Abstract
Understanding the structural dynamics of lead-halide perovskites is essential for their advanced use as photovoltaics. Here, the structural dynamics of the CH3NH3 cation and PbBr6 octahedra in the perovskite CH3NH3PbBr3 were studied via nuclear magnetic resonance (NMR) to determine the mechanism of the transition from the tetragonal to cubic phase. The chemical shifts were obtained by 1H, 13C, and 207Pb magic angle spinning NMR and 14N static NMR. The chemical shifts of the 1H nuclei in CH3 and NH3 remained constant with increasing temperature, whereas those of the 13C and 207Pb nuclei varied near the phase transition temperature (TC = 236 K), indicating that the structural environments of 13C and 207Pb change near TC. The spin–lattice relaxation time T1ρ values for 1H, 13C, and 207Pb nuclei increased with increasing temperature and did not exhibit an abrupt change near TC. In addition, the two lines in the 14N NMR spectra superposed into one line near TC, indicating the occurrence of a phase transition to a cubic phase with higher symmetry than tetragonal. Consequently, the main factor causing the phase transition from the tetragonal to cubic phase near TC is a change in the surroundings of the 207Pb nuclei in the PbBr6 octahedra and of the C–N groups in the CH3NH3 cations.
Similar content being viewed by others
Introduction
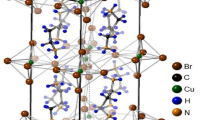
Lead-halide perovskites currently represent the most promising photovoltaic materials for the production of low-cost, high-performance solar cells1,2. In recent years, researchers have succeeded in significantly improving the power conversion efficiency (PCE) of this hybrid perovskite, and rapid advances in this field led to a record-high PCE. The very important for optoelectronic heterostructures are solar cells, photodetectors, and laser diodes3,4,5,6. For this class of materials, which has the general formula CH3NH3PbX3 (X = Cl, Br, and I), an inorganic cage of PbX6 octahedra encloses an organic cation at the CH3NH3+ site7,8,9,10,11,12. The phase transition temperatures of CH3NH3PbBr3, a representative perovskite, are 148.8, 154, and 236.3 K, corresponding to a total of four crystal phases13,14; with decreasing temperature, the cubic phase (I) transforms to a tetragonal phase (II) at 236.3 K, to another tetragonal phase (III) at 154 K, and finally to an orthorhombic phase (IV) at 148.8 K. In tetragonal phase II, the CH3NH3+ ions undergo isotropic reorientation, whereas in the lower-temperature phases, the reorientation of C–N axes seems to be frozen15. All the phase transitions are first-order and order–disorder type, although the highest temperature transition is close to second-order. From the high-temperature cubic phase with freely rotating CH3NH3 cations, this compound enters lower-symmetry tetragonal phases and finally a low-temperature orthorhombic phase with the orientation of CH3NH3 cations fixed at ordered positions16,17,18,19,20. At room temperature, the structure is cubic, the space group is Pm3m, and the lattice constant a = 5.93129 Å and Z = 113. In this crystal structure, there exists a CH3NH3+ cation at the centre of a cube formed by corner-sharing PbBr6 octahedra18,21, as shown in Fig. 1. Below 236 K, the crystal structure has a tetragonal and belongs to the space group I4/mcm with lattice constants a = b = 8.32 Å, c = 11.83 Å, and Z = 422. Throughout the transition from the tetragonal II phase to the tetragonal III phase at 154 K, the full width at half maximum of the Raman ν6 band shows an abrupt increase8. At lower temperatures, CH3NH3PbBr3 undergoes a first-order structural phase transition from the tetragonal III (I4/mmm) phase to the orthorhombic IV (Pnma) phase9.
In a previous nuclear magnetic resonance (NMR) investigation, the temperature dependence of 81Br nuclear quadrupole resonance frequencies and 1H spin–lattice relaxation times in the laboratory frame T1 for CH3NH3PbBr3 were discussed by Xu et al.23 According to their results, the two 81Br NQR lines in phase II were reduced to one line in phase I. The discontinuity of the NQR line at this transition point implied a first-order transition. 1H T1 varied continuously, and no discernible change in the free induction decay was observed during the I–II transition. The phase transition had no significant effect on the motional state of the CH3NH3+ ions. Furthermore, Baikie et al.13 reported that the 1H magic angle spinning (MAS) NMR spectra showed two clear peaks corresponding to the CH3 and NH3 environments in the high-temperature phase, and the 1H and 13C NMR spectra of CH3NH3PbBr3 showed that the CH3NH3+ units undergo dynamic reorientation.
Measuring the spin–lattice relaxation time in the rotating frame T1ρ by MAS NMR allows for the probing of molecular motion in the kHz range, whereas the spin–lattice relaxation time in the laboratory frame T1 measured by static NMR reflects motion in the MHz range. Although the 1H T1 of CH3NH3PbBr3 has been examined by a few research groups, the corresponding phenomena by 1H, 13C, and 207Pb MAS NMR spectra and T1ρ have not been fully studied. In addition, information regarding 14N in the CH3NH3 cation has not yet been discussed.
In the present study, the structural dynamics of the CH3NH3 cation and PbBr6 octahedra in CH3NH3PbBr3 were studied in detail by NMR to resolve the phase transition mechanisms from the tetragonal phase to the higher-temperature cubic phase. The temperature dependences of the chemical shifts and spin–lattice relaxation time in the rotating frame T1ρ were measured using 1H MAS NMR, 13C cross-polarization (CP)/MAS NMR, and 207Pb MAS NMR with emphasis on the role of the CH3NH3 cation and PbBr6 octahedra in CH3NH3PbBr3. In addition, the 14N static NMR spectra of CH3NH3PbBr3 in the laboratory frame were acquired near the phase transition temperature. The abovementioned results help in understanding the thermal stability and the structural dynamics based on the phase transition mechanism, towards the practical application of this material.
Experimental
CH3NH3Br and PbBr2 were dissolved in a dimethylformamide solution and heated the mixed suspension on a hot plate to obtain a transparent solution. Detailed methods for the crystal growth are given elsewhere21,24,25. The CH3NH3PbBr3 single crystals obtained here were orange in colour with a square shape.
Differential scanning calorimetry (DSC) (TA, DSC 25) was conducted at a heating rate of 10 °C/min over a temperature range from 190 to 525 K under nitrogen gas. Thermogravimetric analysis (TGA) was performed on a thermogravimetric analyser (TA Instrument) in an interval from 300 to 780 K at a heating rate of 10 °C/min. Approximately 11.15 mg of CH3NH3PbBr3 was used in each experiment.
NMR measurements were carried out at 9.4 T using a Bruker 400 MHz Avance II + spectrometer at the Korea Basic Science Institute, Western Seoul Center. The 1H, 13C, and 207Pb NMR frequencies were 400.13, 100.61, and 83.75 MHz, respectively. Powdered samples were packed in zirconia MAS rotors with Macor caps, and the MAS rate was set to 10 kHz for the 1H MAS, 13C MAS, and 207Pb MAS NMR measurements to minimise spinning sideband overlap. The spin–lattice relaxation time in the rotating frame T1ρ was measured using an inversion recovery pulse sequence, which employs compensating pulses. The 13C T1ρ values were measured by varying the duration of the 13C spin-locking pulse applied after the CP preparation period. The width of the π/2 pulse used for measuring T1ρ of 1H and 13C was 3.45 µs, and that for measuring T1ρ of 207Pb was 3.5 µs. In addition, 14N NMR spectra of a CH3NH3PbBr3 single crystal were measured with a Larmor frequency of 28.90 MHz in the laboratory frame.
Temperature-dependent NMR spectra were recorded over 180 to 430 K; the NMR spectra and relaxation times could not be measured outside this temperature range because of the limitations of the spectrometer. Sample temperatures were held constant within ± 0.5 K by controlling the nitrogen gas flow and heating current.
Experimental results
Figure 2 shows the DSC and TGA curves obtained under a nitrogen atmosphere. DSC analysis was used to determine the phase transition temperature; only one endothermic peak related to a phase transition was observed at 236 K, which is consistent with previously reported TC values.13,14 The thermal stability of CH3NH3PbBr3 was examined by TGA. The first occurrence of mass loss began at approximately 530 K, which represents the onset of partial thermal decomposition. The mass sharply decreased between 550 and 650 K, with a corresponding mass loss of 22% near 650 K. Optical polarizing microscopy experiments were also conducted to further understand the thermal stability at high temperatures. The colour of the crystal was orange at room temperature, as shown in the inset in Fig. 2. As the temperature increased, the state of the crystal remained the same from 400 to 500 K. Above 550 K, a slight opacity occurred at the bottom of the crystal, and at approximately 600 K, the crystal was nearly opaque.
The 1H NMR spectrum of CH3NH3PbBr3 was recorded by MAS NMR at a frequency of 400.13 MHz. Figure 3 shows the 1H MAS NMR spectrum at 300 K, where the spinning sidebands are marked with open circles and asterisks. The two peaks in the 1H spectrum correspond to CH3 and NH3 environments, with the chemical shifts at δ = 3.27 and 6.36 ppm assigned to 1H in CH3 and NH3, respectively. The chemical shifts remained quasi-constant with increasing temperature, indicating that the structural environments of 1H in the CH3 and NH3 groups were unchanged (see the Supplementary Information). Additionally, the line width (full-width at half-maximum) of the 1H MAS NMR signal at 300 K is approximately 1.62 ppm, which also remained nearly constant with temperature change.
The 1H inversion-recovery curves for both CH3 and NH3 at each temperature were fitted to exponentials to extract T1ρ. The data were well fitted a single exponential, indicating that there is one dominant relaxation mechanism acting per environment. Thus, T1ρ was determined by fitting the decay plots with the equation below26,27.
where P(t) is the magnetisation, t is the spin-locking pulse duration, and P0 is the total nuclear magnetisation of 1H at thermal equilibrium. The recovery curves of 1H in CH3NH3PbBr3 were measured for various delay times at each temperature. Figure 4 (inset) shows the recovery traces for 1H measured for delay times ranging from 1 to 200 ms at 300 K. The intensity of the recovery traces differed with delay time. The T1ρ values obtained from the intensity versus delay time and shown in Fig. 4 reveal that T1ρ increased with temperature because proton hopping was accelerated. This is in agreement with Xu et al.23, who reported that the 1H T1 increased smoothly with increasing temperature through the high-temperature phase transition. The T1ρ values of 1H in CH3 and NH3 in the CH3NH3+ cation show similar trends with temperature and are nearly the same within the error range. The T1ρ values show no change near the phase transition temperature (TC = 236 K). T1ρ increased with increasing temperature, reaching the maximum values of 592 ms and 456 ms for CH3 and NH3, respectively, near 330 K above the phase transition temperature, and then decreased with increasing temperature. Although the structural environment of 1H in the CH3NH3 groups does not change with temperature, their molecular motion increases at high temperatures, as indicated by the T1ρ values. Above TC, the 1H T1ρ value for CH3 slightly exceed that for NH3.
Structural analysis of the 13C and 207Pb nuclei in CH3NH3PbBr3 was performed by MAS NMR, and the corresponding spectra at 300 K are shown as insets in Fig. 5. At room temperature, the 13C and 207Pb MAS NMR spectra show one signal each at chemical shifts of δ = 30.66 and 89 ppm with respect to tetramethylsilane and PbNO3, respectively. Here, the line width for 13C at 300 K is narrow at 2.77 ppm, whereas that for 207Pb is quite broad at 206.24 ppm. Figure 5 shows the 13C and 207Pb chemical shifts of CH3NH3PbBr3 measured as a function of temperature, illustrating that the 13C and 207Pb peak positions moved to higher chemical shifts upon heating. The chemical shifts near TC changed, in contrast to the 1H chemical shifts. The chemical shifts of the 13C and 207Pb signals relative to the reference signal are sensitive to the electronic environment of the nucleus. In particular, the 207Pb chemical shift changed more rapidly than that of 13C near TC. From these results, the phase transition from the tetragonal to cubic phase is thought to arise from a change in the PbBr6 octahedra.
To determine the T1ρ values of 13C and 207Pb in the rotating frame, the nuclear magnetisation was measured as a function of delay time. The signal intensities of the nuclear magnetisation recovery curves could be described by the single exponential function in Eq. (1), and the signal intensity followed this single exponential decay at all temperatures. From these results, the T1ρ values were obtained for 13C and 207Pb in CH3NH3PbBr3 as a function of inverse temperature, as shown in Fig. 6. The 13C and 207Pb T1ρ values for CH3 and PbBr3 seem to follow a similar trend with temperature to that of the 1H T1ρ, where the values increase with increasing temperature and are approximately continuous near TC. In addition, the 207Pb T1ρ values are much lower than 13C T1ρ.
To obtain information concerning possible changes in the surroundings of the 14N ion, static NMR spectra of 14N (I = 1) in the laboratory frame were obtained. Temperature-dependent changes in the 14N resonance frequency are attributable to alterations in the structural geometry, indicating a change in the quadrupole coupling constant of the 14N nuclei. The spectra were obtained by the solid-state echo method using static NMR at a Larmor frequency of 28.90 MHz. Two resonance signals were expected from the quadrupole interactions of the 14N nucleus with spin I = 1. The 14N NMR spectra were shown at 225 and 270 K, and the resonance frequencies referenced with respect to NH4NO3 as a function of temperature are shown in Fig. 7. The line widths are very narrow at all temperatures. The two resonance signals for 14N, which are attributable to NH3, superpose into one line at the transition point of 236 K. This single 14N resonance line indicates that a phase transition takes place to a new phase with a higher symmetry than tetragonal28. In tetragonal phase below TC, the electric field gradient tensors at the N sites vary, reflecting changes in the atomic configuration around the nitrogen. But, there is no electric field gradient tensor at the 14N site in the cubic structure because of the site symmetry of m3m.
Conclusion
Using the information derived from NMR studies near TC (= 236 K), we have probed the structural and dynamic features of CH3NH3PbBr3 in detail and demonstrated its dynamic nature. The ionic dynamics of CH3NH3PbBr3, with emphasis on the role of the CH3NH3 cation and PbBr6 octahedra, were investigated by 1H MAS NMR, 13C CP/MAS NMR, 207Pb MAS NMR, and 14N static NMR as a function of temperature. 1H, 13C, and 207Pb NMR were used to identify the phase transition in CH3NH3PbBr3 by detecting changes in the chemical shifts accompanying a change in crystallographic symmetry. Here, the CH3 and NH3 groups were distinguished based on the 1H chemical shifts. The chemical shifts of the 1H nuclei remained constant at all temperatures, whereas those of the 13C and 207Pb nuclei varied with temperature. The temperature dependence of the chemical shifts was sensitive to the rotation of the PbBr6 octahedra. From these results, it is evident that the structural environments of 13C and 207Pb change near TC. The change in 207Pb chemical shift near TC can be explained by the rotation of PbBr3. This is consistent with the established nature of the phase transition. Additionally, the NMR line widths of 1H, 13C, and 207Pb were 1.62, 2.77, and 206.24 ppm, respectively, and the relaxation time is proportional to the inverse of the line width. Although the chemical shifts of 13C and 207Pb abruptly varied near TC, the 1H, 13C, and 207Pb T1ρ values showed a similar trend with increasing temperature, and their T1ρ values were continuous near TC. These short relaxation times indicate ease of molecular motion. The TGA results also showed that CH3NH3PbBr3 has a high thermal stability.
In addition, the abrupt change occurring in the resonance frequency of the 14N nuclei near TC is attributable to a structural phase transition. The NH3 groups in the structure are coordinated by PbBr6, and thus atomic displacements in the environment of the 14N nuclei with temperature are correlated with PbBr6. The electrostatic interactions governed by hydrogen-bonding interactions between the NH3+ group in the CH3NH3 cation and the PbBr6 octahedra play an important role in the dynamics of the CH3NH3 cations. Consequently, the main factor causing the phase transition from the tetragonal to cubic phase near TC is a change in the surroundings of the 207Pb nuclei in the PbBr6 octahedra and in the surroundings of C–N groups in the CH3NH3 cations. Based on these results, the structural dynamics within the CH3NH3PbBr3 perovskite structure are expected to have a significant effect on the operation mechanism of perovskite solar cells.
References
Yang, W. S. et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234 (2015).
Brenner, T. M., Egger, D. A., Kronik, L., Hodes, G. & Cahen, D. Hybrid organic-inorganic perovskites: low-cost semiconductors with intriguing charge-transport properties. Nat. Tev. Mater. 1, 15007 (2016).
Al-Amri, A. M., Leung, S.-F., Vaseem, M., Shamim, A. & He, J.-H. Fully inkjet-printed photodetector using a grapheme/perovskite/grapheme heterostructure. IEEE Trans. Electron Dev. 66, 2657 (2019).
Al-Amri, A. M., Cheng, B. & He, J.-H. Perovskite methylammonium lead trihalide heterostructures: progress and challenges. IEEE Trans. Nanotechnol. 18, 1 (2019).
Liu, W.-W. et al. Platinum-free ternary metallic selenides as nanostructured counter electrode for high-efficiency dye-sensitized solar cell by interface engineering. ACS Appl. Energy Mater. 3, 3704 (2020).
Lin, C.-H. et al. Giant optical anisotropy of perovskite nanowire array films. Adv. Funct. Mater. 30, 1909275 (2020).
Capitani, F. et al. Locking of methylammonium by pressure-enhanced H-bonding in (CH3NH3)PbBr3 hybrid perovskite. J. Phys. Chem. C 121, 28125 (2017).
Nakada, K., Matsumoto, Y., Shimo, Y., Yamada, K. & Furukawa, Y. Temperature-dependent evolution of Raman spectra of methylammonium lead halide perovskites, CH3NH3PbX3 (X = I, Br). Molecules 24, 626 (2019).
Swainson, I. P., Hammond, R. P., Soulliere, C., Knop, O. & Massa, W. Phase transitions in the perovskite methylammonium lead bromide, CH3ND3PbBr3. J. Solid State Chem. 176, 97 (2003).
Chen, Y. et al. Introduction of nitrogen gas flow and precursor aging process to improve the efficiency of the lead acetate derived CH3NH3PbI3 perovskite solar cells. Solar Energy Mater. Sol. Cells 190, 49 (2019).
Baikie, T. et al. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 1, 5628 (2013).
Varadwaj, P. R., Varadwaj, A., Marques, H. M. & Yamashita, K. Significance of hydrogen bonding and other noncovalent interactions in determining octahedral tilting in the CH3NH3PbI3 hybrid organic-inorganic halide perovskite solar cell semiconductor. Sci. Rep. 9, 50 (2019).
Baikie, T. et al. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 3, 9298 (2015).
Letoublon, A. et al. Elastic constants, optical phonons, and molecular relaxations in the high temperature plastic phase of the CH3NH3PbBr3 hybrid perovskite. J. Phys. Chem. Lett. 7, 3776 (2016).
Knop, O., Wasylishen, R. E., White, M. A., Cameron, T. S. & Van Oort, M. J. M. Alkylammonium lead halides, Part 2, CH3NH3PbX3 (X = Cl, Br, I) perovskites: cuboctahedral halide cages with isotropic cation reorientation. Can. J. Chem. 68, 412 (1990).
Ohmann, R. et al. Real-space imaging of the atomic structure of organic-inorganic perovskite. J. Am. Chem. Soc. 137, 16049 (2015).
Poglitsch, A. & Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimetre-wave spectroscopy. J. Chem. Phys. 87, 6373 (1987).
Onoda-Yamamuro, N., Matsuo, T. & Suga, H. Calorimetric and IR spectroscopic studies of phase transitions in methylammonium trihalogenoplumbates (II). J. Phys. Chem. Solids 51, 1383 (1990).
Weller, M. T., Weber, O. J., Henry, P. F., Di Pumpo, A. M. & Hansen, T. C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 51, 4180 (2015).
Leguy, A. M. A. et al. The dynamics of methylammonium ions in hybrid organic-inorganic perovskite solar cells. Nat. Commun. 6, 7124 (2015).
Park, J.-S. et al. Electronic structure and optical properties of α-CH3NH3PbBr3 perovskite single crystal. J. Phys. Chem. Lett. 6, 4304 (2015).
Zu, F. et al. Constructing CH3NH3PbI3 and CH3NH3PbBr3 perovskite thin film electronic structure from single crystl band structure measurement. J. Phys. Chem. Lett. 10, 601 (2019).
Xu, Q., Eguchi, T., Nakayama, H., Nakamura, N. & Kishita, M. Molecular motions and phase transitions in solid CH3NH3PbX3 (X = Cl, Br, I) as studied by NMR and NQR. Z. Naturforscha 46, 240 (1991).
Saidaminov, M. I. et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 6, 7586 (2015).
Yang, Y. et al. Low surface recombination velocity in solution-grown CH3NH3PbBr3 perovskite single crystal. Nat. Commun. 6, 7961 (2015).
Lim, A. R. Ionic dynamics of the cation in organic-inorganic hybrid compound (CH3NH3)2MCl4 (M = Cu and Zn) by 1H MAS NMR, 13C CP MAS NMR, and 14N NMR. RSC Advances 6, 18656 (2018).
Abragam, A. The Principles of Nuclear Magnetism (Oxford University Press, Oxford, 1961).
Lim, A. R. & Kim, I. G. Phase transition study by using 133Cs and 207Pb nuclear magnetic resonance in a CsPbCl3 single crystal. J. Phys. Soc. Japan 73, 475 (2004).
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2016R1A6A1A03012069 and 2018R1D1A1B07041593).
Author information
Authors and Affiliations
Contributions
A.R.L. designed the project and wrote the manuscript. S.H.K. performed NMR experiments. Y.L.J. suggested the idea and comment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, A.R., Kim, S.H. & Joo, Y.L. Structural dynamics of CH3NH3+ and PbBr3− in tetragonal and cubic phases of CH3NH3PbBr3 hybrid perovskite by nuclear magnetic resonance. Sci Rep 10, 13140 (2020). https://doi.org/10.1038/s41598-020-70128-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70128-5
This article is cited by
-
Thermodynamic Property and Solid-State Molecular Dynamics of Cs2MnBr4·2H2O Crystal
Applied Magnetic Resonance (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.