastfeeding Duration
Child Psychiatry & Human Development (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.