Abstract
Distracted eating can lead to increased food intake, but it is unclear how. We aimed to assess how distraction affects motivated, goal-directed responses for food reward after satiation. Thirty-eight healthy normal-weight participants (28F; 10M) performed a visual detection task varying in attentional load (high vs. low distraction) during fMRI. Simultaneously, they exerted effort for sweet and savory food rewards by repeated button presses. Two fMRI runs were separated by sensory-specific satiation (outcome devaluation) of one of the (sweet or savory) reward outcomes, to assess outcome-sensitive, goal-directed, responses (valued vs. devalued reward, post vs. pre satiation). We could not verify our primary hypothesis that more distraction leads to less activation in ventromedial prefrontal cortex (vmPFC) during goal-directed effort. Behaviorally, distraction also did not affect effort for food reward following satiation across subjects. For our secondary hypothesis, we assessed whether distraction affected other fronto-striatal regions during goal-directed effort. We did not obtain such effects at our whole-brain corrected threshold, but at an exploratory uncorrected threshold (p < 0.001), distraction decreased goal-directed responses (devalued vs. valued) in the right inferior frontal gyrus (rIFG). We continued with this rIFG region for the next secondary hypothesis; specifically, that distraction would reduce functional connectivity with the fronto-striatal regions found in the previous analyses. Indeed, distraction decreased functional connectivity between the rIFG and left putamen for valued versus devalued food rewards (pFWE(cluster) < 0.05). In an exploratory brain-behavior analysis, we showed that distraction-sensitive rIFG-responses correlated negatively (r = − 0.40; p = 0.014) with the effect of distraction on effort. Specifically, decreased distraction-related rIFG-responses were associated with increased effort for food reward after satiation. We discuss the absence of distraction effects on goal-directed responses in vmPFC and in behavior across participants. Moreover, based on our significant functional connectivity and brain-behavior results, we suggest that distraction might attenuate the ability to inhibit responses for food reward after satiation by affecting the rIFG and its connection to the putamen.
Similar content being viewed by others
Introduction
Multi-tasking with electronic devices, such as our smart phones or computers, has become common behavior in everyday life1, and increasingly occurs during consumption of food2,3. Such “distracted eating” has been shown to cause overeating4,5,6, is associated with increased BMI7, and with increased choices of palatable foods8.
However, it is unclear how distraction increases food intake. Previously, we showed that distraction attenuates functional connectivity during tasting between a primary taste region in the insula and a secondary taste region in the OFC, and that lower sweetness activation of the insula under high distraction predicted increased subsequent food intake9. However, this design did not allow for investigation of other processes than taste, such as food-related motivational or decision-making processes that may also be affected by distraction. Previous research has already suggested that distraction can disrupt reward valuation processes10,11. However, this has never been tested using active, motivational responses as happening in daily life eating behavior.
For example, in the act of grasping another crisp out of a bowl, attention to the TV, instead of to the crisp, may bias decision-making processes to the advantage of automatic and reflexive, i.e. habitual, choices. During learning, both humans and rodents form response-outcome (R-O) and stimulus-response (S-R) associations. When a response is made based on the outcome (reward) value of an action, the response is governed by R-O associations, and leads to goal-directed actions. On the other hand, habitual actions can be controlled by S-R associations, which have no associative link with the outcome value of the action. Habitual actions are relatively automatic and therefore less costly compared with goal-directed actions (see12 for a review). When consuming a food to satiation, the outcome value of the eaten food decreases13,14,15, causing people to discontinue eating if they were to act in a goal-directed manner. However, we hypothesize that when distracted, goal-directed control is attenuated, causing people to continue grasping for another crisp in a habitual manner and ignoring the decreased outcome value of the food.
One way to investigate goal-directed versus habitual behavior, is through outcome devaluation. For example, Valentin and colleagues16 used tomato juice (savory) and chocolate milk (sweet) as stimuli and devalued participants on one of these drinks through satiation (i.e. sensory-specific satiation). Before and after the devaluation, participants had to choose one of two rewards in an instrumental choice paradigm. Using fMRI during this task, they found that the ventromedial prefrontal cortex (vmPFC) was involved in goal-directed responses for the juices. Other fMRI studies in humans also found the vmPFC to be involved in encoding reward predictions based on goal-directed R-O associations15,17,18, in line with its role in value-based decision-making19.
To test the effect of distraction on goal-directed responses for food reward, we adapted a similar outcome devaluation paradigm as used previously16,20,21, using sensory-specific satiation. However, instead of a binary choice, participants had to exert effort to obtain a sweet or savory food reward. During this extended period of effort, participants were less or more distracted by a categorical visual detection task of varying attentional load (high, low distraction). After outcome devaluation of the sweet or savory reward, participants performed the same task again. Under high (versus low) distraction, we expected participants to exert less goal-directed effort, and to find less activation in the vmPFC. Due to the nature of the task (effort for reward outcomes instead of binary choice), we also tested the effect of distraction on devalued versus valued food rewards in other fronto-striatal regions, related to other motivational or cognitive control processes than value-based decision-making, in secondary analyses. In further secondary analyses, we assessed whether distraction would affect functional connectivity with the fronto-striatal regions found in the previous analyses (secondary outcomes, see preregistration).
Methods
Participants
Thirty-nine right-handed healthy participants took part in this study and were recruited from a previous study of which the results are presented elsewhere9. All participants were recruited on a voluntary basis. They gave written informed consent and received financial compensation for participating in the study. All methods were carried out in accordance with the policies and principles contained in the Federal Policy for the Protection of Human Subjects and in the Declaration of Helsinki. All experimental protocols were approved by the local ethics committee (CMO region Arnhem-Nijmegen, the Netherlands, protocol nr. 2015–1928).
As a result of one drop-out (i.e. not completing the test session), the final sample size of the study was 38 [age range 19–37; mean age (± SD): 23.8 (3.6); 28 females; mean Body Mass Index (BMI, ± SD): 22.3 (2.01)].
Screening
The two sessions of our previous fMRI study (on the effects of distraction on taste-related neural processing)9 and the session of our current study were preceded by a separate session to screen participants for exclusion criteria. To be eligible to participate in the study, participants had to have a BMI within a range of 18.5–30.0, had to be within 18–35 years old at the time of the intake session [mean (SD) difference in weeks between intake session and current test session: 66.81 (10.26)], and right-handed. Weight change relative to the screening session was assessed at the beginning of the fMRI test session, but never exceeded more than 5 kg. Exclusion criteria were current pregnancy; MRI-incompatibility; diabetes mellitus; history of hepatic, cardiac, respiratory, renal, cerebrovascular, endocrine, metabolic or pulmonary diseases; uncontrolled hypertension; eating, neurological, or psychiatric disorders; current strict dieting; restrained eating score ≥ 20% highest percentile (≥ 3.60 for males and ≥ 4.00 for females) on the Dutch Eating Behavior Questionnaire (DEBQ22; Supplementary Table 1); current psychological or dietary treatment; taste or smell impairments; use of neuroleptica or other psychotropic medication; food allergies relevant to the study, deafness, blindness, and sensori-motor handicaps; drug, alcohol or nicotine addiction; inadequate command of both Dutch and English, and a change in body weight of more than 5 kg in the past 2 months.
Procedure
Participants came to the laboratory for one experimental test session; see Supplementary Fig. S1. To ensure that participants were somewhat hungry during the session, they received a small standardized meal that they consumed 3 h before the session [yogurt drink, strawberry flavor (200 g (g), 850 kJ, 6.0 g. protein, 30.0 g. carbohydrates, 6.0 g. fat; Breaker, Melkunie, Nijkerk, the Netherlands))] Three hours before and after consumption of the yogurt drink, participants were instructed to abstain from eating and from drinking sugared or sweetened drinks (water was allowed), and to refrain from alcohol use (24 h) and recreational drug use (7 days).
After completing an informed consent form and a recent food intake questionnaire, participants practiced the first calibration to balance reward probabilities of the effort task across participants (see “Calibration phase—determination of the reward probability” section). Next, they were asked to choose their preferred sweet and their preferred salty reward [sweet: wine gums, M&Ms, or Skittles; salty: cocktail nuts (crusted peanuts), Pringles (original), or salty crackers (TUC, paprika)]. They rated how hungry, full, and thirsty they felt, and how much they wanted and liked (during tasting) their chosen sweet and salty reward on 100-mm digital visual analogue scales (VAS) ranging from 0 (“not at all”) to 10 (“very (much)”). Following the ratings, participants learned which color (blue, green) would later cue which reward (salty, sweet) through stimulus-outcome learning (see “Stimulus-outcome learning” section). Next, they practiced the task (see below). After this, anthropometric measurements [BMI (weight(kg)/(height (m)2)] and waist-hip ratio [waist(cm)/hip(cm)] were taken. Subsequently, participants performed the task in two fMRI runs (pre- and post-devaluation runs) in the same session, separated by the outcome devaluation phase, which was performed outside the MR scanner. Twice during the task (after block 1 and 2 of the pre- and post-devaluation runs) participants rated how hungry, full, and thirsty they were on VASs. Directly after each run, they received 1/5 of their winnings and consumed the snacks. A high-resolution anatomical scan (T1, see “(f)MRI data acquisition” section) was performed prior to the pre-devaluation task, and a resting state scan followed the post-devaluation task. After the post-devaluation run, participants rated hunger, fullness, thirst, wanting and liking once more, and filled out questionnaires (The Five Facet Mindfulness Questionnaire (FFMQ23), The Three-Factor Eating Questionnaire (TFEQ24), and the Short Food Frequency Questionnaire—Dutch Healthy Diet index (SFFQ-DHD25); Supplementary Table S1 and S2).
Distraction task: categorical visual detection task
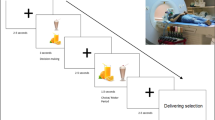
Participants performed a categorical visual detection task during fMRI scanning (Fig. 1, adapted from our previous fMRI study9). Each trial (total duration: 14–18 s) started with a fixation cross (jittered duration of 1–2 s, uniformly distributed), followed by an instruction screen (1 s), indicating the category of pictures for which the participant should count the targets (furniture, tools, or toys), and the speed of the trial (‘>’ for a slow trial, ‘>>>’ for a fast trial. For example, if the instruction screen stated: “category: furniture, >>>”, this meant participants needed to count stimuli in the category furniture, and the pictures would be presented at high speed. In order to keep visual stimulation equal for both trial types, a visual mask always followed a picture. The visual masks were scrambled versions of the stimulus pictures, to keep luminance equal. For the low speed trials, both pictures and visual masks were presented for 750 ms. For the high speed trials, pictures were presented for 75 ms, and the visual mask for 675 ms. Consequently, there were twice as many pictures and visual masks in the high speed trials relative to the low speed trials (12 vs. 6), thus, a higher attentional load. At the end of each trial, participants had to indicate how many target stimuli (i.e. a picture belonging to the instructed category) they had seen by answering a 3-alternative forced choice (3AFC) question on screen (“How many targets did you see?”) with their right hand. One of the three answering options was the correct answer, the other two options varied one or two digits from the correct answer. For example, if the correct answer was “2”, the answering options would be: [0, 1, 2], [1, 2, 3], or [2, 3, 4]. The order of the options on screen was shuffled to ensure participants could not prepare their answer motorically. If they answered within the 1500 ms time limit for responding to the question, the selected answer was presented (500 ms) in bold letters. If participants exceeded the time limit, the text “TOO LATE” was presented (500 ms) and the task continued. Participants made responses using an MRI-compatible button box. Participants received no feedback on whether they answered the target question correctly.
Trial structure for the categorical visual detection task with effort component. Each trial started with a verbal (word) and visual (color) cue indicating which snack could be won (word: sweet or salty, color: blue or green vending machine). An instruction screen followed, indicating the target category (furniture, tools, or toys) and difficulty (low (‘>’) or high (‘>>>’) of the trial. During the trial, target or non-target pictures were presented followed by a visual mask, and participants were instructed to count the pictures belonging to the instructed category. In the low distraction condition, six pictures were presented for 750 ms, giving subjects sufficient time to process the pictures. In the high distraction condition, twelve pictures were presented very briefly (only 75 ms), to increase the attentional load required to process the pictures. Simultaneously, a vending machine was presented on screen, which subjects could tilt by pressing one of two buttons with their left index finger to obtain the cued reward. Each of the two buttons was associated with either the sweet or the salty reward, which implied the vending machine could only be tilted during a trial when the button associated with the cued snack was used. The reward probability increased with the number of button presses subjects exerted (subjects have to exert effort to obtain the rewards), however never fell below 10% or above 90%. At the end of the trial, subjects indicated within 1500 ms how many target pictures they saw with their right hand (3AFC). Pre-devaluation, subjects received feedback on the amount of snacks won every 8 or 9 trials. The post-devaluation phase was performed in nominal extinction (i.e., no feedback was delivered).
Effort task
During the distraction task, participants simultaneously performed the effort task to win sweet or salty rewards (Fig. 1). This task started with an instruction screen (1 s), which followed the instruction screen for the distraction task, and indicated which reward (salty, sweet) the participant could win during the trial. On screen, a vending machine21 was presented with green or blue windows and the text: “snack to win: sweet”, or: “snack to win: salty”. The color of the vending machine was associated with the reward that could be won: the green color was always associated with winning the salty reward, and the blue color with the sweet reward (color-reward associations were counter-balanced). During the trial, the vending machine with the associated color was presented in the top middle of the computer screen, above the stimuli of the distraction task. To win the reward, participants had to exert effort to ‘tilt’ the vending machine to the left or right by repeatedly pressing the corresponding button (tilt left = left button, tilt right = right button) with their left index finger. The left button was always associated with obtaining one reward, the right with the other. Button-reward associations were counter-balanced across participants, and learnt by trial and error at task practice during which participants received direct feedback on whether they won the reward. During trials where the sweet reward could be won, the vending machine tilted only to the side associated with obtaining the sweet reward, and vice versa for the salty reward. Pressing the associated button more often resulted in a larger reward probability, i.e. exerting more effort related to a larger chance of winning the reward. Pressing the corresponding button at least once resulted in a reward probability of 10%. If participants pressed equally often or more often than the maximum number of button presses during the individual calibration (see “Calibration phase—determination of the reward probability” section), the reward probability on a trial would be 90%. Not pressing at all, or pressing any other button, resulted in a reward probability of 0%.
Post-devaluation, participants performed the dual task again during fMRI scanning. The pre- and post-devaluation tasks were similar, except no direct feedback was delivered on whether they won the reward post-devaluation (i.e. nominal extinction). Pre-devaluation, subjects received feedback on the amount of snacks won every 8 or 9 trials. Hence, these trials with feedback were longer (16–18 s instead of 14–15 s).
Outcome devaluation phase
After participants performed the dual task for the first time in the MR-scanner (pre-devaluation run), they were taken to a behavioral lab for the outcome devaluation phase. Sensory-specific satiation for the sweet or savory snack was randomized across participants. Two bowls were placed in front of the participant. One bowl was filled with a fixed amount (10% of participants’ energy need, based on their basal metabolic rate (BMR26) of one of the snacks (sweet or savory, counter-balanced), the other with an excessive amount of the same snack. Participants were asked to eat to satiation and to finish the fixed portion. If this portion did not satiate them, they were instructed to continue eating ad libitum from the other bowl. Unbeknownst to participants, we weighed the bowls before and after consumption to calculate the amount of calories consumed (Supplementary Table S3). Before and after the outcome devaluation phase, participants again rated how much they wanted and liked (after tasting) both rewards.
Dual task: effort + distraction
Pre- and post-devaluation, participants performed in total eight blocks of 25 trials (a total of 200 trials), i.e. four blocks per run. Both pre- and post-devaluation runs had the following numbers of trials: 50 low distraction trials (25 salty reward, 25 sweet reward); 50 high distraction trials (25 salty reward, 25 sweet reward). During task practice, participants performed a total of 32 trials (6 trials: distraction task only; 6 trials: effort task only, 20 trials: full dual task (distraction + effort task)). Category and reward presentation were pseudo-randomized, i.e. the same category and reward were never presented more than 3 times in a row. Moreover, maximally two target stimuli were presented after another.
Calibration phase—determination of the reward probability
Pilot data showed large inter-individual baseline differences in the number of button presses individuals can exert in a certain time frame. Therefore, prior to the pre-devaluation run, we measured participants’ individual maximum button presses in a calibration phase. Whilst in the MR-scanner, participants pressed each of the buttons (left and right) on the button box (used during the effort task) with their left index finger as often as they could during 14 s (the approximate duration of a trial in the dual task). The probability of obtaining a reward during a trial in the dual task was calculated based on the number of button presses participants exerted in the current trial, and their maximum number of button presses. The following formula was used in the calculation:
-
(1)
Reward probability of a trial = minimum probability + ((maximum probability − minimum probability) * (current number of button presses in a trial/maximum number of button presses determined during calibration phase)).
The minimum probability was set to 0.1, given that participants pressed the button corresponding to the reward at least once. The maximum probability was set to 0.9.
Stimulus-outcome learning
To ensure participants learned the associations between the color (stimuli: blue, green) of the vending machine and the rewards (outcomes: sweet, salty), we provided them with three pieces of their preferred sweet and salty reward and paired each piece with digital presentations of the associated color. On screen, a blue or green square was presented (3 s) which was followed by the text: “Now, please eat one of the [X] snacks. Press ENTER on the keyboard after you’ve finished eating it”.[X] was filled with “SWEET” or “SALTY”. In total, 6 stimulus-outcome pairings were presented (3 sweet, 3 salty). Prior to each trial in the actual fMRI task, i.e. at the time of the instruction screen cueing the reward that could be won during the trial, the trial-specific color-reward association was repeated. At the end of the test session, we asked participants to recall the associations.
Behavioral analyses: performance
To test whether participants performed significantly better on the low (versus high) load trials of the distraction task (i.e. correct detection of the number of targets in a trial), we calculated mean weighed accuracies across trials per condition using the following formula:
-
(2)
weighed accuracy = 1 − (mean(abs(actual number of targets − chosen number of targets))).
In this calculation, the absolute difference (abs, i.e. the non-negative value of the difference without regard to its sign) between the correct answer and the answer participants gave was used to account for whether participants were 1 or 2 targets off the correct answer (the latter case being more erroneous than the former). Mean weighed accuracies were analyzed using repeated measures ANOVA (IBM SPSS Statistics 23, Chicago, IL) with attentional Load (high, low), Reward (valued, devalued), and Time (pre-, post-devaluation) as within-subject factors.
Behavioral analyses: devaluation effect
As a subjective measure of devaluation magnitude, we assessed wanting ratings for the sweet and salty snack over time [measured at four time points: at baseline (t0), directly before the devaluation (t1), directly after the devaluation (t2), and after completing the post-devaluation run (t3)]. If the devaluation was successful, we expected the wanting ratings to decrease significantly for the reward that would be devalued, and not for the reward that would remain valued. This was tested using repeated measures ANOVA with within-subject factors Time (t0, t1, t2, t3) and Reward (valued, devalued). Additionally, we tested for pre-experimental wanting differences (wanting at t0) for the valued versus devalued snack using a paired-samples t test.
Effect of distraction on goal-directed effort (repeated button presses)
On each trial of the effort task, participants tilted the vending machine a number of times through button presses, i.e. they exerted effort, to obtain the associated reward. Mean effort values were calculated by averaging the number of button presses across all trials for each condition. To test whether effort decreased significantly for the devalued, but not for the valued reward (i.e. the degree to which effort became goal-directed after devaluation), we assessed the Time (pre, post) × Reward (valued, devalued) interaction using repeated measures ANOVA. The effect of distraction on goal-directed effort was determined by looking at the Load (high, low) × Reward (valued, devalued) × Time (pre, post) interaction (primary objective).
(f)MRI data acquisition
(f)MRI data acquisition was performed in a similar fashion as in our previous fMRI study9: To measure blood oxygen level dependent (BOLD) contrast, whole-brain functional MRI images were acquired on a Siemens 3T Skyra MRI scanner (Siemens Medical system, Erlangen, Germany) using a 32-channel head coil. During the task, 3D echo planar imaging (EPI) scans using a T2 * weighted gradient echo multi-echo sequence (Centre for Magnetic Resonance Imaging, University of Minnesota) were acquired (voxel size 3.5 × 3.5 × 3 mm, TR = 2070 ms, TE = 9 ms; 19.1 ms; 29.2 ms; 39.3 ms, FoV = 224 mm). The slab positioning and rotation (approximate average angle of 14 degrees to AC axis) optimally covered both prefrontal and subcortical brain regions. Before the acquisition of functional images, a high-resolution anatomical scan was acquired (T1-weighted scan, MPRAGE, voxel size 1 × 1 × 1 mm, TR = 2300 ms, TE = 3.03 ms, 192 sagittal slices, flip angle 8°, field of view 256 mm).
(f)MRI image processing
Data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm) and FSL version 5.0.11 (https://www.fmrib.ox.ac.uk/fsl/). The volumes for each echo time were realigned to correct for motion artefacts (estimation of the realignment parameters is done for the first echo and then copied to the other echoes). The four echo images were combined into a single MR volume using an optimized echo weighting method (TE-weighting27,28). Combined functional images were slice-time corrected by realigning the time-series for each voxel temporally to acquisition of the middle slice and spatially smoothed using an isotropic 8 mm full-width at half-maximum Gaussian kernel. Next, ICA-AROMA29 (non-aggressive) was used to reduce motion-induced signal variations in the fMRI data. Subject-specific structural and functional data were then coregistered to a standard structural or functional stereotactic space (Montreal Neurological Institute (MNI) template) respectively. After segmentation of the structural images using a unified segmentation approach, structural images were spatially coregistered to the mean of the functional images. The resulting transformation matrix of the segmentation was then used to normalize the anatomical and functional images into MNI space. The functional images were resampled to 2 × 2 × 2 mm using trilinear interpolation.
Statistical fMRI analysis
Statistical analysis of fMRI data was performed using a general linear model (GLM) approach. The images of both experimental runs (pre- and post-devaluation runs) were combined into one model. At the individual (first) level, subject-level data were analyzed using a fixed effects model, which included four regressors of interest per run. These modeled the trials of valued reward, low load; valued reward, high load; devalued reward, low load; devalued reward, high load. Durations of these regressors represented the moment the first picture of the detection task was presented until presentation of the target question (mean duration: 9.18 s, SD: 0.09 s, SEM: 0.001 s). Parametric modulators reflecting the number of button presses per trial were added to each regressor of interest, to correct for signal change induced by the difference in number of button presses between trials within each condition. Six additional regressors of non-interest were added, representing onsets of the reward instruction screen (one for valued and one for devalued reward trials), and presentation of the target question (“How many targets did you see?”, one for low and one for high load trials). The other two regressors reflected average signal variation in white matter and cerebrospinal fluid regions. All regressors were convolved with the canonical hemodynamic response function. High pass temporal filtering (128 s) was applied to the time series of the functional images to remove low-frequency drifts and correction for serial correlations was done using an autoregressive AR(1) model.
At the group level, we assessed the main effect of Load (high > low load) by contrasting high with low load trials across both runs (pre, post) and rewards (valued, devalued). To identify brain areas sensitive to goal-directed effort, we computed the difference between the two rewards post-devaluation (valued, devalued) relative to their respective baseline (pre-devaluation) during button presses for reward.
To identify our a priori defined vmPFC region in this functional contrast (see preregistration), we used an 8-mm spherical region-of-interest (ROI) centered on coordinates [− 2, 32, − 21] derived from averaged coordinates from previous studies that reported vmPFC responses during instrumental conditioning and/or outcome devaluation16,30,31. We used the Hammersmith atlas to determine whether the activated areas overlapped with the fronto-striatal regions defined in the atlas (bilateral caudate nucleus (regions 34;35), nucleus accumbens (36;37) and putamen (38;39) for striatum, bilateral middle frontal gyrus (28;29)32, precentral gyrus (50:51), straight gyrus (52;53), anterior orbital gyrus (54;55), inferior frontal gyrus (56;57), superior frontal gyrus (58;59), medial orbital gyrus (68;69), lateral orbital gyrus (70;71), posterior orbital gyrus 72;73), subgenual frontal cortex (76;77), subcallosal area (78;79), and pre-subgenual frontal cortex for the frontal regions33.
To investigate the effect of Load on processing in the vmPFC (primary objective) and other fronto-striatal regions (secondary objective) involved in goal-directed control, we assessed the interaction effect of Load (low > high Load) × Reward (valued > devalued) × Time (post > pre). The activated regions for the goal-directed effort contrast (i.e., Time × Reward) were used as ROIs for the Load × Reward × Time interaction contrast. Mean beta weights were extracted from all voxels in the identified ROIs using MarsBar34. These beta-weights were analyzed using ANOVA with the same factors as in the whole-brain analyses (Load, Reward and Time).
Distraction-related functional connectivity analysis
We used a generalized psychophysiological interaction (gPPI35) analysis to investigate whether the (seed) region(s) showing a distraction effect on goal-directed control (Load × Reward × Time, see primary and secondary objectives above) exhibited differential fMRI BOLD connectivity for the same 3-way interaction (secondary objective). Seed regions were determined at a whole-brain uncorrected threshold of p < 0.001. To estimate the neural activity producing the physiological effect in the seed region for each subject, the BOLD signal was extracted from this region and deconvolved36. This was included in the model as the physiological regressor. The task regressors for each of the relevant task conditions (i.e. the psychological regressors: low load, valued reward; low load, devalued reward; high load, valued reward; and high load, devalued reward) were also added to the model. The psychophysiological interaction was entered by multiplying the estimated neural activity (i.e., the physiological regressor) by the duration times for each of the task conditions (i.e., the psychological regressors) separately convolved with the HRF, resulting in nine regressors of interest per run on the first level (i.e., one physiological, four psychological, and four interaction regressors). For each participant, we created a PPI contrast for the 3-way interaction effect of Load (high > low load), Reward (valued > devalued reward), and Time (post > pre devaluation). On the group level, this PPI contrast was analyzed separately using a one-sample t test.
In an exploratory analysis, we repeated the same PPI procedure with the vmPFC cluster found in the Reward * Time contrast as a seed; also if this vmPFC region did not show a Load × Reward × Time effect.
All fMRI analyses were performed on the whole-brain level and with use of ROI analysis where specified. The results of all random effects fMRI analyses were thresholded at p < 0.001 (uncorrected) and statistical inference was performed at the cluster level, family-wise-error-corrected (pFWE < 0.05) for multiple comparisons over the search volume (the whole brain or a smaller search volume based on the defined ROIs).
Analyses: brain-behavior correlations
Exploratory, we investigated whether the effect of Load on goal-directed effort covaried significantly with the effect of Load on processing in areas sensitive to goal-directed control. We executed a repeated measures ANCOVA with Load (high, low), Reward (valued, devalued), and Time (pre, post) as within-subject factors. The (brain) effect of Load in areas sensitive to goal-directed control reflected in the averaged extracted beta weights was used as dependent variable, and the difference score for the same effect on (behavioral) effort as covariate.
Results
Manipulation check: effect of attentional load in behavior
To test whether our load manipulation to assess the effects of distraction (Fig. 1) worked, we compared performance between the high and low load conditions. Indeed, participants were less accurate when answering the 3AFC target question (“How many targets did you see?”) when the visual targets were rapidly presented, i.e. the high load trials [weighed mean accuracy (± SEM): 0.63 (0.02)], than when the visual targets were slowly presented, i.e. the low load trials (weighed mean accuracy (± SEM): 0.81 (0.02)), (F(1,37) = 68.31, p < 0.001). Reward outcome or time did not affect performance (Reward (valued, devalued): F(1,37) = 1.23, p = 0.27; Time (pre, post): F(1,37) < 1, p = 0.63, Table 1).
Manipulation check: effect of devaluation on wanting ratings and effort for food reward in behavior
We assessed the change in wanting ratings over time for the valued versus devalued reward, as a measure of the devaluation magnitude induced by satiation on the (to be) devalued reward. As expected, how much participants wanted the snack decreased significantly for the devalued, but not for the valued, reward after devaluation (Time (t0, t1, t2, t3) × Reward (valued, devalued): F(1,35) = 31.00, p < 0.001, Supplementary Table S2). We further found main effects of Time (F(1,35) = 31.82, p < 0.001) and Reward (F(1,37) = 33.86, p < 0.001), reflecting an overall decrease in wanting for both rewards after the devaluation, and overall lower wanting ratings for the devalued relative to the valued reward. At baseline (t0), wanting did not differ significantly between the rewards (t(1,37) = 1.56, p = 0.13). Thus, the devaluation manipulation was successful. Similar results were found for changes in liking ratings (Supplementary Table S2; Supplementary Results: Liking ratings).
Furthermore, we tested whether the devaluation effect − reflected in the Time (pre, post) × Reward (valued, devalued) interaction − appeared in the number of button presses exerted to obtain the rewards (Table 1). Unexpectedly, this interaction was not significant (F(1,37) < 1, p = 0.57). However, we did find a significant main effect of Load (F(1,37) = 38.59, p < 0.001). Participants pressed more often in the high versus low load condition [Mhighload (± SEM): 40.31 (0.95), Mlowload (± SEM): 39.48 (0.95)]. Furthermore, we found a trend main effect of Reward (F(1,37) = 3.00, p = 0.09). Participants tended to press more often for the valued than for the devalued reward [Mvalued (± SEM): 40.11 (0.90), Mdevalued (± SEM): 39.68 (0.96)].
Primary objective: effect of attentional load on goal-directed effort for food reward in behavior
We tested whether attentional load affected goal-directed effort for the food rewards (i.e., as a function of outcome devaluation). The interaction between Load, Time, and Reward was not significant (F(1,37) < 1, p = 0.88).
Manipulation check: effect of attentional load in fMRI
To test whether our distraction manipulation—operationalized by varying attentional load—activated brain regions typically involved in such tasks, we first assessed the effect of Load (high > low) on a whole-brain corrected threshold (pFWE < 0.05, at the cluster-level). At this threshold, we indeed found an effect of attentional load (high load > low load across reward types and time) in fronto-parietal and visual regions (Table 2, Fig. 2).
Main effect of attentional load (distraction manipulation). Contrast of high versus low (red, respectively), and low versus high load (blue, respectively) trials depicted at a p < 0.001, uncorrected threshold. For whole-brain (FWE < 0.05) corrected effects see Table 2. All statistical parametric maps were overlaid onto a T1-weighted canonical image [ch2better.nii.gz atlas using MRIcron software (https://www.mricro.com/mricron/install.html)]. Slice coordinates were defined in MNI152 space and images are shown in neurological convention (left = left).
Manipulation check: effect of devaluation on effort for food reward in fMRI
To identify brain regions involved in goal-directed effort for the valued versus devalued reward, we assessed baseline-corrected differential responses for these rewards (Time(pre, post) × Reward(valued, devalued) interaction). The valued > devalued contrast showed significant whole-brain responses of a region in the anterior cingulate cortex (ACC: [8, 16, 40], pFWE(cluster) = 0.001, t = 4.76, k = 505, Fig. 3 (left), Table 2). At pFWE < 0.05 (small volume correction using the a priori defined spherical search region described in the “Methods” section), we did not observe a devaluation effect in the vmPFC. Only at an exploratory threshold of p < 0.001 uncorrected, we found increased responses in the vmPFC ([6, 52, − 8]), but unexpectedly for the reverse contrast: devalued > valued rewards (post > pre; see Fig. 3, right).
Time (pre,post) × Reward (valued, devalued) interaction effect (manipulation of goal-directed effort), showing responses of the ACC (left; pFWE (cluster) = 0.001) and vmPFC (right; p < 0.001, uncorrected). Left (yellow): contrast of valued outcome versus devalued outcome trials (val > dev, post > pre). Right (blue): contrast of devalued outcome versus valued outcome trials (dev > val, post > pre). All parametric maps are depicted at a p < 0.001, uncorrected threshold.
Primary objective: effect of attentional load on goal-directed effort for food reward in vmPFC
As a primary objective, we assessed the effect of load on processing related to goal-directed control for food rewards in the vmPFC functional ROI showing the Reward * Time effect (see Fig. 3, right). The extracted beta weights across the vmPFC ROI did not show a Reward * Time * Load interaction (F(1,37) < 1, p = 0.53) in addition to the Reward * Time effect that the voxels were selected on. We only observed a main effect of Reward (F(1,37) = 6.72, p = 0.01) independent of Time, reflecting generally larger vmPFC responses for the devalued than the valued reward.
Exploratory: effect of attentional load on goal-directed effort for food reward in ACC
Because the ACC showed a whole-brain significant Reward * Time effect (see Fig. 3, left), we also exploratory assessed the effect of load on processing related to goal-directed control for food rewards in this region. The averaged extracted beta weights across the ACC cluster showing the effect related to goal-directed control (Time × Reward), did not show a Reward * Time * Load interaction (F(1,37) < 1, p = 0.52) in addition to the Reward * Time effect that the voxels were selected on. We only observed a main effect of Load (F(1,37) = 87.98, p < 0.001), reflecting overall more ACC activity for the high, relative to low, load trials.
Secondary objective: effect of attentional load on goal-directed effort for food reward in other fronto-striatal regions
As a secondary objective, we performed a whole-brain analysis to assess the effect of attentional load on goal-directed effort for food reward in other fronto-striatal regions. However, at pFWE < 0.05 (small volume correction using our anatomically-defined fronto-striatal mask from the Hammersmith atlas described in the “Methods” section), we did not observe an effect of distraction on goal-directed responses in fronto-striatal regions.
Only at an exploratory threshold of p < 0.001, a region in the right inferior frontal gyrus (rIFG) responded ([44, 36, − 2], k = 88, Fig. 4A–C) to the three-way interaction contrast of Load (low > high), Time (post > pre), and Reward (devalued > valued). As mentioned, this cluster was not significant after small volume correction with our bilateral fronto-striatal mask (pFWE(cluster) = 0.19, t = 4.29). Post-hoc whole-brain simple effects showed that this region was activated in the Time(post > pre) × Reward(devalued > valued) interaction under low ([48, 36, 0], k = 122, pFWE(cluster) = 0.11, t = 4.31, Fig. 4A), but not high load. Thus, distraction tended to attenuate responses of the rIFG for devalued versus valued food reward after outcome devaluation.
Effect of load on the outcome devaluation effect [(A) left: Time (pre, post) × Reward (devalued, valued) × Load (low, high)] and the outcome devaluation effect for the low load conditions only [(A) right: two way interaction effect of Time (pre, post) × Reward (devalued, valued)]. Both contrasts showed responses of the rIFG. Under high load, the rIFG did not respond, suggesting that distraction tended to disrupt activation of this region. Bar graphs show parameter estimates in arbitrary units (a.u.) for the effect of load on the outcome devaluation effect (B) and the outcome devaluation effect for low load only (C). The bar graphs are shown for illustrative purposes only, therefore error bars and p values were omitted. All parametric maps are depicted at a p < 0.001, uncorrected threshold.
Secondary objective: distraction-related functional connectivity analysis in fMRI
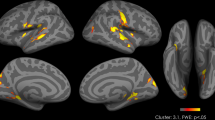
Given the trend effect (p < 0.001 uncorrected) in a fronto-striatal region for the Load * Reward * Time interaction, we used the rIFG cluster in a secondary connectivity analysis (see preregistration). We performed a gPPI analysis to investigate distraction-related (high-low) functional connectivity during goal-directed effort for food rewards. As a seed we used the region in the rIFG that showed an effect of distraction on goal-directed responses for food reward (Fig. 5, top left), extracted at p < 0.001 (uncorrected). The rIFG seed region showed increased functional connectivity with bilateral putamen for valued versus devalued food rewards (Time (post > pre) × Reward (valued > devalued) × Load (low > high) interaction contrast). The left region was significant after small volume correction using our anatomically-defined fronto-striatal mask from the Hammersmith atlas (see “Methods” section; [− 28, − 12, 4], pFWE(cluster) = 0.004, t = 5.16, k = 300, Fig. 5), whereas the right region showed a trend ([22 ,6, − 8], pFWE(cluster) = 0.07, t = 4.14, k = 129, Fig. 5). Thus, distraction attenuated connectivity between the right IFG and left putamen during goal-directed effort for food reward.
Results of the gPPI analysis with the rIFG seed region in the top left (encircled green, extracted from the Time (post > pre) × Reward (valued > devalued) × Load (low > high) interaction contrast). Shown (encircled blue) is the left [− 28, 12, 4] putamen exhibiting significantly (p = 0.004, FWE-corrected at the cluster-level) higher functional connectivity with the seed region under low, relative to high, distraction for valued versus devalued food rewards (effect of load on the outcome devaluation effect). For right [22, 6, − 8] putamen (encircled blue, dashed), this effect was marginally significant [p = 0.07, FWE(cluster)]. Further analysis showed this effect tended to be driven by responses of left putamen in the effect of outcome devaluation under low, but not high, distraction ([− 22, − 12, 2], t = 4.24, k = 29, p < 0.001, uncorrected). All parametric maps are depicted at a p < 0.001, uncorrected threshold. For whole-brain (FWE < 0.05) corrected effects see Table 2.
We further investigated this three-way interaction in rIFG connectivity with putamen by assessing the Time (post > pre) × Reward (valued > devalued) interaction effect separately for the low and high distraction conditions on the whole brain. Under low, but not high, distraction, the left (but not right) putamen showed larger connectivity with the rIFG for the valued relative to the devalued reward, however on a p < 0.001, uncorrected threshold ([− 22, − 12, 2], k = 29, t = 4.24, pFWE(cluster-level) = 0.61).
Exploratory: distraction-related functional connectivity analysis with the vmPFC
Given our a priori interest in the vmPFC, we also used this functional ROI as a seed in a gPPI. This analysis was exploratory, as we only planned to do functional connectivity analyses with fronto-striatal regions showing a Load * Reward * Time interaction as a seed (see preregistration). We did not observe any effects at pFWE < 0.05 (whole brain or small volume corrected using our anatomically-defined fronto-striatal mask from the Hammersmith atlas); see Supplementary Fig. S2 and S3.
Exploratory: brain-behavior correlations
Given the non-significant effects in both the behavioral and fMRI effects of distraction on effort for devalued versus valued reward outcomes across the group, we explored with correlational analyses whether inter-individual variability in both measures were perhaps meaningfully related. Using brain-behavior correlations, we demonstrated that the interaction effect (Load × Reward × Time) in the rIFG co-varied with the same interaction effect on the behavioral effort measure. That is, distraction-related rIFG decreases were associated with continued button presses for food reward after versus before devaluation (relation between Load × Reward × Time covariate [difference score] in button presses and Load × Reward × Time interaction effect in rIFG betas, i.e., four-way interaction: F(1,36) = 6.69, p = 0.01, r = − 0.40). Further analysis showed this relation was present after the outcome devaluation phase (relation between Load × Reward covariate in button presses [difference score] and Load × Reward interaction effect in rIFG betas, i.e., three-way interaction effect, post devaluation: F(1,36) = 5.54, p = 0.02, r = − 0.37), but not prior to the devaluation (relation between Load × Reward covariate in button presses [difference score] and Load × Reward interaction effect in rIFG betas, i.e., three-way interaction effect, pre devaluation: F(1,36) = 2.83, p = 0.10, r = − 0.27). Thus, distraction-induced reductions (low > high load) in rIFG processing for devalued (vs. valued) rewards were associated with increased effort after high (vs. low) distraction for devalued (vs. valued) food reward after outcome devaluation (Fig. 6).
Brain-behavior correlation for the relation between the distraction-sensitive rIFG responses for devalued versus valued food rewards (post-devaluation (devalued reward (low–high distraction) − valued reward (low–high distraction)) − pre-devaluation (devalued reward (low–high distraction) − valued reward (low–high distraction)), and the same effect for continued button presses (effort). The correlation is significant at the highest level (Time (post–pre devaluation) × Reward (devalued-valued reward) × Load (low–high distraction), r = 0.40, p = 0.014). Less distraction-induced goal-directed rIFG responses relate to continued button presses for food reward. Mean rIFG betas are presented in arbitrary units (a.u.).
To investigate whether the effect of distraction on connectivity between the rIFG and putamen correlated with effort, we used a similar approach. This correlation was not significant.
Discussion
To study the effect of distraction on effort for valued and devalued food reward, we used a food-related outcome devaluation paradigm, and manipulated distraction using a categorical visual detection task of varying attentional load. We hypothesized that distraction would disrupt goal-directed control for food reward in terms of button presses (primary objective), by acting on the vmPFC (primary objective) or other fronto-striatal regions (secondary objective), and by affecting functional connectivity with the vmPFC or other fronto-striatal regions resulting from these previous analyses (secondary objective) (see preregistration).
We failed to verify both our primary hypotheses. Specifically, distraction did not affect goal-directed responses behaviorally (i.e., in terms of button presses), neither did distraction affect goal-directed responses in the vmPFC. Already as a result of outcome devaluation, we would have expected to find more activation in the vmPFC for valued than devalued food reward, in line with its role in value-based decision making16,17,18,19. However, the vmPFC did not show this effect and, if anything, the reverse effect (devalued > valued at p < 0.001 uncorrected) occurred. This is likely due to the nature of the task, which was rather different from the instrumental choice paradigms used by aforementioned researchers. For the current research question, both experimental design and ecological-validity required the distraction and food-choice manipulations to co-occur for a continuous time. Specifically, a binary choice is much less likely to be influenced by an attentional load manipulation, as it only requires a brief decision. Repeated button presses, i.e. exerting effort, to obtain the food rewards during high or low distraction therefore offered a better ‘dual task’ manipulation during distraction than a single, binary choice paradigm. Moreover, Valentin and colleagues16 similarly found the vmPFC to show increased responses for devalued > valued rewards, but only in their low probability condition (with the expected valued > devalued direction under high probability). In contrast to Valentin and colleagues’ study, our participants did not receive their food reward immediately, but only after the scanner run. This might have affected the subjective feeling of the probability of getting a food reward. Thus, goal-directed behavior in terms of repeated button presses (“effort”) rather than a binary choice as well as delayed food rewards might be less likely to evoke vmPFC responses. Instead, we found significantly more activation in the ACC after outcome devaluation (valued > devalued); the effect that we would have expected in the vmPFC. Indeed, ACC responses have been associated with effort-based cost–benefit analyses and value-based effort allocation37,38,39, i.e., computations that are likely to be enhanced for valued versus devalued food rewards. Thus, despite the null results in vmPFC, these significant ACC responses might indicate that our devaluation manipulation was successful in terms of value-based effort computations.
Unexpectedly, however, our effort measure in terms of repeated button presses did not reflect goal-directed responses, i.e., differences in valued versus devalued rewards after (vs. before) satiation. This questions the validity of our measure of goal-directed behavior, even though our wanting ratings did show clear differences in valued versus devalued reward following satiation, meaning that the devaluation manipulation was successful. Perhaps the distraction manipulation added noise to our goal-directed effort measure. Distraction did not interact with goal-directed (valued vs. devalued, post vs. pre) button presses across the group as we had expected as part of our primary hypothesis. Instead, distraction showed a main effect on the button presses, meaning that a high attentional load resulted in an increase in button presses compared with low load trials. The participants might have been adapting their button presses to the tempo of the visual detection task (with more targets appearing in the high load condition). This unintentional effect has likely added noise to the effort-based goal-directed responses, perhaps overruling the more subtle devaluation effects.
Similar to the behavioral effect, we did not observe effects of distraction on goal-directed responses in the vmPFC or ACC ROIs, or at a pFWE corrected threshold in other fronto-striatal regions. Instead, high—versus low—distraction tended to reduce goal-directed responses for devalued versus valued food rewards in the rIFG (p < 0.001, uncorrected). Based on previous studies, we did not specifically anticipate finding this region for the effect of distraction on goal-directed responses. Hence, it did not survive multiple comparison correction in our large search volume of fronto-striatal regions. Interestingly, however, the distraction-related rIFG responses correlated significantly with the same effect in our behavioral effort measure (repeated button presses) in an exploratory brain-behavior analysis. Under low, but not high, distraction, larger responses of the rIFG related to less repeated button presses for the devalued versus valued food reward. Activation of the right IFG has been strongly associated with response inhibition40,41. Therefore, this result might reflect worse response inhibition in response to the devalued versus valued food reward under distraction, in line with its negative association with the number of button presses. We speculate that our participants had to suppress their automatic tendency to press for the (now) devalued reward, as their focus was on performing the visual detection task. Our results indicate that for participants with relatively less rIFG activation under high versus low distraction, more effort was exerted for the devalued reward, which suggests worse response inhibition in these individuals. Although the exploratory brain-behavior correlation with the rIFG was significant and seems meaningful, these findings should be interpreted with caution as the distraction effect on goal-directed responses in rIFG was not significant at a corrected threshold. Future research designed to study the role of distraction in response inhibition for devalued versus valued food rewards should confirm the role of the rIFG.
The p < 0.001 uncorrected result in the rIFG for the effects of distraction on goal-directed responses did result in planned functional connectivity analyses with the rIFG as a seed (secondary objective). Under high distraction, we found weaker connectivity between the rIFG and left putamen for valued versus devalued food rewards (post vs. pre satiation). Like the rIFG, the putamen has been associated with successful response inhibition40. This result therefore seems to support the idea that distraction during repeated button presses for food rewards attenuates the mechanism underlying successful response inhibition. Interestingly, the putamen/dorsolateral striatum is also well known for its role in habitual as opposed to goal-directed control12. However, the direction of observed effects does not seem to support this role. Specifically, we would expect that connectivity with a region involved in habitual responses (devalued > valued rewards, post > pre) would be visible under high distraction. Instead, if anything, we found increased connectivity with the putamen for goal-directed (valued > devalued rewards, post > pre) responses under low distraction. Future confirmatory research should clarify the role of rIFG-putamen connectivity in the effects of distraction on goal-directed responses.
In contrast to these significant effects of distraction on rIFG-putamen connectivity across the group, our distraction-related rIFG responses were only significant in a between-subject correlation with behavior. This shows that there is substantial individual variation in the effects of distraction on food (reward)-related processing. These individual differences are in line with our previous study, in which distraction during food consumption attenuated taste-related processes in the insula, but only in relation to increases in food intake9. Future studies should investigate the relative contribution of impairments in taste processing versus response inhibition in distracted (over)eating in real life, and the individual susceptibility to distraction during food-related behavior.
Taken together, our primary hypotheses of distraction attenuating effortful responses for food rewards following sensory-specific satiation in vmPFC and behavior were not confirmed. Secondary analyses did confirm distraction-induced reductions in functional connectivity between the rIFG and the putamen during goal-directed behavior. Exploratory analyses show that distraction might diminish goal-directed responses of the rIFG only in individuals that are more susceptible to the distraction manipulation in terms of their behavioral responses. Both the rIFG and the putamen have been implicated in response inhibition. Therefore, distraction during food-related effort could perhaps attenuate response inhibition, in line with the observed brain-behavior correlation with rIFG responses. Interestingly, impaired response inhibition is also highly implicated in overweight and obesity42. However, before focusing on clinical implications, confirmatory research should be designed to address the role of the rIFG in the (susceptibility for the) effect of attention/distraction on response inhibition during goal-directed responses for food reward.
Data availability
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction upon acceptation.
References
Carrier, L. M., Rosen, L. D., Cheever, N. A. & Lim, A. F. Causes, effects, and practicalities of everyday multitasking. Dev. Rev. 35, 64–78 (2015).
de Graaf, C. & Kok, F. J. Slow food, fast food and the control of food intake. Nat. Rev. Endocrinol. 6, 290–293 (2010).
Teo, E., Goh, D., Vijayakumar, K. M. & Liu, J. C. J. To message or browse? Exploring the impact of phone use patterns on male adolescents’ consumption of palatable snacks. Front. Psychol. 8, 1–7 (2018).
Robinson, E. et al. Eating attentively: a systematic review and meta-analysis of the effect of food intake memory and awareness on eating. Am. J. Clin. Nutr. 97, 728–742 (2013).
Braude, L. & Stevenson, R. J. Watching television while eating increases energy intake. Examining the mechanisms in female participants. Appetite 76, 9–16 (2014).
Morris, J., Vi, C. T., Obrist, M., Forster, S. & Yeomans, M. R. Ingested but not perceived: response to satiety cues disrupted by perceptual load. Appetite 155, 104813 (2020).
Bickham, D. S., Blood, E. A., Walls, C. E., Shrier, L. A. & Rich, M. Characteristics of screen media use associated with higher BMI in young adolescents. Pediatrics 131, 935–941 (2013).
Shiv, B. & Nowlis, S. M. The effect of distractions while tasting a food sample: the interplay of informational and affective components in subsequent choice. J. Consum. Res. 31, 599–608 (2004).
Duif, I. et al. Effects of distraction on taste-related neural processing: a cross-sectional fMRI study. Am. J. Clin. Nutr. 111(5), 950–961 (2020).
Higgs, S. et al. Interactions between metabolic, reward and cognitive processes in appetite control: implications for novel weight management therapies. J. Psychopharmacol. 31, 1460–1474 (2017).
van Dillen, L. F. & van Steenbergen, H. Tuning down the hedonic brain: cognitive load reduces neural responses to high-calorie food pictures in the nucleus accumbens. Cogn. Affect. Behav. Neurosci. 18, 447–459 (2018).
Balleine, B. W. & O’Doherty, J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010).
Rolls, E. T., Rolls, B. J. & Rowe, E. A. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol. Behav. 30, 185–192 (1983).
Fletcher, P. C. et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J. Neurosci. 30, 14346–14355 (2010).
de Wit, S., Corlett, P. R., Aitken, M. R., Dickinson, A. & Fletcher, P. C. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J. Neurosci. 29, 11330–11338 (2009).
Valentin, V. V., Dickinson, A. & O’Doherty, J. P. Determining the neural substrates of goal-directed learning in the human brain. J. Neurosci. 27, 4019–4026 (2007).
Gläscher, J., Hampton, A. N. & O’Doherty, J. P. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb. Cortex 19, 483–495 (2009).
Tanaka, S. C., Balleine, B. W. & O’Doherty, J. P. Calculating consequences: brain systems that encode the causal effects of actions. J. Neurosci. 28, 6750–6755 (2008).
Rangel, A. Regulation of dietary choice by the decision-making circuitry. Nat. Neurosci. 16, 1717–1724 (2013).
Hogarth, L., Chase, H. W. & Baess, K. Impaired goal-directed behavioural control in human impulsivity. Q. J. Exp. Psychol. 65, 305–316 (2012).
Morris, R. W., Quail, S., Griffiths, K. R., Green, M. J. & Balleine, B. W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol. Psychiatry 77, 187–195 (2015).
Van Strien, T., Frijters, J., Bergers, G. & Defares, P. B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 5, 295–315 (1986).
Baer, R. A., Smith, G. T., Hopkins, J., Krietemeyer, J. & Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment 13, 27–45 (2006).
Stunkard, A. J. & Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 29(1), 71–83 (1985).
van Lee, L. et al. Evaluation of a screener to assess diet quality in the Netherlands. Br. J. Nutr. 115, 517–526 (2016).
Schofield, W., Schofield, C. & James, W. Basal metabolic rate—review and prediction together with annotated bibliography of source material. Hum. Nutr. Clin. Nutr. S1, 5–96 (1985).
Poser, B. A., Versluis, M. J., Hoogduin, J. M. & Norris, D. G. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magn. Reson. Med. 55, 1227–1235 (2006).
Posse, S. Multi-echo acquisition. Neuroimage 62, 665–671 (2012).
Pruim, R. H. R. et al. NeuroImage ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
Daw, N. D., O’Doherty, J. P., Dayan, P., Seymour, B. & Dolan, R. J. Cortical substrates for exploratory decisions in humans. Nature 441, 876–879 (2006).
Kim, H., Shimojo, S. & O’Doherty, J. P. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 4, 1453–1461 (2006).
Hammers, A. et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 19, 224–247 (2003).
Gousias, I. S. et al. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage 40, 672–684 (2008).
Brett, M., Anton, J. L., Valabregue, R. & Poline, J. B. Region of interest analysis using an SPM toolbox. Neuroimage 16, 497 (2002).
McLaren, D. G., Ries, M. L., Xu, G. & Johnson, S. A. A generalized form of context-dependent psychophysiological interactions (gPPI). Neuroimage 61, 1277–1286 (2012).
Gitelman, D. R., Penny, W. D., Ashburner, J. & Friston, K. J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 19, 200–207 (2003).
Kurniawan, I. T., Guitart-Masip, M., Dayan, P. & Dolan, R. J. Effort and valuation in the brain: the effects of anticipation and execution. J. Neurosci. 33, 6160–6169 (2013).
Shenhav, A., Botvinick, M. & Cohen, J. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013).
Vassena, E., Holroyd, C. B. & Alexander, W. H. Computational models of anterior cingulate cortex: at the crossroads between prediction and effort. Front. Neurosci. 11, 1–9 (2017).
Zandbelt, B. B. & Vink, M. On the role of the striatum in response inhibition. PLoS ONE 5, 1–11 (2010).
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J. & Robbins, T. W. Erratum: stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 1329–1329 (2003).
Stice, E. & Burger, K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 68, 38–53 (2019).
Acknowledgements
This work was supported by a public-private Food, Cognition, and Behavior grant funded by the Netherlands Organization for Scientific Research (NWO, Grant 057-14-001) with financial support of Mars Wrigley. We would like to thank Britt Lambregts, Marlou Lasschuijt and Lieke van Lieshout for their help in conducting the study. Preregistration: https://osf.io/ad2qk.
Author information
Authors and Affiliations
Contributions
I.D., J.W., K.d.G., P.A.M.S., and E.A. designed research; I.D. performed research; I.D., J.W. and E.A. analyzed data; I.D. and E.A. wrote the first version of the paper. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duif, I., Wegman, J., de Graaf, K. et al. Distraction decreases rIFG-putamen connectivity during goal-directed effort for food rewards. Sci Rep 10, 19072 (2020). https://doi.org/10.1038/s41598-020-76060-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76060-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.