Abstract
High-grade serous ovarian cancer (HGSOC) is the most common subtype of epithelial ovarian cancer and early detection is challenging. TP53 mutations are a hallmark of HGSOC and detection of these mutations in liquid-based Pap samples could provide a method for early diagnosis. Here we evaluate the use of IBSAFE, an ultra-sensitive droplet digital PCR (ddPCR) method, for detecting TP53 mutations in liquid-based Pap samples collected from fifteen women at the time of diagnosis (diagnostic samples) and/or up to seven years prior to diagnosis (archival samples). We analysed tumours for somatic TP53 mutations with next generation sequencing and were able to detect the corresponding mutations in diagnostic samples from six of eight women, while one patient harboured a germline mutation. We further detected a mutation in an archival sample obtained 20 months prior to the ovarian cancer diagnosis. The custom designed IBSAFE assays detected minor allele frequencies (MAFs) with very high assay sensitivity (MAF = 0.0068%) and were successful despite low DNA abundance (0.17–206.14 ng, median: 17.27 ng). These results provide support for further evaluation of archival liquid-based Pap samples for diagnostic purposes and demonstrate that ultra-sensitive ddPCR should be evaluated for ovarian cancer screening in high-risk groups or in the recurrent setting.
Similar content being viewed by others
Introduction
While the incidence and mortality of cervical cancer have decreased radically since the introduction of the Papanicolaou test (Pap test)1,2,3, overall survival from ovarian cancer has not changed substantially over the past 50 years4. High-grade serous ovarian cancer (HGSOC) confers an overall 5-year survival rate around 45%, but outcomes vary greatly between disease stages, with 5-year survival rates above 70% in stage I and II disease. However, symptoms of HGSOC often present in late stages (III and IV) of the disease, resulting in 5-year survival rates between 26–42%5. None of the approaches aimed at early detection, including serum CA-125 and trans-vaginal ultrasound have been successfully applied in a screening setting due to limited specificity and sensitivity6,7,8.
HGSOC is believed to arise in the fallopian tube epithelium9, and mutations in the tumour suppressor gene TP53 are believed to be a very early event in the carcinogenesis of HGSOC10. A recent study by Labidi-Galy et al. (2017) showed shared TP53 mutations in patient-matched pre-cancerous and cancerous lesions (including so-called p53 signatures, Serous Tubal Intraepithelial Carcinoma (STIC) lesions and invasive carcinomas) from nine patients with HGSOC, providing support for the possibility of discovering tumour-driving mutations in early stages of the disease11. Apart from frequent mutations in TP53, these cancers are characterised by few recurrent mutations and instead harbour wide-spread chromosomal instability12. Recent studies have investigated chromosomal instability and levels of somatic copy number alterations as a prognostic tool and therapeutic target in HGSOC13,14.
A promising development occurred in 2013, when Kinde et al. showed that somatic mutations in DNA shed from endometrial and ovarian cancers could be detected in standard liquid-based Pap test specimens by massively parallel sequencing15. While highly sensitive for endometrial cancer, the method was not able to detect more than 41% of ovarian cancers using a panel of 12 genes commonly mutated in these tumours. Subsequent studies from this research group in collaboration with others have attempted to increase the sensitivity for detection of ovarian cancer by introducing new procedures including lavage of the uterine cavity16, by combining Pap test and plasma sampling and by complementing the mutation assay with an assay for aneuploidy17. However, clinical sensitivity remains a challenge with this approach, and will require extensive modelling before application in clinical diagnostics18,19,20. Moreover, these approaches have only been applied in symptomatic patients, at the time of diagnosis, and have so far not been evaluated in pre-symptomatic women prior to the time of diagnosis.
Droplet Digital PCR (ddPCR) provides an alternative to sequencing-based methods, with the advantages of increased sensitivity, rapid turnover time and ease of use21. Analysis of circulating tumour DNA using ddPCR has shown great potential for prognostication and monitoring of treatment response in several tumour types including gynaecological cancers22,23,24.
In this study, we analysed liquid-based archival Pap samples (archival samples) from fifteen women collected approximately two to seven years before they were diagnosed with HGSOC, and from nine of these women also liquid-based diagnostic Pap samples (diagnostic samples) collected at the time of the HGSOC diagnosis. Mutations in TP53 were identified by next generation sequencing (NGS) of tumour tissue obtained at surgery. We used the ultra-sensitive ddPCR IBSAFE technology for mutation detection in Pap samples and used a commercially available approach from Bio-Rad as a control where applicable. The analysis of liquid Pap samples from pre-symptomatic women subsequently diagnosed with HGSOC has, to our knowledge, not been previously reported.
Results
Patient cohort
A total of 20 archival samples were obtained from 15 patients from cohorts 1 and 2 (Fig. 1A–C). Fresh frozen tumour tissue was available from 11 patients, while FFPE tumour tissue was available from four patients. Corresponding blood was available from 14 patients, while nine patients from cohort 1 had diagnostic samples. Patient characteristics are provided in Table 1. The time from the collection of archival samples to the time of HGSOC diagnosis ranged from 20 to 95 months; two patients had more than one archival sample and the remaining patients had a single archival sample. DNA concentrations in diagnostic and archival samples varied between 5.2–55.2 ng/µl (median 16.3 ng/µl) and 0 (too low)-19.4 ng/µl (median 2.755 ng/µl) respectively but were not linked to disease stage. DNA concentrations in archival samples were lower than in diagnostic samples (P = 0.02).
Tumour sequencing and analysis
Paired tumour and blood samples were analysed for TP53 mutations using the INVIEW Oncopanel All-in-one from GATC (Supplementary Table S1). One patient lacked a corresponding blood sample; the tumour sequence was therefore analysed using a normal control constructed from five patients (patients 2, 3, 4, 6 and 9) within cohort 1 and two patients from cohort 2 (patients 11 and 14). At least one mutation was identified for each patient using GEMINI and a hard filter minor allele frequency (MAF) cut-off of 5%. Ten missense mutations, two nonsense mutations, and three frameshift deletions were identified. Mutations were dispersed across the TP53 gene, with no overlap between patients. Patient 4 displayed two mutations in positions adjacent to each other, which was handled as a single mutation in the downstream analysis (Table 2). All mutations but one were previously recorded in COSMIC (Catalogue Of Somatic Mutations In Cancer); however five of the patients scored neutral or NA in the Functional Analysis through Hidden Markov Models (FATHMM)25 scoring by GEMINI (GEnome MINIng). MAFs in the tumours ranged between 8–85% with a median of 64% (Table 3).
Mutation screening in diagnostic and archival samples using IBSAFE and Bio-Rad ddPCR
We analysed both diagnostic and archival samples using IBSAFE, an ultra-sensitive ddPCR-based method. We were also able to analyse three diagnostic samples (from patients 3, 8 and 9) with the Bio-Rad-based ddPCR assay to serve as an additional control for the ddPCR approach. Using IBSAFE we were able to detect a tumour MAF comparable to that of the NGS approach (Table 3, Fig. 2A). The Bio-Rad assay performed equally well in the tumour setting. The measured MAFs of the IBSAFE method ranged between 8–86% with a median of 63%.
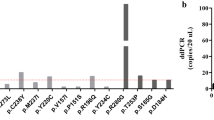
Minor allele frequencies (MAFs) (primary y-axis) for tumours (A), diagnostic samples (B) and archival samples (C). Amount of DNA tested (secondary y-axis) from archival samples (C). Note the log scale on the y-axis. Red, next-generation sequencing; green, IBSAFE assays; purple, Bio-Rad assays; blue: amount of DNA in ng.
The IBSAFE method was able to detect tumour-identified mutations in seven of nine diagnostic samples, however one mutation was determined to be a germline event (see below); hence, true somatic mutations were detected in six of eight diagnostic samples. Furthermore, IBSAFE detected the tumour-identified mutation in diagnostic samples from two of three patients with stage II disease (patients 5 and 8). The Bio-Rad assay failed to detect any mutations in the diagnostic sample from patient 3 but was able to detect mutations in the diagnostic samples from patients 8 and 9 (Table 3, Fig. 2B). The calculated MAF in the diagnostic sample from patient 8 (0.0089%) was below the theoretical limit of detection of the Bio-Rad assay21.
Despite the age and the low abundance of DNA in the archival samples, the IBSAFE method was successful in all the individual assays, measuring concentrations comparable to the QUBIT assay (Tables 1 and 3, Fig. 2C). Furthermore, we were able to detect a tumour corresponding mutation in the archival samples from patients 1 and 13 (sample 13C). Patient 13 was diagnosed with a stage IIIB tumour and had a total of three archival samples (13A, B and C) collected 46, 32 and 20 months prior to diagnosis. A TP53 mutation with a MAF of 0.042% was detected in sample 13 C obtained 20 months prior to diagnosis, with a total of 31.75 ng of DNA tested. The mutation was however not detectable in the earlier samples (13 A and B).
The MAF of 46% in the sample from patient 1 led us to suspect a germline TP53 mutation, which was confirmed using a normal tissue sample (endometrial biopsy), from the same patient (data not shown).
No mutations were detected in the remaining archival samples (Table 3, Fig. 2C).
Discussion
In this study we evaluated the ability of IBSAFE, an ultra-sensitive ddPCR method, to detect known TP53 mutations in Pap samples from patients with HGSOC collected at the time of diagnosis and approximately two to seven years prior to diagnosis.
We were able to detect somatic TP53 mutations in diagnostic samples from six of nine women using IBSAFE. TP53 mutations were not detectable in two of the diagnostic samples, most likely due to the MAF being below the limit of detection of the IBSAFE method. The MAF estimates suggest that there is a huge variance in detectable somatic DNA mutations ranging from 0.0068% to 7.9%.
Importantly, IBSAFE was able to detect mutations with an in-sample limit of detection of 1 in 50,000. This sensitivity is much higher than that of conventional NGS18,19,20,26. Moreover, the MAF estimates of IBSAFE were higher than those of the commercial approach for the diagnostic samples, despite exhibiting similar MAFs in the tumour setting, possibly indicating higher in-sample sensitivity. This is probably due to the experimental set-up in which the Bio-Rad assay is optimised for 100 ng DNA inputs. In reality, DNA inputs from liquid biopsies, such as plasma and Pap samples, are limited and vary greatly between patients. Therefore, a method that relies less on the amount of DNA input will allow for an improved limit of detection in low abundance DNA samples. Of note, IBSAFE was able to perform in samples with as little as 0.17 ng of DNA input. However, due to the small number of samples tested no significance comparisons between the two ddPCR methods could be performed. Furthermore, a direct comparison of MAFs is complicated due to differences between the methodologies. Such a comparison would require a larger study containing also healthy controls and is beyond the scope of this proof-of-principle study.
Notably, we were able to detect a tumour corresponding mutation in an archival sample collected 20 months before diagnosis from a patient subsequently diagnosed with a stage IIIB tumour, while we did not detect any mutations in the two other samples collected at time points earlier than 20 months prior to diagnosis. Although the archival samples in our study were obtained within the suggested median time frame proposed by Labidi-Galy et al. (2017) and IBSAFE assays were successful in all the archival samples, we did not detect any TP53 mutation in the remaining samples from the other women also obtained more than 20 months prior to diagnosis. We therefore speculate that the time-frame to detect HGSOC-derived mutations in Pap samples may be narrower. One explanation might be that although TP53 precursor lesions have been suggested to be present approximately seven years prior to overt HGSOC, these precursors might not shed cells or DNA until later in the tumorigenic process. Importantly however, a 20-month window for early detection may confer a better prognosis for the patient, as suggested by the diagnosis in this case being stage IIIB HGSOC. Unfortunately, the limited number of patients in the present study precluded the possibility of exploring this further, and a larger study, with multiple archival samples per patient, collected at time-points closer to the time of diagnosis would be required to address this.
Although sequencing costs have been reduced during the last decade and population-wide screens for rare disorders have been suggested, deep sequencing is still not feasible for population-wide screens of genetically complex somatic diseases like cancer27,28.
Digital droplet PCR offers a clinically feasible platform, with ease of use, fast turnover and high sensitivity21. Although the assay sensitivity is high, one shortcoming of ddPCR is the limited number of mutations that can be detected in a single low abundance DNA sample. For ddPCR to be applicable in a clinical setting, attention should be given to collecting as much DNA as possible from liquid biopsies such as plasma or Pap samples. One such approach was illustrated by Wang et al., who evaluated a Tao brush for sampling in close proximity to the tumour17, which was found to improve the limit of detection. Other methods, such as a lavage of the uterine cavity have also been reported to improve detection of ovarian cancer16. However, for a diagnostic test to be successfully implemented in a screening setting, it is crucial that the sampling procedure imposes minimal stress to the patient.
Another limitation of conventional ddPCR is the ability to detect only a single mutation per assay. However, the emergence of multiplexing of ddPCR assays may allow for several mutations to be screened simultaneously29. This has recently proved efficient when genotyping KRAS mutations in non-small cell lung cancer30, and could possibly increase the utility of TP53 screening even further, as mutations span the entire TP53 gene31.
Although the size of the cohort limited the power of the present study, we were able to analyse both diagnostic and archival (pre-diagnostic) samples successfully despite low amounts of DNA. We were able to detect true somatic tumour corresponding mutations in diagnostic samples from six of eight patients. Of note, detection of low abundance TP53 mutant DNA in Pap samples from two patients with stage IIA HGSOC emphasises the potential of the method for early detection. These findings may also support the use of such a sensitive method in the recurrent setting, where patients can be monitored for treatment response on a regular basis based on a known TP53 mutation. Furthermore, we successfully detected a tumour-derived mutation in an archival sample collected 20 months prior to diagnosis from a non-symptomatic woman, and the IBSAFE ddPCR assay performed well in all archival samples. To our knowledge, this is the first time an ovarian cancer-derived mutation has successfully been identified in a pre-diagnostic Pap sample from a non-symptomatic woman.
Although the present study is based on a small number of patients, we believe that an ultra-sensitive ddPCR method should be evaluated in a larger cohort of patients with a greater number of serial pre-diagnostic samples collected prior to the time of diagnosis to provide a better resolution of the diagnostic potential of TP53 testing in Pap samples for the pre-symptomatic detection of HGSOC.
Methods
Patients
We identified 79 women with HGSOC from an on-going study prospectively recruiting women with a suspected adnexal tumour at the Department of Obstetrics and Gynaecology in Lund at Skåne University Hospital in the southern Swedish healthcare region. Among these, liquid-based archival Pap samples collected before the ovarian cancer diagnosis were available from nine women from the cervical cancer screening program within the region of Malmö, Sweden at the Department of Medical Microbiology, Lund University Hospital, Sweden (cohort 1). Further, we identified an additional six women from whom matched liquid-based archival Pap samples and tumour tissue were available (cohort 2). All tumours were classified according to WHO 201432 and staged according to the International Federation of Gynaecology and Obstetrics (FIGO) criteria33. The study was approved by the Ethics Committee at Lund University (Sweden) and was performed in accordance with the Declaration of Helsinki. The patients provided written informed consent.
Sequencing of tumours
Tumour DNA was extracted using the AllPrep DNA/RNA Mini kit and blood DNA was extracted using the QIAmp DNA Blood Midi kit, both from Qiagen (Sollentuna, Sweden). Formalin-fixed paraffin embedded DNA was extracted using the QIAamp DNA FFPE Tissue Kit from Qiagen.
Paired tumour/blood samples were sequenced using the INVIEW Oncopanel All-in-one (Supplementary Table S1) and analysed using the in-house analysis pipeline at GATC (Ebersberg, Germany). Samples were aligned against the human reference genome hg19 (chromosomes only, UCSC) with Burrows–Wheeler Aligner (version 0.7.15). Local realignment was carried out using the Genome Analysis Tool Kit (GATK, version 3.7), and duplicate reads were removed using Picard (version 1.131). For one patient lacking paired normal DNA, a pooled reference genome was constructed from seven patients with available blood samples. One patient had two tumour samples, one from each ovary. Bam files were analysed using the Bcbio-nextgen pipeline (version 1.1.0) for paired tumour samples34, with Mutect2 from GATK35, Freebayes36, VarDict37 and Varscan238 as mutation callers. Mutations were annotated using the Variant Effect Predictor39 and classified using the GEMINI framework40 to filter out possible germline mutations.
DNA extraction from liquid-based Pap samples
Diagnostic samples were collected using a ThinPrep (Hologic Inc., Sollentuna, Sweden) brush at time of diagnosis and were kept in DNAgard (Sigma Stockholm, Sweden). Archival samples were collected using the BD SurePath liquid-based Pap test (Becton Dickson, Stockholm, Sweden) until 2014, after which ThinPrep was used. Following pathology review, residual materials were transferred to new tubes and centrifuged. Residual liquid was removed and the cell pellets were stored at −80 °C. Each cell pellet was resolved in 420 µl Specimen Transport Medium buffer (STM) (Qiagen, Hilden, Germany) and used for extraction of DNA with the QIAmp DNA Mini kit (Qiagen), using the manufacturer’s instructions but with two additional washes with each wash buffer. DNA was quantified using Qubit HS DNA kit (Thermo Fisher, Göteborg, Sweden).
Droplet Digital PCR of Pap samples
All chemicals, primers and equipment were purchased from Bio-Rad (Solna, Sweden) and used according to the manufacturer’s instructions, unless otherwise stated. Primers for mutations were designed using Bio-Rad’s online tool (San Diego, CA, USA) (Supplementary Table S2).
Droplet digital PCR of diagnostic samples was performed using a QX100™ Droplet Digital PCR system (Bio-Rad) according to the manufacturer’s instructions. Briefly, a 22 µl PCR reaction was prepared for each sample using 100 ng of diagnostic sample DNA in a final concentration of 1x ddPCR Supermix with no dUTP, primers (450 nM), probes (250 nM), and restriction enzyme (HaeIII or MseI (5U), Thermo Fisher). Subsequently droplets were generated and transferred to a 96-well PCR plate (VWR, Spånga, Sweden). The plate was heat-sealed with pierceable foil (VWR), and amplification performed using a C1000 Touch deep-well thermal cycler (Bio-Rad). The cycling conditions were as follows: an initial denaturation cycle of 10 min at 95 °C, followed by 40 cycles of denaturation for 30 s at 94 °C, annealing for 60 s at 55 °C (ramping rate set to 2 °C/s), and a final incubation for 10 min at 98 °C, ending at 4 °C. The plate was transferred to the QX100 droplet reader and analysed using the automated settings of the QuantaSoft analysis software (Bio-Rad, Version 1.4.0.99). Patient-matched tumour DNA was used as a positive control and Human Genomic Female DNA (Promega, Nacka, Sweden) was used as a negative control.
Detection of TP53 mutations in archival samples using IBSAFE
Diagnostic and archival samples were analysed for their respective tumour TP53 mutation using IBSAFE (SAGA Diagnostics, Lund, Sweden). IBSAFE utilises ddPCR droplets together with a proprietary methodology that allows for ultra-sensitive detection of mutations to a lower limit of detection of ~0.001% MAF. 120 ng of diagnostic sample DNA and varying amounts of archival sample DNA (0.17–206.14 ng) were analysed using IBSAFE by SAGA Diagnostics. IBSAFE reactions were performed in duplicate or quadruplicate. Patient specific tumour DNA (positive control) as well as normal Human Genomic DNA (Promega) (negative control) samples were included in every run to confirm assay performance: for all IBSAFE assays, zero false positive signals were present in the negative control analyses of at least 80,000 normal haploid human genome copies.
Statistics
Statistical tests were performed in R (version 3.3.3) using a two-sided Mann-Whitney U test with a significance threshold of 0.05.
Data availability
All data, materials and results are kept at the Division of Oncology and Pathology, Department of Clinical Sciences, Skåne University Hospital, Lund University, Lund, Sweden and can be made available upon reasonable request to the corresponding author.
References
Papanicolaou, G. N. & Traut, H. F. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med 121, 211–224 (1997).
Peirson, L., Fitzpatrick-Lewis, D., Ciliska, D. & Warren, R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2, 35, https://doi.org/10.1186/2046-4053-2-35 (2013).
Benard, V. B. et al. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007–2012. MMWR Morb Mortal Wkly Rep 63, 1004–1009 (2014).
Davidson, B. & Trope, C. G. Ovarian cancer: diagnostic, biological and prognostic aspects. Womens Health (Lond) 10, 519–533, https://doi.org/10.2217/whe.14.37 (2014).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 68, 284–296, https://doi.org/10.3322/caac.21456 (2018).
Buys, S. S. et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 305, 2295–2303, https://doi.org/10.1001/jama.2011.766 (2011).
Kamal, R., Hamed, S., Mansour, S., Mounir, Y. & Abdel Sallam, S. Ovarian cancer screening-ultrasound; impact on ovarian cancer mortality. Br J Radiol 91, 20170571, https://doi.org/10.1259/bjr.20170571 (2018).
Henderson, J. T., Webber, E. M. & Sawaya, G. F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 319, 595–606, https://doi.org/10.1001/jama.2017.21421 (2018).
Kurman, R. J. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol 24(Suppl 10), x16–21, https://doi.org/10.1093/annonc/mdt463 (2013).
Kim, J., Coffey, D. M., Ma, L. & Matzuk, M. M. The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice. Endocrinology 156, 1975–1981, https://doi.org/10.1210/en.2014-1977 (2015).
Labidi-Galy, S. I. et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 8, 1093, https://doi.org/10.1038/s41467-017-00962-1 (2017).
Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615, https://doi.org/10.1038/nature10166 (2011).
Berger, A. C. et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell 33, 690–705 e699, https://doi.org/10.1016/j.ccell.2018.03.014 (2018).
Song, Q., Zhang, W. & Sun, Y. Haploinsufficiency and mutation are two sides of the cancer coin as cause and therapeutics target. Transl Cancer Res 6 (Suppl 3) S590–S593, https://doi.org/10.21037/tcr.2017.05.12 (2017).
Kinde, I. et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med 5, 167ra164, https://doi.org/10.1126/scitranslmed.3004952 (2013).
Maritschnegg, E. et al. Lavage of the Uterine Cavity for Molecular Detection of Mullerian Duct Carcinomas: A Proof-of-Concept Study. J Clin Oncol 33, 4293–4300, https://doi.org/10.1200/JCO.2015.61.3083 (2015).
Wang, Y. et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med 10, doi:wa (2018).
Kinde, I., Wu, J., Papadopoulos, N., Kinzler, K. W. & Vogelstein, B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA 108, 9530–9535, https://doi.org/10.1073/pnas.1105422108 (2011).
Chin, E. L., da Silva, C. & Hegde, M. Assessment of clinical analytical sensitivity and specificity of next-generation sequencing for detection of simple and complex mutations. BMC Genet 14, 6, https://doi.org/10.1186/1471-2156-14-6 (2013).
Pecuchet, N. et al. Analysis of Base-Position Error Rate of Next-Generation Sequencing to Detect Tumor Mutations in Circulating DNA. Clin Chem 62, 1492–1503, https://doi.org/10.1373/clinchem.2016.258236 (2016).
Hindson, B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83, 8604–8610, https://doi.org/10.1021/ac202028g (2011).
Pereira, E. et al. Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers. PLoS One 10, e0145754, https://doi.org/10.1371/journal.pone.0145754 (2015).
Huang, A. et al. Detecting Circulating Tumor DNA in Hepatocellular Carcinoma Patients Using Droplet Digital PCR Is Feasible and Reflects Intratumoral Heterogeneity. J Cancer 7, 1907–1914, https://doi.org/10.7150/jca.15823 (2016).
Mattox, A. K. et al. Applications of liquid biopsies for cancer. Sci Transl Med 11, https://doi.org/10.1126/scitranslmed.aay1984 (2019).
Shihab, H. A. et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 34, 57–65, https://doi.org/10.1002/humu.22225 (2013).
Stasik, S. et al. An optimized targeted Next-Generation Sequencing approach for sensitive detection of single nucleotide variants. Biomol Detect Quantif 15, 6–12, https://doi.org/10.1016/j.bdq.2017.12.001 (2018).
Kingsmore, S. F. et al. Next-generation community genetics for low- and middle-income countries. Genome Med 4, 25, https://doi.org/10.1186/gm324 (2012).
van Dijk, E. L., Auger, H., Jaszczyszyn, Y. & Thermes, C. Ten years of next-generation sequencing technology. Trends Genet 30, 418–426, https://doi.org/10.1016/j.tig.2014.07.001 (2014).
Dobnik, D., Stebih, D., Blejec, A., Morisset, D. & Zel, J. Multiplex quantification of four DNA targets in one reaction with Bio-Rad droplet digital PCR system for GMO detection. Sci Rep 6, 35451, https://doi.org/10.1038/srep35451 (2016).
Pender, A. et al. Efficient Genotyping of KRAS Mutant Non-Small Cell Lung Cancer Using a Multiplexed Droplet Digital PCR Approach. PLoS One 10, e0139074, https://doi.org/10.1371/journal.pone.0139074 (2015).
Ahmed, A. A. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 221, 49–56, https://doi.org/10.1002/path.2696 (2010).
Kurman, R. J., Carcangiu, M. L., Herrington, C. S. & Young, R. H. In WHO Classification of Tumours of Female Reproductive Organs (eds Kurman, R. J., Carcangiu, M. L., Herrington, C. S. & Young, R. H.) (2014).
Prat, J. & Oncology, F. C. O. G. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 124, 1–5, https://doi.org/10.1016/j.ijgo.2013.10.001 (2014).
BCbio-nextgen, https://github.com/bcbio/bcbio-nextgen (2018).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–1303, https://doi.org/10.1101/gr.107524.110 (2010).
Garrison E, M. G. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207.3907 [q-bio.GN] (2012).
Lai, Z. et al. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res 44, e108, https://doi.org/10.1093/nar/gkw227 (2016).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22, 568–576, https://doi.org/10.1101/gr.129684.111 (2012).
Cunningham, F. et al. Ensembl 2015. Nucleic Acids Res 43, D662–669, https://doi.org/10.1093/nar/gku1010 (2015).
Paila, U., Chapman, B. A., Kirchner, R. & Quinlan, A. R. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol 9, e1003153, https://doi.org/10.1371/journal.pcbi.1003153 (2013).
Acknowledgements
We wish to thank the patients who participated in the study and we acknowledge RCC South for providing infrastructure for collection and biobanking of samples. This study was supported by grants to Dr. Hedenfalk from the Swedish Cancer Society, the G Nilsson Cancer Foundation, the B Kamprad Foundation, the Cancer and Allergy Foundation, the Sten K Johnson Foundation, King Gustaf V’s Jubilee Foundation, the Lund University Hospital Research Foundation and governmental funding of clinical research within the National Health Services (ALF). Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
N.S.A., L.M.d.l.F., A.M., S.M., P.K. and I.H. planned the study. L.M.d.l.F., S.M., A.M., P.K. and I.H. were responsible for ethical approvals. PK collected liquid-based diagnostic Pap samples. NSA planned and performed experiments, and reviewed experiments conducted by SAGA Diagnostics. O.F. supplied liquid-based archival Pap samples. NSA and L.M.d.l.F. wrote the manuscript while IH supervised the writing of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arildsen, N.S., Martin de la Fuente, L., Måsbäck, A. et al. Detecting TP53 mutations in diagnostic and archival liquid-based Pap samples from ovarian cancer patients using an ultra-sensitive ddPCR method. Sci Rep 9, 15506 (2019). https://doi.org/10.1038/s41598-019-51697-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51697-6
This article is cited by
-
Current and Emerging Applications of Droplet Digital PCR in Oncology: An Updated Review
Molecular Diagnosis & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.