Abstract
The presence of acute myocardial infarction (AMI) confers a poor prognosis in atrial fibrillation (AF), associated with increased mortality dramatically. This study aimed to evaluate the predictive value of CHADS2 and CHA2DS2-VASc scores for AMI in patients with AF. This retrospective study enrolled 5140 consecutive nonvalvular AF patients, 300 patients with AMI and 4840 patients without AMI. We identified the optimal cut-off values of the CHADS2 and CHA2DS2-VASc scores each based on receiver operating characteristic curves to predict the risk of AMI. Both CHADS2 score and CHA2DS2-VASc score were associated with an increased odds ratio of the prevalence of AMI in patients with AF, after adjustment for hyperlipidaemia, hyperuricemia, hyperthyroidism, hypothyroidism and obstructive sleep apnea. The present results showed that the area under the curve (AUC) for CHADS2 score was 0.787 with a similar accuracy of the CHA2DS2-VASc score (AUC 0.750) in predicting “high-risk” AF patients who developed AMI. However, the predictive accuracy of the two clinical-based risk scores was fair. The CHA2DS2-VASc score has fair predictive value for identifying high-risk patients with AF and is not significantly superior to CHADS2 in predicting patients who develop AMI.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a common cardiac rhythm disturbance with an age-related increase in both women and men. The arrhythmia is also a major cardiac cause of stroke1. Given that AF has become a major cardiovascular challenge in the last two decades, it is crucial to have an updated picture of its medical, social and economic impact of AF to plan appropriate interventions2.
The CHADS2 and CHA2DS2-VASc scoring systems have been proved efficacy to stratify stroke and thromboembolism risk in patients with non-valvular AF (NVAF). According to recent large clinical trials, patients identified as high-risk category using the CHADS2 score comprise many AF patients at risk of fatal and devastating strokes. Thus, the CHA2DS2-VASc score is recommended by current major guidelines to identify the “truly low-risk patients” with AF3. Except for preventing stroke in AF patients, the CHADS2 and CHA2DS2-VASC scores have been reported recently to predict cardiovascular4 and cerebrovascular events5.
Coronary artery disease (CAD) and AF have close relationship and interact with each other. CAD is considered a risk factor for AF as well as a disease for which the adverse outcome is modulated by AF. Reports from several clinical trials have proved that AF is associated with an increased risk of incident MI6,7,8. Furthermore, the coexistence of the two diseases increases the risk of future cardiovascular events and stroke dramatically9. Subsequently, the number and complexity of therapies increase and the potential for significant adverse interactions grow. In addition, CAD and stroke share a number of common cardiovascular risk factors, including sex, age, obesity, hypertension, diabetes mellitus (DM), and congestive heart failure (CHF). Identification of high-risk AF patients is a needed first step to develop cost-effective approaches for prevention of CAD. For example, Ankle-Brachial Index (ABI) is a non-invasive tool in evaluating cardiovascular risk, useful in predicting MI in NVAF patients10. The CHADS2 and CHA2DS2-VASc scores include similar risk factors for the development of CAD. Therefore, unsurprisingly, there have been recent reports about the two stroke risk scoring systems used to predict severity or even outcome of CAD11. Most previous studies mainly focused on AF after CAD and demonstrated AF adversely influences the outcomes in patients with CAD. However, studies concerned with the cardiovascular risk stratification and identification AF patients at higher risk to experience acute myocardial infarction (AMI) using the CHADS2 and CHA2DS2-VASc scores are relatively sparse. The objective of our study was to investigate the predictive value of CHADS2 and CHA2DS2-VASc scores for AMI risk in AF, and subsequently compare the accuracy of the CHADS2 score with CHA2DS2-VASc score in predicting the AMI incidence.
Subjects and Methods
This was a retrospective study based on electronic hospital databases of our hospital. This study enrolled 5140 consecutive patients with NVAF who presented to our department of cardiology from November 2013 to October 2016. The definition of NVAF was in accordance with 2014 AHA/ACC/HRS guideline for the management of patients with AF1. Patients were excluded if they had rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair, and death in hospital. To be eligible for our study, AMI was comprised of ST-segment elevation myocardial infarction (STEMI) and non-STEMI within the previous 30 days. The following information was collected from the database: age, gender, history of CAD, CHF, hypertension, DM, stroke or transient ischemic attack (TIA), thromboembolism, vascular disease (including prior myocardial infarction, peripheral artery disease, or complex aortic plaque), hyperlipidaemia, hyperuricemia, hyperthyroidism, hypothyroidism and obstructive sleep apnea (OSA). From the baseline clinical characteristics of each patient, the pre-AMI CHADS2 and CHA2DS2-VASc scores were calculated according to the ESC guidelines for the management of AF12. The study was approved by the research ethics committee of Xuzhou Central Hospital and all patients provided their written informed consent. All methods were carried out in accordance with the approved guidelines and regulations.
Statistical Analysis
Quantitative variables are expressed as mean ± standard deviation for parametric variables and were analyzed using the independent-samples t test. In addition, nonparametric variables are presented as median with interquartile ranges. Categorical variables are expressed as numbers and percentages, and were compared using the chi-square test. The relationships between CHADS2 score and AMI rate as well as the relationships between CHA2DS2-VASc score and AMI rate were examined by Kruskal-Wallis non-parametric H test. The multivariate logistic regression analysis was used to evaluate the associations of the baseline clinical characteristics, CHADS2 and CHA2DS2-VASc scores with the prevalence of AMI, respectively. We identified the optimal cut-off values of the CHADS2 and CHA2DS2-VASc scores each based on receiver operating characteristic (ROC) curves to predict the risk of AMI. The area under the curve (AUC) is a rough guide for quantifying the discriminatory capacity of a diagnostic test ranked as: excellent (0.9–1), good (0.8–0.89), fair (0.7–0.79), poor (0.6–0.69), or fail/no discriminatory capacity (0.5–0.59)13. The differences between the areas under the two ROC curves were assessed by a univariate z-score test. The agreement on identification of AMI risk determined by the two scores was tested by the Cohen’s kappa coefficient (κ) and McNemar test was used to compare the differences in risk. The agreement of κ 0.81–1.00 is interpreted as almost perfect agreement, 0.61–0.80 as substantial agreement, 0.41–0.60 as moderate agreement, 0.21–0.40 as fair agreement, and ≤0.20 as slight agreement14. Assessing value of CHADS2 and CHA2DS2-VASc scores in AMI prediction using net reclassification improvement (NRI) and integrated discrimination improvement (IDI)15. Whether NRI and IDI were statistically significant was analyzed using the Z-test. The analyses were performed using the SPSS 21.0 and ROCKIT 0.9β statistical software, and a two-sided P-value < 0.05 was considered statistically significant.
Results
A total of 5140 AF patients were enrolled in this study. These patients were divided into two groups: 300 in the AMI group and 4840 in the non-AMI group. Patient demographics, cardiovascular diseases and cardiovascular risk factors of the two groups are shown in Table 1. Of the 300 AMI patients, 87 (29.0%) were female, with a mean age of 67.4 ± 10.9 years. But in the non-AMI group, 1867 (38.6%) were female, with a mean age of 59.9 ± 12.4 years. In comparison with the non-AMI group, the AMI group had a higher prevalence of hypertension, DM, CHF, CAD, prior MI, stroke/TIA, thromboembolism, vascular disease and hyperlipidaemia. AMI rate positively correlated with the CHADS2 score and the CHA2DS2-VASc score.
Multivariable models of the difference between baseline clinical characteristics and prevalence of AMI in patients with AF are shown in Table 2. Multivariate logistic regression analysis showed that advanced age, male, and history of hypertension, DM, hyperlipidaemia, stroke/TIA and prior MI were independent risk factors for AMI (P < 0.05). The associations of CHADS2 and CHA2DS2-VASc scores with the prevalence of AMI were assessed by multivariable logistic regression in different models (Table 3). The unadjusted logistic regression analysis revealed that both CHADS2 score (odds ratio 2.166, 95%CI 1.987–2.362, P < 0.001) and CHA2DS2-VASc score (odds ratio 1.673, 95%CI 1.566–1.789, P < 0.001) were associated with an increased odds ratio of the prevalence of AMI in patients with AF. Furthermore, the adjusted logistic regression analysis revealed that both CHADS2 score (odds ratio 2.120, 95%CI 1.942–2.315, P < 0.001) and CHA2DS2-VASc score (odds ratio 1.639, 95%CI 1.532–1.753, P < 0.001) were associated with an increased odds ratio of the prevalence of AMI in patients with AF, after adjustment for hyperlipidaemia, hyperuricemia, hyperthyroidism, hypothyroidism and OSA.
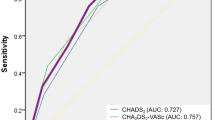
ROC analysis indicated that the AUC for CHADS2 score predicting AMI was 0.787 (0.763–0.812), P < 0.001 (Fig. 1). The optimal cutoff value was CHADS2 ≥ 2, with a sensitivity of 75.3% and a specificity of 71.1%. The AUC for CHA2DS2-VASc score predicting AMI was 0.750 (0.722–0.777), P < 0.001. The optimal cutoff value for CHA2DS2-VASc score displaying the best predictive value was ≥3, with a sensitivity of 75.0% and a specificity of 64.1%. The difference between the two areas under the curves was not significant (Z = 1.947, P > 0.05). To follow up on this reclassification, we calculated category-based NRI and absolute IDI. The CHA2DS2-VASc score was chosen as reference. The CHADS2 score resulted in a NRI of 0.069 (P = 0.002) and an IDI of 0.074 (P > 0.05).
Receiver operating characteristic curves (ROC) for CHADS2 and CHA2DS2-VASc scores for prediction of acute myocardial infarction (AMI) in patients with atrial fibrillation (AF). The area under the receiver operating characteristic curve (AUC) for CHADS2 score predicting AMI is 0.787 (0.763–0.812), P < 0.001. The sensitivity and specificity for a CHADS2 score ≥2 are 75.3% and 71.1%, respectively. The AUC for CHA2DS2-VASc score is 0.750 (0.722–0.777), P < 0.001. The sensitivity and specificity for a CHA2DS2-VASc score ≥3 are 75.0% and 64.1%, respectively. The difference between the two areas under the curves is not significant (P > 0.05).
The risk estimates of AMI were stratified by the cut-offs of CHADS2 and CHA2DS2-VASc scores, using the baseline characteristics of 5140 AF patients, respectively. CHADS2 score rated 1624 patients (31.6%) as having high risk of AMI, versus 1941 (37.8%) who were considered high risk by CHA2DS2-VASc score. Both CHADS2 and CHA2DS2-VASc scores indicated that 1418 (27.6%) patients were at high risk of AMI and 2993 (58.2%) patients were at low risk of AMI. CHA2DS2-VASc score rated 523 patients (10.2%) as having high risk of AMI, who were considered low risk by the CHADS2 score. On the contrary, CHADS2 score rated 206 (4.0%) patients at high risk of AMI, but considered low risk by the CHA2DS2-VASc score. The Kappa coefficient showed substantial concordance between the two tests (Kappa = 0.688, P < 0.001) and the consistency rate between the two scores was 85.8%. The differences in risk discrimination of high risk AMI patients between the two scores were significant (McNemar test, P < 0.001). The AMI rate was significantly higher in patients with CHADS2 ≥ 2 compared with those with CHADS2 < 2 (13.9% [226/1624] versus 2.1% [74/3516], P < 0.001). In addition, patients with CHA2DS2-VASc ≥ 3 had a significantly higher AMI compared with patients with CHA2DS2-VASc < 3 (11.6% [225/1941] versus 2.3% [75/3199], P < 0.001).
Discussion
The major findings of this study are as follows: (i) AMI rate positively correlates with the CHADS2 and CHA2DS2-VASc scores; (ii) CHADS2 and CHA2DS2-VASc scores are independent predictors of AMI in AF patients; (iii) accuracy of CHADS2 score in predicting high-risk AF patients who developed AMI is similar to CHA2DS2-VASc score; (iv) the predictive accuracy of the two scores is fair; (v) the best cutoff value to predict AMI is CHADS2 ≥ 2 or CHA2DS2-VASc ≥ 3. The study suggests that CHADS2 and CHA2DS2-VASc scores may be useful as AMI risk indices for patients with AF, although the statistical impact is fair.
AF and CAD are closely associated. Data from recent studies in different patient populations have shown that AF and CAD coexist in a large percentage of patients (18–34%)16. CAD can promote AF due to its setting of consequential physiopathological changes, including inflammation, fibrosis, hypertrophy and atrial ischemia1. AF population with a history of CAD has been reported to be associated with recurrent AF episodes, heart failure and increased short-term and long-term mortality17. Furthermore, the morbidity and mortality of MI-associated stroke is often high, and the risk of post-MI stroke may be highest over the first 3 months18. Stable CAD was common in Chinese AF patients who were more likely to be older and to have more co-morbidities. Additionally, stable CAD was strongly associated with a higher risk of 1-year all-cause mortality19. A previous clinical trial proved that coronary artery calcium was independently associated with an increased risk of AF20. But whether CAD is the underlying pathophysiologic link between coronary artery calcium and AF is still controversial21.
AF patients with CAD have more risk factors and comorbidities than patients without CAD. Increased risk of stroke and other thrombo-embolic events, left ventricular dysfunction, aggravation of heart failure and hospitalizations related to AF complicating MI result in significant reduced exercise capacity, degraded quality of life and long-term death. For instance, the development of AF during index hospitalisation for MI was associated with increased risk of sudden cardiac death22. Coexistence of atherosclerotic risk factors, systemic inflammation and platelet activation can promote a pro-thrombotic state and eventually MI in AF. In AF patients, impaired artery dilatation predisposes to atherosclerotic complications is associated with increased risk of cardiovascular events23. There are other mechanisms for the increased MI risk in patients with AF. For example, episodes of poorly controlled AF with high ventricular rates may result in type 2 MI24. Shibata et al. reported that AF was the most frequent cause of coronary artery embolism which was recognized as an important nonatherosclerotic cause of AMI25.
The data from our study demonstrates that, the prevalence of hypertension, DM, CHF, CAD, prior MI, stroke/TIA, thromboembolism, vascular disease and hyperlipidaemia among 300 AF patients with AMI were higher than the patients without AMI. The present data also indicated that advanced age, male, and history of hypertension, DM, hyperlipidaemia, stroke/TIA and prior MI were independent risk factors for AMI in AF patients.
Much earlier prevention of the MI might reduce the burden of AF to protect the patients, not only from the progression of AF from an easily treated condition to an utterly refractory problem, but also from the risk of bleeding with triple therapy (vitamin K antagonist, aspirin, and clopidogrel). It is an enormous challenge to the management of AF patients at risk or with previous MI, because of the complexity of antithrombotic treatment to prevent both thromboembolic and cardiac events. Previous studies showed that oral anticoagulants alone were not enough to lower the risk of MI in AF26. Of note, recent studies have demonstrated that combined anticoagulant and antiplatelet therapy is independently associated with significantly increased risk for bleeding compared with anticoagulant therapy alone in AF patients27. Moreover, the antithrombotic treatment of AF may complicate coronary revascularization and related antiplatelet treatment11. Therefore, the attention must be directed towards preventing CAD at an early stage in order to improve the treatment and prognosis of AF, with more focus on the AMI risk stratification. Our data illustrated that AMI rate positively correlated with the CHADS2 score and the CHA2DS2-VASc score. Furthermore, both CHADS2 score and CHA2DS2-VASc score were independently associated with an increased prevalence of AMI.
Except for preventing stroke in AF patients, several studies recently have reported that the CHADS2 and CHA2DS2-VASc scores can also predict severity and outcomes of stroke and thromboembolic events28 in patients with AF and those without AF29, 30. Compared with HF with concomitant AF, the risk of thromboembolism was higher among HF patients without AF at high CHA2DS2-VASc scores31. In eight cohort studies (7 prospective and 1 retrospective) of 31,509 patients with CAD, CHADS2 score was associated with increased mortality and stroke/TIA incidence in patients without AF. But no significant association was found between CHADS2 score and stroke/TIA incidence in patients with AF32. Furthermore, CHA2DS2-VASc score was strongly predictive of stroke and embolic events in a retrospective cohort of 465 patients with cardiac myxomas following surgical treatment33. Hoshino T et al. found that CHADS2 and CHA2DS2-VASc scores were useful in predicting functional status after stroke in CAD patients34. Both the CHADS2 and CHA2DS2-VASc scores were used to predict contrast-induced nephropathy (CIN) in patients with CAD who underwent urgent percutaneous coronary intervention (PCI)35. Chou RH et al. enrolled 539 stable CAD patients who underwent elective PCI and reported that CHADS2 score independently increased the risk of CIN. Moreover, the predictive accuracy of CHADS2 score was not inferior to either R2CHADS2 score or Mehran’s risk score36. In addition, Subjects who underwent coronary artery bypass surgery with higher CHADS2 scores had significantly higher all-cause mortality and cardiovascular mortality37. The present results showed that the AUC for CHADS2 score was 0.787 with a similar accuracy of the CHA2DS2-VASc score (AUC 0.750) in predicting “high-risk” AF patients who developed AMI. Adding extra points for MI (e.g. age ≥75, prior myocardial infarction, etc.) did not improve the predictive accuracy of CHA2DS2-VASc. However, the predictive accuracy of the two clinical-based risk scores was fair (AUC 0.7–0.79). Although the CHA2DS2-VASc score might be more inclined to classify AF patients as high-risk AMI than the CHADS2 score did. The discriminative and reclassification power of CHADS2 and CHA2DS2-VASc scores was assessed using NRI and IDI. When CHADS2 score ≥2 and CHA2DS2-VASc score ≥3 were chosen as the best predictive cutoff values, CHADS2 score significantly improved risk classification for AMI by assessment of NRI. However, insignificant IDI for CHADS2 score was showed. Now that the CHADS2 and CHA2DS2-VASc scores have proved useful not only to assess the stroke, but also to assess the AMI risk, and to drive therapeutic choices, our future clinical trials are going to examine the usefulness of addition of antiplatelet drugs to oral anticoagulants in reducing the risk of both MI and stroke in AF patients.
Study limitations
The main limitation of this study is related to its retrospective nature. The history of AMI was ascertained at the time of admission. CHADS2 and CHA2DS2-VASc scores were calculated retrospectively after the AMI had occurred. Precise information about the pre-AMI status was unavailable. It should also be noted that, because this was a hospital-based observational study, the characteristics of the patients with AF admitted to the Department of cardiology, Xuzhou Central Hospital might differ from those of the general population. Additionally, these data derived from a single medical center survey might exist selection bias. Although we adjusted for several variables, residual and unmeasured confounding might not be fully reflected. Treatment related to the incidence of AMI was not studied, especially for anticoagulant and antiplatelet therapy. Thus, we could not exclude the possibility that treatment influenced predictive value of the two scores. Finally, since all patients in our study were Asians, the results were not directly translatable to other ethnicities. As a result, further prospective multicenter and larger-scale studies are needed to clarify our conclusions.
Conclusions
Focusing specifically on risk stratification of AMI by the CHADS2 and CHA2DS2-VASc scores as well as means for optimizing outcomes in the treatment of AF is the significance of our study. Even if the accumulated evidence has shown that CHA2DS2-VASc is better at identifying ‘truly low-risk’ patients with AF who develop stroke and thromboembolism, our data demonstrate that CHA2DS2-VASc is not significantly superior to CHADS2 for predicting AMI in AF patients.
References
January, C. T. et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 64, e1–76 (2014).
Zoni-Berisso, M., Lercari, F., Carazza, T. & Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 6, 213–220 (2014).
Pisters, R., Nieuwlaat, R., Lane, D. A., Crijns, H. J. & Lip, G. Y. Potential net clinical benefit of population-wide implementation of apixaban and dabigatran among European patients with atrial fibrillation: A modelling analysis from the Euro Heart Survey. Thromb Haemost. 109, 328–336 (2013).
Kang, I. S., Pyun, W. B. & Shin, G. J. Predictive value of CHADS2 score for cardiovascular events in patients with acute coronary syndrome and documented coronary artery. Korean J Intern Med. 31, 73–81 (2016).
Hsu, C. Y. et al. Usefulness of the CHADS2 Score for Determining Risk of Seizure in Patients With Atrial Fibrillation. Am J Cardiol. 118, 1340–1344 (2016).
Chao, T. F. et al. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2-VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm. 11, 1941–1947 (2014).
Soliman, E. Z. et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 174, 107–114 (2014).
Soliman, E. Z. et al. Atrial Fibrillation and Risk of ST-Segment-Elevation Versus Non-ST-Segment-Elevation Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 131, 1843–1850 (2015).
Akao, M. Atrial fibrillation and coronary artery disease: Resembling twins? J Cardiol. 63, 169–170 (2014).
Violi, F. et al. Ankle-Brachial Index and cardiovascular events in atrial fibrillation. The ARAPACIS Study. Thromb Haemost. 115, 856–863 (2016).
Zielonka, A. et al. Atrial fibrillation in outpatients with stable coronary artery disease: results from the multicenter RECENT study. Pol Arch Med Wewn. 125, 162–171 (2015).
Camm, A. J. et al. Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association. Europace. 14, 1385–1413 (2012).
Wingert, N. C. et al. The ACS NSQIP Risk Calculator Is a Fair Predictor of Acute Periprosthetic Joint Infection. Clin Orthop Relat Res. 474, 1643–1648 (2016).
Kêkê, L. M. et al. Body mass index and childhood obesity classification systems: A comparison of the French, International Obesity Task Force (IOTF) and World Health Organization (WHO) references. Rev Epidemiol Sante Publique. 63, 173–182 (2015).
Pencina, M. J., D’Agostino, R. B. Sr. & Steyerberg, E. W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 30, 11–21 (2011).
Senoo, K. et al. Coronary artery diseases in Japanese patients with nonvalvular atrial fibrillation. J Cardiol. 63, 123–127 (2014).
Hersi, A. et al. Prognostic significance of prevalent and incident atrial fibrillation amongpatients hospitalized with acute coronary syndrome: findings from the GulfRACE-2 Registry. Angiology. 63, 466–471 (2012).
Goldstein, L. B. & El Husseini, N. Neurology and cardiology: points of contact. Rev Esp Cardiol. 64, 319–327 (2011).
Bai, Y. et al. Clinical characteristics and one year outcomes in Chinese atrial fibrillation patients with stable coronary artery disease: a population-based study. J Geriatr Cardiol. 13, 665–671 (2016).
O'Neal, W. T. et al. Coronary Artery Calcium and Risk of Atrial Fibrillation (From the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 114, 1707–1712 (2014).
Jain, A. et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging. 4, 8–15 (2011).
Murakami, N. et al. Distinct risk factors of atrial fibrillation in patients with and without coronary artery disease: a cross-sectional analysis of the BOREAS-CAG Registry data. Open Heart. 4, e000573 (2017).
Perri, L., Pastori, D., Pignatelli, P., Violi, F. & Loffredo, L. Flow-mediated dilation is associated with cardiovascular events in non-valvular atrial fibrillation patients. Int J Cardiol. 179, 139–143 (2015).
Kolodgie, F. D., Virmani, R., Finn, A. V. & Romero, M. E. Embolic myocardial infarction as a consequence of atrial fibrillation: a prevailing disease of the future. Circulation. 132, 223–226 (2015).
Shibata, T. et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation. 132, 241–250 (2015).
Pastori, D. et al. Inadequate anticoagulation by vitamin K antagonists is associated with major adverse cardiovascular events in patients with atrial fibrillation. Int J Cardiol. 201, 513–516 (2015).
Verheugt, F. W. Antithrombotic therapy during and after percutaneous coronary intervention in patients with atrial fibrillation. Circulation. 128, 2058–2061 (2013).
Parsons, C. et al. CHA2DS2-VASc Score: A Predictor of Thromboembolic Events and Mortality in Patients With an Implantable Monitoring Device Without Atrial Fibrillation. Mayo Clin Proc. 92, 360–369 (2017).
Hong, H. J. et al. Early neurological outcomes according to CHADS2 score in stroke patients with non-valvular atrial fibrillation. Eur J Neurol. 19, 284–290 (2012).
Tu, H. T. et al. Pre-stroke CHADS2 and CHA2DS2-VASc scores are useful in stratifying three-month outcomes in patients with and without atrial fibrillation. Cerebrovasc Dis. 36, 273–280 (2013).
Melgaard, L. et al. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA. 314, 1030–1038 (2015).
Zhou, X. et al. Usefulness of CHADS2 score for prognostic stratification of patients with coronary artery disease: A systematic review and meta-analysis of cohort studies. Int J Cardiol. 228, 906–911 (2017).
Yin, L. et al. Usefulness of CHA2DS2-VASc Scoring Systems for Predicting Risk of Perioperative Embolism in Patients of Cardiac Myxomas Underwent Surgical Treatment. Sci Rep. 6, 39323 (2016).
Hoshino, T., Ishizuka, K., Shimizu, S. & Uchiyama, S. CHADS2, CHA2DS2-VASc, and R2CHADS2 Scores Are Associated With 3-Month Functional Outcome of Stroke in Patients With Prior Coronary Artery Disease. Circ J. 78, 1481–1485 (2014).
Kurtul, A., Yarlioglues, M. & Duran, M. Predictive Value of CHA2DS2-VASC Score for Contrast-Induced Nephropathy After Percutaneous Coronary Intervention for Acute Coronary Syndrome. Am J Cardiol. 119, 819–825 (2017).
Chou, R. H. et al. CHADS2 score predicts risk of contrastinduced nephropathy in stable coronary artery disease patients undergoing percutaneous coronary interventions. J Formos Med Assoc. 115, 501–509 (2016).
Lu, D. Y. et al. Usefulness of the CHADS2 Score for Prognostic Stratification in Patients With Coronary Artery Disease Having Coronary Artery Bypass Grafting. Am J Cardiol. 119, 839–844 (2017).
Acknowledgements
The present work was supported in part by grants from the Jiangsu Provincial Medical Youth Talent (QNRC2016383), Xuzhou Municipal Bureau of Science and Technology (No. KC16SH042), Natural Science Foundation of Jiangsu Province (BK20131122) and Xuzhou Science and Technology Grant (KC14SX012).
Author information
Authors and Affiliations
Contributions
Hui Pang conceived the idea for the study and contributed to the design of the research. Bing Han and Qiang Fu were involved in data collection. Zhenkun Zong performed statistical analysis. Hui Pang wrote the main manuscript text and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pang, H., Han, B., Fu, Q. et al. Predictive value of CHADS2 and CHA2DS2-VASc scores for acute myocardial infarction in patients with atrial fibrillation. Sci Rep 7, 4730 (2017). https://doi.org/10.1038/s41598-017-04604-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04604-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.