Abstract
Tacrolimus (TAC) pharmacokinetics is influenced by the donor CYP3A5 genotype and the age of pediatric liver recipients. However, an optimization of a genotype-based algorithm for determining TAC starting is needed to earlier achieve stable target levels. As the graft itself is responsible for its metabolism, the Graft-to-Recipient Weight Ratio (GRWR) might play a role in TAC dose requirements. A single-center study was carried out in a cohort of 49 pediatric recipients to analyse the impact of patient and graft characteristics on TAC pharmacokinetics during the first 15 post-transplant days. Children < 2 years received grafts with a significantly higher GRWR (4.2%) than children between 2–8 (2.6%) and over 8 (2.7%). TAC concentration/weight-adjusted dose ratio was significantly lower in recipients from CYP3A5*1/*3 donors or with extra-large (GRWR > 5%) or large (GRWR 3–5%) grafts. The donor CYP3A5 genotype and GRWR were the only significant predictors of the TAC weight adjusted doses. Patients with a GRWR > 4% had a higher risk of acute rejection, observed in 20/49 (41%) patients. In conclusion, TAC starting dose could be guided according to the donor CYP3A5 genotype and GRWR, allowing for a quicker achievement of target concentrations and eventually reducing the risk of rejection.
Similar content being viewed by others
Introduction
The introduction of the calcineurin inhibitor drug tacrolimus (TAC) has improved patient survival in pediatric liver transplant patients. Nevertheless, the best way of employing this drug is still a matter of intense debate1,2, since TAC is characterized by a narrow therapeutic index, a high inter- and intra-individual pharmacokinetic, pharmacodynamic and pharmacogenetic variability and by significant adverse effects3.
Therefore, TAC therapeutic drug monitoring (TDM) in whole blood is essential to optimize clinical outcomes and minimize adverse effects. Nevertheless, the use of the TDM results for dose adjustment is time consuming before a stable and optimum TAC blood concentration can be reached and may lead to over or under-dosing determining drug toxicity or acute rejection. Therefore, better strategies for TDM are required to establish the optimal TAC dosage, especially in the early post-transplant period4.
Orally administered TAC, a lipophilic drug, has a variable absorption, first pass metabolism and limited bioavailability3. CYP3A4 and CYP3A5 are the primary enzymes responsible for TAC metabolism in the liver and to a lesser extent in enterocytes5. It is already well known that CYP3A5*3 allele, that is found in 90% of the Caucasian population, determines a lower TAC catabolism, whilst the *1 allele is related to a fast metabolism and, consequently, to a relatively lower TAC exposure6: non-expresser patients are poor metabolizers, have higher TAC blood concentrations and should be given the standard recommended starting dose, whereas individuals carrying at least one CYP3A5*1 allele are intermediate or extensive metabolizers and require a higher tacrolimus dose. The role of CYP3A5 genotype was established by a systematic review carried out on 596 pediatric transplant recipients7. In the most recent consensus report on TAC personalized therapy, CYP3A5 genotype is indicated as the main pharmacogenetic biomarker to be used in clinical practice for determination of the initial dose of tacrolimus in order to rapidly reach a therapeutic concentration2. Particularly, the donor CYP3A5 genotype was reported to influence TAC pharmacokinetics in liver transplant patients, whereas the recipient CYP3A5 genotype had no impact on TAC blood concentration8,9,10,11,12. Beyond CYP3A5, the determination of CYP3A4*22 genotype could explain the residual variability in TAC pharmacokinetics3. Lastly, ABCB1 (encoding P-glycoprotein, P-gp) polymorphisms seem to be less relevant in TAC pharmacokinetics13.
Apart from the genetic make-up, clinical and graft factors may affect TAC concentrations, e.g. patient age and gender, transplant type, baseline renal and liver function, concomitant use of corticosteroids or other drugs, serum albumin concentration and hematocrit, food administration or diarrhoea14. Among these, the recipient age has been pointed out as a main factor influencing TAC concentrations: younger pediatric patients require higher TAC doses than do older patients or adults to achieve similar TAC concentrations, probably due to a wider volume of distribution or a faster drug metabolism12,15,16.
In this regard, a sliding scale algorithm for a genotype guided TAC starting dose, stratified by the recipient age, was designed by Min et al., with the lowest dose in older (> 6 years) CYP3A5 non-expressers and the highest dose in younger (≤ 6 years) CYP3A5 expressers. This genotype-guided dosing was compared to a standard dosing in a cohort of pediatric solid organ transplant patients17, obtaining an earlier attainment of target levels and stable therapeutic concentrations with significantly fewer out-of-range results.
Indeed, TAC clearance could be dependent on the graft size, as the graft itself is responsible for its metabolism. This implies that, theoretically, the best marker of graft size could well be the Graft-to-Recipient Weight Ratio (GRWR), indicating the normalized size of the graft based on the patient’s weight. GRWR remains the most commonly used parameter for decision making in transplant surgery to calculate the optimal graft size for the recipient, so far due to its simplicity and practicality for clinical application. GRWR should be within the range 1% to 3% to avoid major complications related to size mismatching between the graft and the recipient, which may result in graft failure or even patient death if not appropriately handled18. GRWR has recently been correlated to TAC pharmacokinetics in a large Japanese cohort of pediatric living donor liver transplants19.
A further refinement and optimization of a genotype-based algorithm for determining TAC starting dose would be of advantage, in order to reach an earlier achievement of stable target immunosuppression levels and avoid adverse effects.
We present here our single-center study designed to evaluate the influence genetic polymorphisms and other patient and graft factors have onTAC blood levels in a cohort of pediatric liver transplant patients in the first two post-transplant weeks.
Materials and methods
Patient enrolment and data collection
All pediatric patients who consecutively underwent liver transplantation from January 2007 to December 2014 at AOU Città della Salute e della Scienza di Torino, Italy, were assessed for eligibility. This study is an extended pharmacokinetic and pharmacogenetic analysis on a previously published study by our group, which was limited to the first post-transplant day12 and did not take graft weight into account.
Inclusion criteria were as follows: (a) age < 18 years old at listing; (b) oral TAC immunosuppressive monotherapy and (c) informed consent of legal guardian. Exclusion criteria were as follows: (a) contra indications to oral tacrolimus; (b) multiple organ transplants or retransplants; and (c) declined consent.
Donor and recipient DNA samples were provided by the Regional Transplantation Center (CRT Piemonte e Valle d'Aosta, Italy), extracted after enrollment and analyzed.
Donor, recipient and graft characteristics, along with laboratory data, were collected from the medical records.
For the analysis, the recipients were divided into groups according to their age: under 2 years of age, between 2 and 8 and older than 8 years. GRWR was calculated using the formula graft weight/recipient weight and it was expressed as a percentage. The liver transplants were categorized into the following three groups according to the GRWR:
-
small-for-size graft, if GRWR was < 1%;
-
size-matched graft, if GRWR was between 1 and 3%;
-
large-for-size graft, if GRWR was between 3 and 5%;
-
extra-large-for-size graft, if GRWR was > 5%.
The study was approved by the Institutional Review Board (Comitato Etico Interaziendale AOU Città della Salutee della Scienza di Torino, Italy) and written informed consent was obtained from all patients’ parents. The study was carried out in compliance with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines. No organs from executed prisoners were used.
Immunosuppression protocol
The recipients received basiliximab at transplant (day 0) and on day 4 and/or steroids as induction. All patients were postoperatively administered oral TAC as capsules; naso-gastric administration was used in few cases.
The initial target TAC dose was 0.075 mg/Kg every 12 h, subsequently adjusted according to TAC whole blood concentrations (C0). The target C0 for the first 2 weeks was set between 8 and 12 ng/mL, in line with our center’s protocol and in agreement with other study protocols.
Any suspicion of acute rejection was assessed by liver biopsy. All histologically proven episodes of acute rejection were treated by increasing the TAC dose and/or a bolus dose of steroids.
Tacrolimus pharmacokinetic analysis
TAC daily doses (mg/Kg/die), trough TAC blood concentrations (ng/mL) and the concentration/weight-adjusted dose ratio (C/D/Kg) were determined daily during the first 2 post-liver transplantation weeks. C/D/Kg was used as a marker of TAC bioavailability to avoid any confounding effect due to the different TAC pro-kilo doses and dose variations during treatment.
Blood sampling was performed at the end of the inter-dose interval (just before the new drug intake), with sodium/EDTA Vacutainer tubes. The TAC concentrations were determined by the Masstrak Immunosuppressants XE kit (Chromsystems, Gräfelfing, Germany), based on a UltraPerformance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS) method.
Genotype identification
Donor and recipient DNA samples were genotyped for CYP3A5*3, CYP3A4*22, ABCB1 3435C > T and 1199A > G using the polymerase chain reaction (PCR) with TaqMan (Thermo Fisher Scientific). The other most common polymorphisms of ABCB1, i.e. 2677G > T and 1236 C > T, were not analyzed, since they are generally reported to be in linkage disequilibrium with 3435C > T polymorphism14.
Statistical analysis
Data were analyzed by SPSS 24.0 software package (IBM, Armonk, NY). Categorical variables were summarized as counts and percentages and continuous variables as means + /- SD or medians and inter-quartiles ranges. Differences between groups in the continuous variables were determined by the Mann–Whitney or Kruskal–Wallis tests when more than one group was involved. The Fisher exact test or χ2-square test were used for categorical data. Univariate and multivariate linear regression analysis were performed to test the predictive value of variables showing significant correlation with TAC concentrations. Absence of significant collinearity of covariates and significant correlation with the dependent variables were considered as preliminary assumptions for regression analysis. In case of collinearity, only the variable with the highest predictive value by univariate regression was included in the multivariate analysis. All the tests were two tailed and a p value of ≤ 0.05 was considered statistically significant. The confidence intervals (CI) were calculated at the 95% level. ROC curve was used to identify cut-off values.
Results
Patient and graft characteristics
A total of 49 pediatric patients on TAC immunosuppressive monotherapy were included in this study.
Patient and graft characteristics are summarized in Table 1. All liver transplants were from deceased donors. Twenty-five recipients were under 2 years of age (51%), 16 were between 2 and 8 (32.7%) and 8 (16.3%) were older than 8 years old. The most frequent indications for liver transplant were biliary atresia, 26 pts (53.1%), hepatoblastoma, 12 pts (24.5%) and sclerosing cholangitis, 5 pts (10.2%). Other causes of end-stage liver disease were: Alagille syndrome, 1 pt (2%), progressive familial intrahepatic cholestasis, 3 pts (6.2%), cystic fibrosis, 1 pt (2%), and urea cycle disorder, 1 pt (2%). Induction immunosuppressive therapy was administered as follows: basiliximab in 33/49 (67%), basiliximab and steroids in 7/49 (14%) and steroids in 9/49 (19%).
The CYP3A5, CYP3A4 and ABCB1 genotype frequencies did not deviate from the Hardy–Weinberg equilibrium. Genotype frequency was not significantly different between transplant recipients and donors and showed no association with age or gender. The total allelic frequency was similar to that observed in other populations made up in great majority of Caucasian subjects1,20,21,22.
Patients’ weight and graft weight resulted significantly correlated (r = 0.815, r2 0.664, p value < 0.001), indicating a not perfectly proportional graft matching.
The median GRWR was 3.3% (IQR 2.5–4.4). Among all the enrolled patients, respectively 43%, 41% and 16% showed a GRWR between 1 and 3% (normal), 3% and 5% (large) or more than 5% (extra-large). No small-for-size grafts (GRWR < 1%) were transplanted.
GRWR was statistically significantly correlated with the recipient age (r = 0.479, r2 = 0.229, p value < 0.001). Median GRWRs were 4.2% (IQR 3.45–5.17), 2.6% (IQR 2.01–4.13) and 2.7% (IQR 1.59–2.98) for patients’ age groups < 2 years old, between 2 and 8 and > 8 years old, respectively (p value = 0.002). Children under 2 years of age received liver grafts with a higher GRWR than children between 2–8 (p value = 0.011) and over 8 (p value = 0.001). There was no difference in the GRWR values between children 2–8 years old and those over 8 years (p value = 0.417).
Whole, reduced and split livers respectively represented 45%, 5% and 50% of size-matched grafts (GRWR 1–3%), 16%, 16% and 68% of large grafts (GRWR 3–5%) and 62.5%, 12.5%, and 25% of extra-large grafts (GRWR > 5%).
Median values of ALT values were significantly higher in patients who received large and extra-large grafts. There was a statistically significant correlation between ALT and GRWR on the 1st post-transplant day (r2 = 0.155;p value = 0.043), thereafter this correlation did not reach statistical significance.
Tacrolimus pharmacokinetics and patient characteristics
Figure 1 shows the TAC whole blood concentrations during the first 15 post-transplant days. There was a gradual increase in the number of patients who achieved the target C0 level (8–12 ng/mL) during this period, most likely due to TDM-guided dose adjustments, i.e. the percentage of patients who achieved target therapeutic ranges at day 1 and 15 increased from 23.6% to 42.9%.
In the first 7 days of treatment, there was a significant correlation with the initial (day 1) dose corrected by graft weight (r between 0.316 and 0.480, p values between 0.008 and 0.044 in the range of 2–8 days), with the dose corrected by the patients’ weight (r between 0.247 and 0.402, p values between 0.001 and 0.019) and with the graft weight (r between − 0.328 and − 0.389, p values between 0.006 and 0.023 in the range of 4–6 days). These data suggest that graft weight may affect the achievement of the desired TAC concentrations.
Attention was then focused on the factors affecting the inter-individual variability in the TAC C/D/Kg. Female recipients showed significantly higher C/D/Kg on the 1st post-transplant day (p value = 0.03) than male patients. TAC C/D/Kg was significantly lower in children under 8 years of age, particularly in those under 2, from the 1st post-transplant day to the 7th (Table 2). Those patients who received livers from adult (p value = 0.046) and female donors (p value = 0.048) had a significantly higher TAC C/D/Kg on the 1st post-transplant day and on the 3rd and 4th post-transplant days, respectively.
The levels of ALT were not correlated to TAC C/D/Kg, whereas a higher value of PT-INR on the 1st and the 5th post-transplant days correlated to a higher TAC C/D/Kg in the time-lapse between the 2nd and the 4th day (p values between 0.002 and 0.010) and between the 10th and the 15th day (p values < 0.01), respectively. Corticosteroids administered as induction therapy did not influence TAC C/D/Kg.
When both donor and recipient genotypes were taken into consideration, it was observed that the TAC C/D/Kg were significantly lower in patients who received a liver from CYP3A5*1/*3 donors compared to those who were recipients from donors homozygous for the *3 allele. This difference was statistically significant from the first post-transplant day through to the 10th (Table 2). Conversely, the recipient CYP3A5 genotype had no significant influence on TAC levels in the early post-transplant period. Recipients receiving organs from *1/*3 donors had significantly higher TAC dose requirements than recipients from *3/*3 donors, regardless of the recipients’ genotype. Neither the donor nor recipient CYP3A4 or ABCB1 polymorphisms influenced significantly the TAC dose requirements in the first 15 post-transplant days. TAC C/D/Kg tended to be higher in 3435 C/T than in 3435 C/C recipients, in 3435 T/T than in 3435 C/T patients and in 1199 G/A than in 1199 G/G recipients, although these differences did not reach the statistical significance.
The type of transplanted graft did not influence the TAC blood levels, whereas TAC C/D/Kg was significantly lower in extra-large or large grafts from the 3rd to the 9th post-transplant day (Table 2).
The TAC C/D/Kg data were stratified by the main parameters showing a statistically significant association over a longer time range (recipients’ age, donor CYP3A5 genotype and GRWR), as reported in Table 2.
Predictive factors of tacrolimus pharmacokinetics
Univariate and a multivariate linear regression analysis were performed to identify the most relevant independent factors affecting TAC C/D/Kg. The univariate analysis confirmed the results from the correlation tests. Factors which showed at least one significant predictive power on TAC C/D/Kg during the first 15 post-transplant days included the recipient age, the donor gender and CYP3A5 genotype, the GRWR and the PT-INR.
The multivariate analysis documented that the recipient age, the donor CYP3A5 genotype and gender were independent predictors of TAC C/D/Kg on the 1st post-transplant day, whereas only the recipient age, the donor CYP3A5 genotype and PT-INR at day 1 remained statistically significant on the 2nd day. The recipient age and the PT-INR at day 1 were no longer significant predictors from the 3rd and the 4th day respectively, whereas from the 3rd day the GRWR started to be a significant predictor of TAC C/D/Kg. The donor CYP3A5 genotype and GRWR remained significant predictors of the TAC C/D/Kg up until the 10th post-transplant day, when PT-INR at day 5 remained the only predictor of TAC C/D/Kg (Table 3).
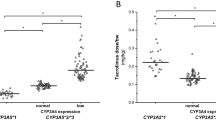
The influence GRWR has on TAC pharmacokinetics according to the donor CYP3A5 polymorphism was analyzed. Recipients from *1/*3 donors rarely achieved TAC concentrations within the therapeutic range, whereas only patients with extra-large grafts did not reach adequate target TAC concentrations among recipients from *3/*3 donors. Indeed, patients who received an extra-large-for-size graft required higher TAC doses than recipients with a large or size-matched graft regardless the donor CYP3A5 genotype. Figure 2 shows the impact of GRWR on TAC blood concentrations stratified by the donor CYP3A5 genotype on the 10th post-transplant day. Nevertheless, the differences in TAC concentrations between GRWR groups resulted not statistically significant (p value = 0.095), probably due to the low sample size and the variability in the administered dose between groups.
Graft size correlation with tacrolimus kinetics and the weight-adjusted dose required
A linear regression analysis was performed between the GRWR and the weight-adjusted dose during treatment, to estimate the impact graft size has on the need for dose adjustments during treatment. Noteworthy is that the GRWR was a statistically significant predictor of the weight adjusted dose between the 10th and the 15th, indicating a predictive value on the required TAC dose. CYP3A5 genotype also resulted to be an independent predictor of the weight-adjusted dose of TAC (Table 4).
Clinical significance
Among the enrolled patients, 20 out of 49 (41%) patients developed acute rejection during the first 15 post-transplant days, at a median of 9 post-transplant days (range 7–30). None of these events led to graft failure or patient death.
Genetic, anthropometric or demographic variables were not significantly associated with the onset of acute rejection.Only ALT levels on the 1st post-transplant day were significantly higher in patients who developed acute rejection in the following days. The protocol assumption of corticosteroids as induction immunosuppression did not prevent the onset of acute rejection.
Patients with acute rejection had a lower TAC C/D/Kg than patients with no rejection, even if this did not reach statistical significance. This trend was present from the first to the 10th post-transplant day, followed by a reversed trend. Acute rejection was not significantly associated with recipient or donor genotype, age or gender. Noteworthy was the fact that acute rejection occurred with statistical significance more frequently in the group of patients who received an extra-large graft (GRWR > 5%). The logistic regression analysis evidenced that only the GRWR had a statistically significant predictive value for the occurrence of acute rejection (p value = 0.005). Patients with a GRWR > 4% had a higher risk of acute rejection than those with a ratio < 4% (AUROC 0.742, p value = 0.004), with a 70% sensitivity and an 82% specificity (Positive Predictive Value = 73.7% and a Negative Predictive Value = 80%).
Incidences of early post-transplant surgical complications such as hepatic artery thrombosis, portal vein thrombosis, biliary complications, intra-abdominal bleeding or intestinal perforation were all comparable in our cohort regardless the GRWR. For the entire cohort, 1-, 3-, 5-year patient and graft survival rates after liver transplant were 95.8%. Patient and graft survival did not differ among the three groups of patients stratified by GRWR: 1-, 3-, and 5-year patient and graft survival rates were 95.2%, 95.0%, and 100% respectively, for children who received size-matched, large and extra-large grafts.
Discussion
Currently, immunosuppressive treatment with calcineurin inhibitors requires continuous monitoring and dose adjustments, particularly in the first two weeks of treatment, in order to avoid graft rejection or treatment toxicity. This optimization goal is currently achieved through the adoption of a routine TDM. In our study, despite the effectiveness of the TDM in gradually optimizing the posology, many patients had TAC concentrations out of the therapeutic ranges during the first days. This appeared to be due to inadequate TAC starting dose, which is usually determined based on patients’ weight.
The TAC pharmacokinetic analysis we performed in the first two post-transplant weeks confirmed some results from our previous study12, where the donor CYP3A5 genotype and the recipient age explained part of the inter-individual variability in TAC concentrations. In the present study, we confirmed that patients receiving a liver from donors heterozygous for the CYP3A5*3 allele have higher dose requirements compared to those who are homozygous for the CYP3A5*3 allele, as previously reported8,9,10,11, suggesting that the hepatic metabolism is determinant on TAC blood concentrations immediately after the transplant, regardless of the impaired hepatic synthetic function, which characterizes the early post-transplant period. On the other hand, the recipient CYP3A5 resulted not significantly associated with TAC pharmacokinetics, suggesting that the extrahepatic metabolism may have a limited impact, even in the first few days. On the contrary, CYP3A4 genotype showed no significant impact on TAC metabolism in our cohort, probably due to the low sample size (the stronger effect of CYP3A5 could mask factors with lower impact). However, although a minor role of CYP3A4 polymorphisms on TAC pharmacokinetics has been recently reported in the consensus report on TAC personalized therapy2, data are more controversial in pediatric liver transplant patients, whereas it may be more relevant in kidney transplant patients23,24,25.
Moreover, although P-glycoprotein (encoded by ABCB1 gene) may have a role in TAC disposition (particularly in lymphocytes29,30), we did not observe any significant association with dose requirements, in line with previous reports26,27,28.
We confirmed the impact of the recipient age, suggesting that children under 8 years of age should be given higher TAC doses, particularly if they are under 2, whereas metabolic pathways in patients over 8 years of age may be more similar to adults31,32,33.
Nevertheless, when the pharmacokinetic analysis was extended to cover the first two post-transplant weeks, we observed that the impact of the aforementioned factors had on TAC pharmacokinetics changed during the first post-transplant days and most likely reflected changes in the patient’s physiology and pharmacokinetics. Indeed, the patients’ gender and age had no significant effect on the TAC exposure, whereas an increase in the influence of GRWR on TAC blood concentrations was observed. The recipient age could play an important role in determining TAC concentrations during the first two post-transplant days, probably due to differences in the volume of distribution, whereas the graft size could become a better predictive factor of TAC concentrations later. According to this hypothesis, younger recipients might require higher TAC doses since they generally receive relatively larger livers. As already reported, the standard liver volume to body weight ratio (as well as GRWR) of children decreases as age increases until approximately the age of 16 years old34, therefore GRWR could explain the age-related variability in TAC pharmacokinetics. Conversely, our results showed that the type of transplant graft (whole/reduced/split liver) had no relevant influence on TAC blood levels, as previously reported35,36.
Our evidence about the impact of GRWR on TAC metabolism is in line with a recent study on a large Japanese cohort19, and suggests that patients who receive a larger graft, due to their higher amount of hepatic tissue (and hence enzymes), have enhanced TAC metabolism. This evidence is theoretically quite reasonable, so much that graft weight is a key variable for the calculation of the metabolic capability of individuals, and it is considered as a main feature for the physiologically based pharmacokinetic (PBPK) modeling37. Therefore, a GRWR-based TAC initial dose could be more accurate than the current standard weight-based dose.
Nevertheless, probably due to the low sample size (and so low statistical power), the significant correlation between GRWR and TAC C/D/Kg was observed up to the 10th post-transplant day (and then it lost of significance), whereas in the Japanese study it occurred from 6 to 12 months after transplant19. Other factors could contribute to this difference with the Japanese study. The study by Shoji et al.19 was focused on transplants from living donors, while our patients received graft from deceased donors. The median graft size was larger in our study (GRWR 3.3% vs 2.37%) and a different distribution in GRWR groups was observed, e.g. no small-for-sizegrafts were observed in our population.
On the other hand, the regeneration of the transplanted liver may be relatively fast in the first post-transplant weeks, as already reported previously38,39,40,41,42: this may occur very early in the first post-transplant weeks, with 80% of volumetric recovery after 1 week from transplant38 and graft size normalization within the 2nd post-transplant month. Moreover, in our sample, we observed higher ALT levels, underlying more cytolysis, and probably a consequent reduction in the amount of functional liver tissue, in patients with higher GRWR. These concomitant mechanisms, e.g. liver regeneration and cytolysis, may progressively reduce the differences in TAC metabolism between small-for-size and large or extra-large-for-size grafts: the interindividual variability in GRWR would rapidly decrease within the first weeks according to the initial graft size, reaching a very low variability after two months, thus explaining both our results and those by Shoji et al.19.
Most important, in our cohort the vast majority of acute rejections and TDM-guided dose adjustments were applied around the 9th day of treatment: acute rejection can slightly decrease the hepatic metabolism of TAC (this was not statistically significant, but a reversion in the trend was observed compared with the previous days), leading to an increase in the TAC C/D/Kg in patients with rejection. Interestingly, acute rejection was more frequent in patients with high GRWR, so this phenomenon would also explain the change in the predictive power of GRWR for TAC C/D/Kg.
Finally, the dose adjustments applied (particularly around the 9th post-transplant day), based on TDM results and/or on the occurrence of acute rejection, may also cause a slight bias in the C/D/Kg; in fact, the observed trough TAC blood levels in the following days cannot be considered as the steady-state concentrations, causing fluctuations in the ratio until a new steady-state is reached.
Therefore, the most reliable data in terms of prediction of C/D/Kg are surely the ones within the 9th post-transplant day, before the introduction of these sources of bias.
Safety criteria of GRWR for pediatric patients are greatly influenced by the age of recipients and still represent a matter of intense debate, especially for infant recipients, where the impact of graft-to-recipient size mismatching on outcomes remains unclear. Liver transplant techniques using living-related grafts, reduced-size livers, and split livers have been widely exploited due to the shortage of size-matched cadaveric organs. Nevertheless, the reasonable range of GRWR in infants has not been well defined. The reduction in the graft mass may be an adequate strategy in selected cases to limit collateral risks of oversizing, but it should not be proposed systematically as it may incur an added risk of complications caused by greater parenchymal surface and suboptimal vascular flow. In our series, the high median GRWR was dependent on the surgical decision to prefer whole liver transplants in children < 2 years old and to eventually use temporary tension-free abdominal closure in cases of increased abdominal pressure. Our data showed that all the patients who received a large o extra-large graft achieved excellent post-transplant survival outcomes, suggesting that the accumulation of technical experience is relevant for the successful implant of large/extra-large grafts in infants. Nevertheless, our personal experience with this research showed that the onset of acute rejection was significantly associated with the patient’s GRWR, meaning that patients who have a larger than proportional graft size also run a higher risk of developing rejection, i.e. we observed that a GRWR cut-off value of > 4% was strongly predictive of acute rejection. This observation is in line with those of Kiuchi et al., who found that acute rejection was more frequent in larger-for-sizegrafts18. Other authors observed that acute rejection rate was not associated to GRWR43, however, these different findings could be related to the adoption of different induction immunosuppression protocols.
Acute rejection was not significantly associated to other donor/recipient and graft characteristics, but it occurred more frequently in patients with lower TAC blood concentrations, even if this association did not reach statistical significance, probably due to the small sample size. This indicated that acute rejection might depend on the size of the graft, not only because there is more tissue capable of metabolizing the TAC, leading to lower systemic concentrations, but probably also because an extra-large sized graft means more allo-antigens and a more extended tissue damage, as sustained by the correlation between ALT levels and the GRWR on the 1st post-transplant day. Moreover, a larger liver could mean also a less homogeneous drug penetration in the first days after transplantation, so it could take longer to achieve therapeutic levels in the whole graft. Nevertheless, the surgical decision to use large or extra-large grafts should not be limited on the basis of our results, if it is supported by a valid technical experience. On the other hand, we believe that the TAC starting dose should be increased when a pediatric liver transplant is performed by using a proportionally large or extra-large graft.
The multivariate regression analysis suggests that the best predictors of the final TAC dose needed to reach therapeutic concentrations are GRWR and the donor CYP3A5 genotype. Therefore, the TAC starting dose could be successfully guided and individualized according to the donor metabolic activity and graft size, allowing to achieve the TAC concentrations in the therapeutic in a shorter time and reduce the risk of acute rejection.
Our findings suggest that it may be of advantage stratifying genotype-guided dosing by the graft size (GRWR) instead of the recipient age17. We reported in Fig. 3 a proposal for an algorithm for individualized TAC starting dose on the basis of our results, including the donor CYP3A5 and the GRWR, where the recommended doses need to be validated in larger sample sizes. This hypothesis might lay the groundwork for a future prospective trial that would allow implementation of an efficacious guided dosing for the TAC starting dose and the subsequent dose titrations in the early post-transplant period.
Prior studies in adults reported a higher risk of rejection, poor graft outcomes and longer hospital stay in patients with sub-therapeutic or supra-therapeutic concentrations compared to those with therapeutic concentrations44,45,46. Confirmation of whether a more precise individualized dosing has a potential to improve patients’clinical outcomes and reduce hospital length of stay and costs associated with complications is to be obtained through further research.
In conclusion, defining the donor genetic profile (CYP3A5 genotype) and the graft size (GRWR) of the liver transplant before starting the TAC–based immunosuppression may be an attractive option for the prediction of the individual dose required and could well allow for a quicker achievement of target blood concentrations. We confirmed that the testing of the donor CYP3A5 genotype should be routinely implemented by clinical pharmacology and pharmacogenetics laboratories for all children. Although the donor CYP3A5 genotype seems to be the most relevant predictive factor on TAC pharmacokinetics, we suggest that GRWR, which is readily available to clinicians at the time of transplant, could be used not only for its surgical impact, but also as a predictive factor to guide the TAC starting dose.
The findings of this study should be an incentive for developing TAC dosing algorithms, incorporating clinical and genetic factors, in order to minimize the inter-individual variability in TAC concentrations and potentially reduce post-transplant complications. Multicenter large-scale studies are required to more comprehensively explore the impact of the use of large grafts on TAC pharmacokinetics and the risk of acute rejection in infants < 5 kg.
Abbreviations
- TAC:
-
Tacrolimus
- TDM:
-
Therapeutic drug monitoring
- GRWR:
-
Graft-to-Recipient Weight Ratio
- C/D/Kg:
-
Concentration/weight-adjusted dose ratio
References
Birdwell, K. A. et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 98, 19–24 (2015).
Brunet, M. et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther. Drug Monit. 41, 261–307 (2019).
Venkataramanan, R. et al. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 29, 404–430 (1995).
Johnston, A. & Holt, D. W. Immunosuppressant drugs–the role of therapeutic drug monitoring. Br. J. Clin. Pharmacol. 52(Suppl 1), 61S-73S (2001).
Sattler, M., Guengerich, F. P., Yun, C. H., Christians, U. & Sewing, K. F. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab. Dispos. 20, 753–761 (1992).
Kuehl, P. et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 27, 383–391 (2001).
Hendijani, F., Azarpira, N. & Kaviani, M. Effect of CYP3A5*1 expression on tacrolimus required dose for transplant pediatrics: a systematic review and meta-analysis. Pediatr. Transplant. 22, e13248 (2018).
Provenzani, A. et al. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int. J. Mol. Med. 28, 1093–1102 (2011).
Barrera-Pulido, L. et al. Clinical relevance and prevalence of polymorphisms in CYP3A5 and MDR1 genes that encode tacrolimus biotransformation enzymes in liver transplant recipients. Transplant. Proc. 40, 2949–2951 (2008).
Goto, M. et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 14, 471–478 (2004).
Wei-lin, W. et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transplant. 12, 775–780 (2006).
Calvo, P. L. et al. Donor CYP3A5 genotype influences tacrolimus disposition on the first day after paediatric liver transplantation. Br. J. Clin. Pharmacol. 83, 1252–1262 (2017).
Jun, K. R. et al. Tacrolimus concentrations in relation to CYP3A and ABCB1 polymorphisms among solid organ transplant recipients in Korea. Transplantation 87, 1225–1231 (2009).
Provenzani, A. et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J. Gastroenterol. 19, 9156–9173 (2013).
Murry, D. J. et al. Liver volume as a determinant of drug clearance in children and adolescents. Drug. Metab. Dispos. 23, 1110–1116 (1995).
Kearns, G. L. et al. Developmental pharmacology drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349, 1157–1167 (2003).
Min, S. et al. A randomized clinical trial of age and genotype-guided tacrolimus dosing after pediatric solid organ transplantation. Pediatr. Transplant. 22, e13285 (2018).
Kiuchi, T. et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 67, 321–327 (1999).
Shoji, K. et al. Graft-to-Recipient Weight Ratio associated with tacrolimus metabolism following pediatric living donor liver transplantations. J. Pediatr. Pharmacol. Ther. 24, 138–147 (2019).
Sinues, B. et al. CYP3A5*3 and CYP3A4*1B allele distribution and genotype combinations: differences between Spaniards and Central Americans. Ther. Drug. Monit. 29, 412–416 (2007).
Gervasini, G. et al. Differences in CYP3A5*3 genotype distribution and combinations with other polymorphisms between Spaniards and Other Caucasian populations. Ther. Drug. Monit. 27, 819–821 (2005).
Hustert, E. et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 11, 773–779 (2001).
Staatz, C. E., Goodman, L. K. & Tett, S. E. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin. Pharmacokinet. 49, 141–175 (2010).
Roy, J. N. et al. CYP3A4, CYP3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 16, 659–665 (2006).
Andrews, L. M. et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br. J. Clin. Pharmacol. 85, 601–615 (2019).
Cho, J. H. et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transplant. Proc. 44, 109–114 (2012).
Provenzani, A. et al. The effect of CYP3A5 and ABCB1 single nucleotide polymorphisms on tacrolimus dose requirements in Caucasian liver transplant patients. Ann. Transplant. 14, 23–31 (2009).
Shi, Y. et al. Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients. Gene 512, 226–231 (2013).
Capron, A. et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. 11, 703–714 (2010).
Tron, C. et al. Tacrolimusdiffusion across the peripheral mononuclear blood cell membrane: impact of drug transporters. Fundam. Clin. Pharmacol. 33, 113–121 (2019).
Wallemacq, P. E. et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur. J. Drug. Metab. Pharmacokinet. 23, 367–370 (1998).
Wallemacq, P. E. & Verbeeck, RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin. Pharmacokinet. 40, 283–295 (2001).
Jusko, W. J. et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin. Pharmacol. Ther. 57, 281–290 (1995).
Jalil, M. H. et al. Population pharmacokinetic and pharmacogenetic analysis of tacrolimus in paediatric liver transplant patients. Br. J. Clin. Pharmacol. 77, 130–140 (2014).
Wallin, J. E. et al. Population pharmacokinetics of tacrolimus in pediatric liver transplantation: early posttransplantation clearance. Ther. Drug. Monit. 33, 663–672 (2011).
Urata, K. et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 21, 1317–1321 (1995).
Li, G. F. et al. Impact of ethnicity-specific hepatic microsomal scaling factor, liver weight, and cytochrome P450 (CYP) 1A2 content on physiologically based prediction of CYP1A2-mediated pharmacokinetics in young and elderly Chinese adults. Clin. Pharmacokinet. 58, 927–941 (2019).
Haga, J. et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver. Transpl. 14, 1718–1724 (2008).
Kawasaki, S. et al. Liver regeneration in recipients and donors after transplantation. Lancet 339, 580–581 (1992).
Humar, A. et al. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver. Transpl. 10, 374–378 (2004).
Nadalin, S. et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver. Transpl. 10, 1024–1029 (2004).
Olthoff, K. M. et al. Liver regenerationafterliving donortransplantation:adult-to-adultliving donorliver transplantationcohort study. Liver. Transpl. 21, 79–88 (2015).
Wan, P. et al. Influence of graft size matching on outcomes of infantile living donor liver transplantation. Pediatr. Transplant. 19, 880–887 (2015).
Borra, L. C. et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol. Dial. Transplant. 25, 2757–2763 (2010).
Sapir-Pichhadze, R. et al. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 85, 1404–1411 (2014).
Rehman, S. et al. Effect of different tacrolimus levels on early outcomes after kidney transplantation. Ann. Transplant. 19, 68–75 (2014).
Acknowledgments
The authors wish to thank Ms. Barbara Wade for her linguistic advice.
Funding
This study was supported by internal funding.
Author information
Authors and Affiliations
Contributions
M.P. and P.L.C. designed the study, M.P. and A.D.N. performed the study, analysed the data and wrote the paper. R.C. and L.S. performed the genetic and pharmacokinetic analysis and interpreted the data. A.P. and M.A. collected the data. D.D.O., A.D., S.C., F.T., R.R. and P.L.C. critically reviewed the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinon, M., De Nicolò, A., Pizzol, A. et al. Early impact of donor CYP3A5 genotype and Graft-to-Recipient Weight Ratio on tacrolimus pharmacokinetics in pediatric liver transplant patients. Sci Rep 11, 443 (2021). https://doi.org/10.1038/s41598-020-79574-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79574-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.