Abstract
Only a minority of cases of differentiated thyroid carcinoma (DTC) have a poor clinical outcome. Clinical outcomes and molecular aspects were assessed in: 144 DTC ≤ 40 mm without distant metastases (group 1); 50 DTC > 40 mm without distant metastases (group 2); and 46 DTC with distant metastases (group 3). Group 3 had a worse outcome than the other two groups: during the follow-up, patients more frequently had persistent disease, died, or underwent further treatment. The outcomes did not differ between groups 1 and 2. Group 3 had a higher prevalence of TERT promoter mutations than group 2 (32.6% vs 14%). Group 1 had a higher frequency of BRAF mutations than groups 2 or 3 (61.1% vs 16.0% and 26.1%, respectively), while RAS mutations were more common in group 2 than in groups 1 and 3 (16.0% vs 2.1% and 6.5%, respectively). Groups 1 and 2 shared the same outcome, but were genetically distinct. Only lymph node involvement, distant metastases, older age and (among the molecular markers) TERT promoter mutations were independent predictors of a worse outcome. Metastatic DTC had the worst outcome, while the outcome was identical for large and small non-metastatic DTC, although they showed different molecular patterns. TERT promoter mutations emerged as an independent factor pointing to a poor prognosis.
Similar content being viewed by others
Introduction
Differentiated thyroid cancer (DTC), which includes papillary and follicular thyroid cancer (PTC and FTC, respectively), is the most common endocrine malignancy, accounting for about 3.1% of cancers in 2018. Its worldwide incidence has been increasing rapidly in the last four decades, and the age-standardized mortality rate for DTC is estimated at about 0.42 per 100,000 population a year1,2.
DTC is characterized by an excellent prognosis, with a 5-year overall survival rate of about 97% for PTC, and 89% for FTC. Some patients nonetheless develop aggressive tumors with poor clinical outcomes. The most important clinical and pathological features conferring a more aggressive phenotype are: age at diagnosis; primary tumor size; soft tissue invasion; and distant metastases (found in about 2–5% of cases)3,4,5. These variables, and other risk factors are weighted differently in the numerous staging systems available (such as the European Organization for Research and Treatment of Cancer [EORTC]; the Age, Grade, Extent, Size [AGES]; the Age, Metastases, Extent, Sex [AMES]; the Metastases, Age, Completeness of resection, Invasion, Size [MACIS] criteria; the Memorial Sloan Kettering Cancer Center [MSKCC] or the National Thyroid Cancer Treatment Cooperative Study [NTCTCS] systems), depending on the prognostic importance attributed to them, but none of these approaches have proved clearly superior. The 2009 version of the American Thyroid Association’s guidelines strongly support risk classification, considering not only the mortality risk, but also the risk of recurrent and persistent structural disease. The latter risk has a greater impact in thyroid cancer management, given that disease-specific mortality is usually low. The American Joint Committee on Cancer (AJCC) staging system was developed primarily to predict mortality risk, however, and (even now in its 8th edition) tumor extension and distant metastases have remained as prognostic factors over the years, while the age threshold of prognostic significance has shifted up to 55 years6,7,8. In the TNM classification, a larger tumor size, in the absence of signs of its extension into other tissues, is not considered a negative prognostic factor for survival. Some risk classifications (like the MSKCC, AGES and MACIS) suggest otherwise, and some large studies have shown that size can affect patient survival4,9,10,11, and may be a predictor of distant metastasis12. In their recent monocentric series of 5897 patients with a median follow-up of 177 months, Ito et al. found cancer > 4 cm in size an independent predictor of a worse cause-specific survival (CSS), together with metastatic disease and older age4. In short, there is still uncertainty about the influence of tumor size on patient outcome and survival, and large tumors have not been sufficiently investigated in the literature.
In the last few years, much effort has gone into characterizing the molecular drivers responsible for the onset of DTC in an attempt to clarify patients’ prognosis. The mutations most often considered concern BRAF and RAS, and—more recently—the telomerase reverse transcriptase (TERT) promoter. A great body of evidence has demonstrated that TERT promoter mutations in DTC are associated with a higher stage at diagnosis, and with distant metastases (even when they are only discovered at cytology)13,14,15. More importantly, TERT promoter mutations are an independent factor for predicting patient mortality16 and disease-free survival (DFS)13,15,17.
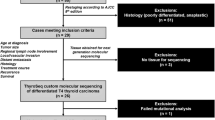
A greater knowledge of the clinical, pathological and molecular features of DTC might improve the diagnostic frame and lead to customized therapies. On these premises, the aims of the present single-center, retrospective study were: (1) the clinical, pathological and molecular characterization (based on BRAF, RAS, TP53, PTEN, PIK3CA genes, and TERT promoter analysis) of selected cases of DTC with or without distant metastases, grouped by primary tumor size: group 1 included patients with tumors ≤ 40 mm in size and no distant metastases; group 2 included patients with tumors > 40 mm in size, with no distant metastases; and group 3 included patients with metastatic DTC, regardless of tumor size.
Results
Clinical and pathological characteristics, and outcomes
The clinical characteristics of the three groups are summarized in Table 1. The sample of patients included: 144/240 (60%) in group 1, 50/240 (20.8%) in group 2, and 46/240 (19.2%) in group 3.
The patients in group 3 were more frequently males (p < 0.01), and were older than those in the other two groups (p < 0.01). Group 3 included a higher proportion of cases of widely invasive FTC (WI-FTC), with 72.7% in group 3 and 37.5% in group 2 (there were no cases of FTC in group 1). Patients in group 3 were also more likely to undergo a second treatment (80.0% versus 9.0% in group 2, and 5.6% in group 1, p < 0.01), and to have a worse outcome, with higher rates of persistence/recurrence or death (78.2% versus 6.0% in group 2, and 4.9% in group 1, p < 0.01). Patients with metastatic tumors (group 3) had a shorter DFS (p < 0.01) (Fig. 1), and a worse CSS (p < 0.01) (Fig. 2) then the other two groups. No differences in patient outcomes emerged between groups 1 and 2.
Disease-free survival in the three groups (p < 0.01), MedCalc Statistical Software version 19.1.7 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Cause-specific survival in the three groups (p < 0.01), MedCalc Statistical Software version 19.1.7 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Molecular characteristics
The molecular characteristics of the three groups are summarized in Table 2.
BRAF mutations
BRAF mutations were detected in 88/144 (61.1%) of patients in group 1, 8/50 (16.0%) in group 2, and 12/46 (26.1%) in group 3 (p < 0.01). The BRAF mutation was significantly more frequent in group 1 than in the other two groups (p < 0.01). The type of BRAF mutation was related to the histotype: BRAF V600E was detected in all but one PTC, while BRAF K601E was the only BRAF mutation found in cases of FTC.
RAS mutations
RAS mutations were detected in 3/144 (2.1%) cases in group 1, 8/50 (16.0%) in group 2, and 3/46 (6.5%) in group 3. They were more common in large, non-metastatic tumors than in the other two groups (p = 0.006).
TERT promoter mutations
TERT promoter mutations were detected in 5/133 (3.8%) patients in group 1, 7/50 (14.0%) in group 2, and 15/46 (32.6%) in group 3, and the rising frequency from group 1 to group 3 was statistically significant (p < 0.01). The TERT promoter showed the C228T mutation in 23/27 cases (85.2%), and the C250T mutation in 4/27 (14.8%).
As concerns the histotype, 17/27 (62.9%) patients with a TERT promoter mutation had PTC, while the other 10/27 (37.1%) had FTC.
Among the 27 patients with TERT mutations, 8 (29.6%) also had BRAF mutations, and 4/27 (14.8%) also had RAS mutations. In one case (of WI-FTC), we found a homozygous TERT promoter mutation together with BRAFK601E and PIK3CA mutations (this case had been reported in 201118, but TERT analysis had not been done at the time). A combination of TERT and PTEN mutations was also found in one case of large FTC without distant metastases.
TP53, PTEN and PIK3CA mutations
A TP53 mutation was found on exon 6 in only 1/133 patients (0.8%) in group 1 (G293R), and in 1/44 (2.3%) in group 2 (D208N). PTEN mutations were detected on exon 5 in 1/50 patients (2%) in group 2 (D93N). PIK3CA mutations were detected in 1/43 patients (2.3%) in group 2 (R516K) who had PTC, and in 2/40 (5.0%) of patients in group 3 (one carrying an E545A mutation, the other carrying a H1047R mutation), both cases of WI-FTC.
Clinical and molecular correlations
In the series as a whole, and within the three groups, RAS, PTEN, TP53 and PIK3CA mutations were not associated with patient outcomes.
BRAF mutations were also unassociated with outcomes in the series as a whole, but in group 1 they were weakly associated with a worse prognosis. Indeed, of the 137 patients with a remission or indeterminate response at the end of the follow-up, 81 (59.1%) were BRAF-mutated, as opposed to 7/7 (100%) with persistence of disease (p = 0.03).
BRAF was not associated with outcome in groups 2 or 3.
In the series as a whole, TERT-mutated patients were older at diagnosis (63 versus 47 years, p < 0.01), and had larger tumors (44 versus 16 mm, p < 0.01), and a greater tumor extension (T4: 11.5% versus 3.5%, p < 0.01). They more frequently had distant metastases (53.8% versus 15.3%, p < 0.01), and their disease was more advanced (Stage IV 40.7% versus 9.9%, p < 0.01). Accordingly, TERT promoter mutations was the only molecular event statistically associated with patient outcomes. TERT-mutated patients more frequently needed a second treatment (14/27 [51.8%] TERT-positive versus 34/202 [16.8%] TERT-negative patients mutated; p < 0.01), and had a worse outcome: a biochemical or structural incomplete response or disease-related death occurred in 28/202 patients (13.9%) without TERT promoter mutations as opposed to 18/27 (66.7%) TERT-mutated patients (p < 0.01). They also had a shorter DFS (p = 0.002).
Whether alone or in association with other molecular events, TERT promoter mutations correlated with the worst outcomes: they were detectable in 18/46 patients (39.1%) with persistent disease, and only in 9/183 patients (4.9%) with an excellent response (p < 0.01).
At univariate analysis, male sex, older age at diagnosis, type of cancer, TNM parameters, tumor stage, need for a second treatment, and TERT mutations were all associated with a worse outcome (p < 0.01).
At multivariate analysis, only TERT promoter mutations (odds ratio [OR] 8.7997; 95% confidence interval [CI] 1.9877–38.9567), age (OR: 1.0420; 95% CI 1.0023–1.0814), lymph node involvement (OR 4.2993; 95% CI 1.2530–14.7517), FTC histology (OR 1.6232; 95% CI 0.381–7.7926), and distant metastases (OR 40.3925; 95% CI 13.5846–120.1033) emerged as independent factors for a poor prognosis (p < 0.01) (Table 3).
TERT promoter mutations also influenced outcomes for patients in groups 2 and 3. In group 2, patients with persistent disease or disease-related death were more frequently TERT-mutated than patients in remission or an indeterminate status: 2/3 (66.7%) versus 5/47 (10.6%), respectively (p = 0.0073). In group 3, none of the patients with a biochemical remission or indeterminate response (0/10) were TERT-mutated, while 41.7% of the patients with TERT promoter mutations were among those with a worse prognosis (15/36) (p = 0.01).
Discussion
Although the mortality rate for DTC has remained stable, its incidence has been rising rapidly in recent decades due to the increasing use of neck ultrasound for thyroid diseases and other, unrelated conditions7,19,20,21,22,23. Many authors have focused on defining clinical, pathological and molecular characteristics of DTC useful for the purposes of identifying the minority of aggressive tumors associated with a higher likelihood of progression or death. This would enable customized treatment decisions and follow-up protocols, possibly right from the time of diagnosis. With this in mind, various classification systems have been proposed, such as the TNM classification (useful for predicting the risk of death) and the ATA grouping score (suitable for establishing the risk of persistence/recurrence)6,8. From a clinical standpoint, the presence of distant metastases and a tumor size greater than 40 mm have conventionally been associated with a poor prognosis, warranting radical surgery associated to radioactive iodine (RAI) therapy6,7. The impact of tumor size per se on patient mortality is still debated, however.
Given these premises, we examined a group of cases with distant metastases (regardless of primary tumor diameter), and a group with non-metastatic DTC larger than 40 mm in size, seeking to establish whether these forms of DTC, which are known to be more aggressive, share similar molecular features and clinical courses. We compared the characteristics of these “high-risk” groups with those of a group of cases of DTC not exhibiting these two features of aggressiveness. All patients had been diagnosed, treated and followed up according to the same standards of care at the same institution.
As expected, our findings confirm the impact of distant metastases on a patient’s clinical outcome24, as metastatic disease is associated with a significantly worse DFS and CSS. None of the patients without metastases died of their disease regardless of the size of their DTC. The presence of distant metastases was also the most influential independent prognostic factor in terms of poor prognosis, with an OR of around 40.0. Patients with distant metastases, were more often male, older at the time of their diagnosis, with lymph node involvement. They were more likely to need of a second treatment, and they had the worst outcome. Patients with large, but non-metastatic DTC shared the same outcome as those with small non-metastatic lesions. In other words, a large tumor size was not a risk factor in our series when considered alone. A shorter median follow-up for the group with large but not metastatic tumors may have influenced this result, although the 4.3-year median follow-up for this group should have been long enough for most relapses to become apparent25.
In recent years, several studies have suggested that certain molecular events—mainly BRAF, RAS, TP53 and TERT promoter mutations, alone or in combination—may be useful for stratifying the behavior of DTC, and predicting which tumors are more likely to recur and/or be fatal26,27,28.
The BRAF V600E mutation is the most common molecular event in DTC (found in 40–60% of cases). Its presence has been historically associated with less-differentiated phenotypes carrying a poor prognosis, but its role is still debated29,30,31,32,33. The most important multicenter retrospective studies on the role of BRAF in DTC found it associated with key negative prognostic factors. Xing et al. reported, for instance, that it was not independently associated with cancer-related death, but it was independently associated with disease persistence/recurrence34,35. Some monocentric retrospective studies confirmed the BRAF mutation as an independent negative prognostic factor related to persistent disease36, and to aggressive characteristics such as loss of the ability to concentrate radioiodine, or acquisition of a capacity for glucose uptake37. On the other hand, prospective studies on patients who underwent fine needle aspiration biopsy for molecular definition and total thyroidectomy failed to confirm an role of BRAF mutations on patient outcomes38,39.
These different findings may stem from the fact that, particularly in the setting of prospective studies, the early detection of a BRAF mutation (particularly at cytology) prompts early surgery, so this genetic alteration may not have the time to confer the aggressive tumor characteristics found in retrospective studies. We confirmed the published data on the prevalence of BRAF mutations34, but only in our group 1 (61.1%), while it was markedly lower in groups 2 and 3 (16.0% and 26.1%, respectively). This is in line with reports on other metastatic DTC series in which this genetic event was not associated with the tumor’s potential to spread to distant sites34,40. In short, the mutation does not give the cancer cells a metastatic advantage. Our study has a possible bias, however, regarding the lack of FTCs among the consecutively-selected cases in group 1. This could have increased the frequency of BRAF mutations in group 1 by comparison with the other groups, since this mutation is uncommon in FTCs41.
Our data revealed a higher prevalence of the RAS mutation than reported elsewhere in the literature34,42,43, particularly in our group 2 (16.0% of cases revealed RAS mutations). We found no association between these mutations and the patients’ clinical features, probably because of the small size of this sample.
TERT promoter mutations in DTC were first described in 201344,45. The literature has since consistently demonstrated an association between TERT promoter mutations and poor outcomes. This mutation in DTC patients is associated with older age, larger tumors, distant metastases, and advanced stage at diagnosis13,16,17. In our series too, the frequency of TERT promoter mutations rose with DTC aggressiveness and risk of progression: it was 3.8% group 1, 14.0% in group 2, and 32.6% in group 3.
In line with previous reports, TERT promoter mutations in our series as a whole were associated with older age at diagnosis, more advanced tumor stage, more frequent need for a second treatment, and worse outcomes (around 67% of patient had persistent disease or died of their disease). At multivariate analysis, TERT promoter mutations was the only variable independently associated with a negative prognosis.
There are reports in the literature on associations between TERT mutations and other oncogenic molecular events. This condition was found related to an aggressive DTC behavior, including distant metastases and a lack of radioiodine uptake capability13,37,45. We found no such negative impact of simultaneous mutations on the prognosis for patients with DTC, by comparison with those harboring a single TERT promoter mutation. It could be useful to consider larger series in order to clarify the real impact of multiple mutations. The other molecular events considered here—PIK3CA, PTEN and TP53 mutations—seem to be rare, and almost exclusive to high-risk DTC, as previously reported17. It is worth noting that the presence of a TERT promoter mutation was able to influence the prognosis even in our group 3, with metastatic disease.
It has been reported that age, tumor size, extrathyroidal extension, and nodal and distant metastases are predictors of a patient’s mortality risk46,47,48,49,50,51. Our data, focusing on tumor size and distant metastases, only confirm the latter as a predictor of mortality.
It is noteworthy that patients with larger DTCs shared much the same outcome as those with smaller lesions, though they differed considerably from a molecular standpoint. RAS and TERT mutations were more common in group 2, while BRAF mutations were more prevalent in group 1. Group 2 had a molecular profile more similar to that of group 3, except for a higher frequency of RAS mutations in the former. TERT promoter mutations were more frequent than in group 1, but still lower than in metastatic patients. Based on our results and the conflicting data in the literature regarding its prognosis, we wonder whether large DTCs may be a sort of intermediate entity, in between small indolent DTCs and those with a poor outcome. Our data suggest a shift in the molecular profile from DTC < 4 cm in size to metastatic tumors, with large DTCs seeming to come somewhere in between, in terms of their molecular features, although they have much the same outcomes as the smaller, non-metastatic tumors. It may be that many other molecular events need to accumulate in large tumors to have any metastatic potential and affect the prognosis. The clinical importance of cancer size is still debated. Most authors have observed an indolent behavior of microcarcinomas52, but some have reported distant metastases already at diagnosis or during the follow-up, and a poor prognosis even for tumors less than 10–15 mm in size53,54,55. Little is known as yet about the molecular patterns of such small but aggressive DTCs. In our series, the median size of tumors in group 3 (with metastatic disease) was smaller than in group 2, and ranged very widely, from 2 up to 90 mm, although their molecular pattern and other characteristics associated with poor outcome (lymph node involvement) were very different. These data suggest that some DTCs are inherently aggressive, already developing aggressive mutations even while they remain small in size.
A better knowledge of the molecular pattern of advanced thyroid disease could help us to identify promising therapeutic targets for advanced DTC. The treatment of choice for metastatic thyroid cancer is RAI therapy, but as DTC progresses, it can lose its capacity for iodine uptake56, becoming refractory to RAI. Other therapies involving tyrosine kinase inhibitors can be attempted in such cases. The loss of iodine uptake capacity in some thyroid cancers has been associated with involving BRAF V600E57 or TERT promoter mutations, or both58. Phase II trials with the BRAF V600E inhibitors vemurafenib59 and dabrafenib60 (two FDA-approved drugs for treating BRAF V600E-mutated melanoma) obtained promising results in thyroid cancers with this mutation. Vemurafenib also proved capable of restoring radio-iodine uptake, to some degree at least61. As discussed earlier, however, few cases of metastatic thyroid disease are associated with BRAF V600E mutations, so other therapeutic targets are needed. Given the frequency of its mutations in metastatic cancers, and their prognostic role in thyroid cancer, the TERT promoter could be a future therapeutic target. Telomerase inhibitors (e.g. Imetelstat62), drugs inducing telomere dysfunction (e.g. 6-thio-2′-deoxyguanosine63), and adenoviral gene therapies that induce telomerase promoter-driven oncolytic activity64 are currently under investigation as potential anticancer agents. Based on the latest literature (as confirmed by the present study), telomerase could be a promising therapeutic target for RAI-refractory thyroid cancer, but no data are available as yet.
Our study confirms the prognostic impact of distant metastases, but not primary cancer size on patient outcomes. From the available literature, it is still hard to say whether large tumor size has an impact on patients’ prognosis. Our data suggest that larger DTCs differ in their molecular features from smaller ones, but are not more aggressive. We found a higher prevalence of TERT promoter mutations in patients with metastatic disease. This mutation confirmed its association with tumor aggressiveness, and was the only molecular event capable of significantly and independently influencing DTC outcome.
Methods
Patients
We selected 240 consecutive patients with a histological diagnosis of DTC who underwent total thyroidectomy from 2007 to 2016, and were followed up by the Endocrinology and Radiotherapy Units in Padua. We first ensured that adequate frozen material was available in the Tissue Bank after first surgery for all cases. Then patients were grouped as follows: group 1, DTC with the largest tumor focus ≤ 40 mm, without distant metastases; group 2, DTC with the largest tumor focus > 40 mm, without distant metastases; and group 3, metastatic DTC at diagnosis or detected during the follow-up, regardless of tumor size.
Clinical data were obtained from the electronic medical records. Surgical pathology specimens were analyzed by two expert pathologists (FG and GP). All pathological samples were reviewed and a histological diagnosis was established following the 4th edition of the WHO classification (WHO-2017)65. Pathological staging was done according to the 8th edition of the TNM staging system8. If there were multiple foci, the largest tumor dimension was considered.
All patients underwent total thyroidectomy and RAI treatment (median dose 200 mCi). When further treatments were indicated, these involved surgery, RAI treatments, external beam radiation, bisphosphonates (in cases of multiple bone lesions), and tyrosine kinase inhibitors (in cases of progressive metastatic RAI-refractory disease).
Following recent ATA guidelines6, we identified four possible outcomes, defining response as: excellent; biochemical incomplete; structural incomplete; or indeterminate. The median patient follow-up was 79.8 months (12.9–237 months).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All patients participating in the study gave their written informed consent. The Ethical Committee for Clinical Experimentation at Padua Hospital approved the study protocol (Ref. 121).
DNA extraction and mutation analysis
Genomic DNA was extracted from frozen tissues after surgery using the DNeasy Blood and Tissue kit (Qiagen, Italy), according to the manufacturer’s protocol. Mutation analyses were performed in all patients for BRAF (NM_004333.4). and for N-RAS (NM_002524.3; exons 2 and 3), K-RAS (NM_033360.2; exons 2 and 3) and H-RAS (NM_005343.2; exons 2 and 3). TP53 (exons 5, 6,7 and 8) in 133/144 (92.4%) patients in group 1, 44/50 (88%) in group 2, and 44/46 (95.6%) in group 3. PTEN (exons 5, 7 and 8) was tested in all patients in groups 1 and 2, and in 45/46 (97.8%) in group 3. PIK3CA (exons 9 and 20) was tested in all patients in group 1, 43/50 (86.0%) in group 2, and 40/46 (86.9%) in group 3. The TERT proximal promoter (NM_198253.2) was tested in 133/144 patients (92.4%) in group 1, and in all patients in groups 2 and 3, by direct sequencing (ABI PRISM 3130, Applied Biosystems, Foster City, CA), as previously described elsewhere66,67.
Statistical analysis
Categorical data were summarized using frequencies and percentages. Distributions of the continuous variables were assessed, and data were summarized accordingly. The comparison of continuous variables (age at diagnosis, tumor size, follow-up) between the three groups was done with the Kruskal–Wallis test. Group comparisons of the categorical variables were done with the χ2 test. A p < 0.05 was considered statically significant. DFS and CSS were calculated with the Kaplan–Meier method. A multivariate analysis was conducted with a logistic regression analysis. The Med Calc software version 19.1.7 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020) was used for the statistical analysis.
References
Global Cancer Observatory. https://gco.iarc.fr/. (Accessed 7 May 2020)
La Vecchia, C. et al. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 136, 2187–2195 (2015).
Hay, I. D. et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J. Surg. 26, 879–885 (2002).
Ito, Y. et al. Overall survival of papillary thyroid carcinoma patients: A single-institution long-term follow-up of 5897 patients. World J. Surg. 42, 615–622 (2018).
Lo, C. Y., Chan, W. F., Lam, K. Y. & Wan, K. Y. Follicular thyroid carcinoma: The role of histology and staging systems in predicting survival. Ann. Surg. 242, 708–715 (2005).
Haugen, B. R. et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016).
Cooper, D. S. et al. Revised American Thyroid Association Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009).
Tuttle, R. M., Haugen, B. & Perrier, N. D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why?. Thyroid 27, 751–756 (2017).
Londero, S. C. et al. Papillary thyroid carcinoma in Denmark, 1996–2008: Outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid 25, 78–84 (2015).
Krämer, J. A. et al. Primary tumour size is a prognostic parameter in patients suffering from differentiated thyroid carcinoma with extrathyroidal growth: Results of the MSDS trial. Eur. J. Endocrinol. 163, 637–644 (2010).
Nam, S. H. et al. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 87, 158–164 (2018).
Tam, S. et al. Survival in differentiated thyroid cancer: Comparing the AJCC cancer staging seventh and eighth editions. Thyroid 28, 1301–1310 (2018).
Liu, R. & Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 23, R143–R155 (2016).
Censi, S. et al. Prognostic significance of TERT promoter and BRAF mutations in TIR-4 and TIR-5 thyroid cytology. Eur. J. Endocrinol. 181, 1–11 (2019).
Melo, M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, E754–E765 (2014).
Melo, M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, E754–E765 (2014).
Kim, T. H. et al. Refining dynamic risk stratification and prognostic groups for differentiated thyroid cancer with TERT promoter mutations. J. Clin. Endocrinol. Metab. 102, 1757–1764 (2017).
Pennelli, G. et al. BRAFK601E mutation in a patient with a follicular thyroid carcinoma. Thyroid 21, 1393–1396 (2011).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015)
Davies, L. & Welch, H. G. Increasing incidence of thyroid cancer in the United States, 1973–2002. J. Am. Med. Assoc. 295, 2164–2167 (2006).
Vaccarella, S. et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: A population-based study in selected high-resource countries. Thyroid 25, 1127–1136 (2015).
Vaccarella, S. et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 375, 614–617 (2016).
Davies, L. & Welch, H. G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 140, 317–322 (2014).
Shoup, M. et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J. Am. Coll. Surg. 197, 191–197 (2003).
Durante, C. et al. Papillary thyroid cancer: Time course of recurrences during postsurgery surveillance. J. Clin. Endocrinol. Metab. 98, 636–642 (2013).
Ibrahimpasic, T. et al. Genomic alterations in fatal forms of non-anaplastic thyroid cancer: Identification of MED12 and RBM10 as novel thyroid cancer genes associated with tumor virulence. Clin. Cancer Res. 23, 5970–5980 (2017).
Agrawal, N. et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690 (2014).
Charles, R. P., Silva, J., Iezza, G., Phillips, W. A. & McMahon, M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol. Cancer Res. 12, 979–986 (2014).
Garnett, M. J. & Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6, 313–319 (2004).
Alzahrani, A. S. et al. Single point mutations in pediatric differentiated thyroid cancer. Thyroid 27, 189–196 (2017).
Kim, T. H. et al. The association of the BRAFV600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer 118, 1764–1773 (2012).
Xing, M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 28, 742–762 (2007).
Xing, M. M. et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA J. Am. Med. Assoc. 309, 1493–1501 (2013).
Melo, M. et al. TERT, BRAF, and NRAS in primary thyroid cancer and metastatic disease. J. Clin. Endocrinol. Metab. 102, 1898–1907 (2017).
Xing, M. et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 33, 42–50 (2015).
Elisei, R. et al. The BRAFV600E mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: Single-institution results from a large cohort study. J. Clin. Endocrinol. Metab. 97, 4390–4398 (2012).
Barollo, S. et al. BRAF in primary and recurrent papillary thyroid cancers: The relationship with 131I and 2-[18F]fluoro-2-deoxy-D-glucose uptake ability. Eur. J. Endocrinol. 163, 659–663 (2010).
Galuppini, F. et al. BRAF analysis before surgery for papillary thyroid carcinoma: Correlation with clinicopathological features and prognosis in a single-institution prospective experience. Clin. Chem. Lab. Med. 54, 1531–1539 (2016).
Damiani, L. et al. Evaluation of the role of BRAF V600E somatic mutation on papillary thyroid cancer disease persistence: A prospective study. Eur. Thyroid J. 7, 251–257 (2018).
Sancisi, V., Nicoli, D., Ragazzi, M., Piana, S. & Ciarrocchi, A. BRAFV600E mutation does not mean distant metastasis in thyroid papillary carcinomas. J. Clin. Endocrinol. Metab. 97, E1745–E1749 (2012).
Prete, A. et al. Update on Fundamental Mechanisms of Thyroid Cancer. Frontiers in Endocrinology 11, (2020).
Prior, I. A., Lewis, P. D. & Mattos, C. A comprehensive survey of ras mutations in cancer. Can. Res. 72, 2457–2467 (2012).
Howell, G. M., Hodak, S. P. & Yip, L. RAS mutations in thyroid cancer. Oncologist 18, 926–932 (2013).
Sandin, S. & Rhodes, D. Telomerase structure. Curr. Opin. Struct. Biol. 25, 104–110 (2014).
Liu, X. et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 20, 603–610 (2013).
Konturek, A., Barczyński, M., Nowak, W. & Richter, P. Prognostic factors in differentiated thyroid cancer—A 20-year surgical outcome study. Langenbeck’s Arch. Surg. 397, 809–815 (2012).
Pelizzo, M. R. et al. Papillary thyroid microcarcinoma (PTMC): Prognostic factors, management and outcome in 403 patients. Eur. J. Surg. Oncol. 32, 1144–1148 (2006).
Bellantone, R. et al. Prognostic factors in differentiated thyroid carcinoma: A multivariate analysis of 234 consecutive patients. J. Surg. Oncol. 68, 237–241 (1998).
Eustatia-Rutten, C. F. A. et al. Survival and death causes in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 91, 313–319 (2006).
Lundgren, C. I., Hall, P., Dickman, P. W. & Zedenius, J. Clinically significant prognostic factors for differentiated thyroid carcinoma: A populationed-based, nested case-control study. Cancer 106, 524–531 (2006).
Mazzaferri, E. L. & Jhiang, S. M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 97, 418–428 (1994).
Oda, H. et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid 26, 150–155 (2016).
Pellegriti, G. et al. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: Study of 299 cases. J. Clin. Endocrinol. Metab. 89, 3713–3720 (2004).
Roti, E. et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: Results of a retrospective study in 243 patients. J. Clin. Endocrinol. Metab. 91, 2171–2178 (2006).
Baudin, E. et al. Microcarcinoma of the thyroid gland: The gustave-roussy institute experience. Cancer 83, 553–559 (1998).
Durante, C. et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 91, 2892–2899 (2006).
Mian, C. et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin. Endocrinol. (Oxf) 68, 108–116 (2008).
Liu, J. et al. The genetic duet of BRAF V600E and TERT promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer. J. Nucl. Med. 61, 177–182 (2020).
Brose, M. S. et al. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 1272–1282 (2016).
Shah, M. H. et al. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J. Clin. Oncol. 35, 6022–6022 (2017).
Dadu, R. et al. Efficacy and tolerability of vemurafenib in patients with BRAFV600E positive papillary thyroid cancer: Cancer center off label experience. J. Clin. Endocrinol. Metab. 100, 77–81 (2015).
Tefferi, A. et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 373, 908–919 (2015).
Zhang, G. et al. Induction of telomere dysfunction prolongs disease control of therapy-resistant melanoma. Clin. Cancer Res. 24, 4771–4784 (2018).
Seimiya, H. Crossroads of telomere biology and anticancer drug discovery. Cancer Sci. https://doi.org/10.1111/cas.14540 (2020).
IARC Publications Website—WHO Classification of Tumours of Endocrine Organs. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Endocrine-Organs-2017. (Accessed 1 Nov 2020).
Barollo, S. et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid 24, 809–819 (2014).
Muzza, M. et al. Telomerase in differentiated thyroid cancer: Promoter mutations, expression and localization. Mol. Cell. Endocrinol. 399, 288–295 (2015).
Acknowledgements
The authors thank Frances Coburn for text editing.
Funding
This research received a Grant for a Ph.D from Sanofi Genzyme Italy.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.M., F.V. and S.C.; Methodology, C.M. and F.V.; Formal analysis, Y.H.Z. and J.M.; Investigation, L.B., S.B., F.G., C.B., J.M., S.C., and G.P.; Resources, M.I., C.B., F.G., and N.A., Data curation, L.B., S.B., Y.H.Z., S.C., and M.I.; Writing—original draft preparation, F.V., S.W.F. and S.C.; Writing—review & editing, S.C., C.M., G.P. and M.I.; Supervision, C.M.; Project administration, C.M.; Funding acquisition, S.W.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vianello, F., Censi, S., Watutantrige-Fernando, S. et al. The role of the size in thyroid cancer risk stratification. Sci Rep 11, 7303 (2021). https://doi.org/10.1038/s41598-021-86611-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86611-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.