Abstract
Brain infiltration of peripheral immune cells and their interactions with brain-resident cells may contribute to Alzheimer’s disease (AD) pathology. To examine these interactions, in the present study we developed a three-dimensional human neuroimmune axis model comprising stem cell-derived neurons, astrocytes and microglia, together with peripheral immune cells. We observed an increase in the number of T cells (but not B cells) and monocytes selectively infiltrating into AD relative to control cultures. Infiltration of CD8+ T cells into AD cultures led to increased microglial activation, neuroinflammation and neurodegeneration. Using single-cell RNA-sequencing, we identified that infiltration of T cells into AD cultures led to induction of interferon-γ and neuroinflammatory pathways in glial cells. We found key roles for the C-X-C motif chemokine ligand 10 (CXCL10) and its receptor, CXCR3, in regulating T cell infiltration and neuronal damage in AD cultures. This human neuroimmune axis model is a useful tool to study the effects of peripheral immune cells in brain disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of the present study are available within the paper, Supplementary Information files or publicly available. RNA-seq data have been deposited to the National Center for Biotechnology Information (GSE239509). The scRNA-seq was performed in five independent batches. All the relevant raw data (fastq files)/sparse matrix/R objective files are available at https://github.com/jospark09/PiChip. Unfiltered all-condition-combined list deposited; PiChip.list.ordered.Not.filtered.rds. Filtered Seurat cell type annotated R objective files deposited; PiChip.filtered_seurat.cell.type.annotated.rds. Fig. 1 and Extended Data Fig. 1e objective files deposited; PiChip.Fig.1 and extended Fig.1.rds. Microglia only re-clustered and subcluster-annotated file deposited; Mic.only.type.annotated.Fig.4.rds. T cell only re-clustered and subclustered-annotated file deposited; T.only.type.annotated.Fig.4.rds. Further information on research design is available in the Nature Research Reporting Summary linked to this article. Source data are provided with this paper.
Code availability
The code for analysis and generating scRNA-seq figures can be found at https://github.com/jospark09/PiChip and https://zenodo.org/record/8150286.
References
Jorfi, M., Maaser-Hecker, A. & Tanzi, R. E. The neuroimmune axis of Alzheimer’s disease. Genome Med. 15, 6 (2023).
Bettcher, B. M., Tansey, M. G., Dorothée, G. & Heneka, M. T. Peripheral and central immune system crosstalk in Alzheimer disease—a research prospectus. Nat. Rev. Neurol. 17, 689–701 (2021).
Arlehamn, C. S. L., Garretti, F., Sulzer, D. & Sette, A. Roles for the adaptive immune system in Parkinson’s and Alzheimer’s diseases. Curr. Opin. Immunol. 59, 115–120 (2019).
Wu, K.-M. et al. The role of the immune system in Alzheimer’s disease. Ageing Res. Rev. 70, 101409 (2021).
Cao, W. & Zheng, H. Peripheral immune system in aging and Alzheimer’s disease. Mol. Neurodegen. 13, 51 (2018).
Rogers, J., Luber-Narod, J., Styren, S. D. & Civin, W. H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 9, 339–349 (1988).
Itagaki, S., McGeer, P. L. & Akiyama, H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci. Lett. 91, 259–264 (1988).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
Merlini, M., Kirabali, T., Kulic, L., Nitsch, R. M. & Ferretti, M. T. Extravascular CD3+ T cells in brains of Alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener. Dis. 18, 49–56 (2018).
Unger, M. S. et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 89, 67–86 (2020).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Ferretti, M. T. et al. T-cell brain infiltration and immature antigen-presenting cells in transgenic models of Alzheimer’s disease-like cerebral amyloidosis. Brain Behav. Immun. 54, 211–225 (2016).
Browne, T. C. et al. IFN-γ production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. 190, 2241–2251 (2013).
MacPherson, K. P. et al. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol. Dis. 102, 81–95 (2017).
Shukla, A. K. et al. CD11a expression distinguishes infiltrating myeloid cells from plaque‐associated microglia in Alzheimer’s disease. Glia 67, 844–856 (2019).
Monsonego, A. et al. Aβ-induced meningoencephalitis is IFN-γ-dependent and is associated with T cell-dependent clearance of Aβ in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 103, 5048–5053 (2006).
Machhi, J. et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflamm. 18, 272 (2021).
Pietronigro, E. et al. Blockade of α4 integrins reduces leukocyte–endothelial interactions in cerebral vessels and improves memory in a mouse model of Alzheimer’s disease. Sci. Rep. 9, 12055 (2019).
Gate, D. et al. CD4+ T cells contribute to neurodegeneration in Lewy body dementia. Science 374, 868–874 (2021).
Wareham, L. K. et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol. Neurodegen. 17, 23 (2022).
Götz, J., Bodea, L.-G. & Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 19, 583–598 (2018).
Scearce-Levie, K., Sanchez, P. E. & Lewcock, J. W. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat. Rev. Drug Discov. 19, 1–16 (2020).
Blanchard, J. W., Victor, M. B. & Tsai, L.-H. Dissecting the complexities of Alzheimer disease with in vitro models of the human brain. Nat. Rev. Neurol. 18, 25–39 (2021).
Choi, S. H., Kim, Y. H., Quinti, L., Tanzi, R. E. & Kim, D. Y. 3D culture models of Alzheimer’s disease: a road map to a ‘cure-in-a-dish’. Mol. Neurodegen. 11, 75 (2016).
Choi, S. H. et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278 (2014).
Kim, Y. H. et al. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat. Protoc. 10, 985–1006 (2015).
Kwak, S. S. et al. Amyloid-β42/40 ratio drives tau pathology in 3D human neural cell culture models of Alzheimer’s disease. Nat. Commun. 11, 1377 (2020).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951 (2018).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293.e9 (2017).
McQuade, A. et al. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol. Neurodegen. 13, 67 (2018).
Doorn, K. J. et al. Microglia in olfactory bulb of AD and PD patients. Brain Pathol. 24, 152–165 (2014).
Anderson, K. G. et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9, 209–222 (2014).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Takata, K. et al. Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem Biophys. Res. Commun. 301, 699–703 (2003).
Smit, M. J. et al. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 102, 1959–1965 (2003).
Lund, H. et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 9, 4845 (2018).
Lee, S. M., Hudobenko, J., McCullough, L. D. & Chauhan, A. Microglia depletion increase brain injury after acute ischemic stroke in aged mice. Exp. Neurol. 336, 113530 (2021).
Laurent, C. et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 140, 184–200 (2016).
Xu, L. et al. T‐cell infiltration, contribution and regulation in the central nervous system post‐traumatic injury. Cell Prolifer. 54, e13092 (2021).
Kaya, T. et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 25, 1446–1457 (2022).
Schulz, O., Hammerschmidt, S. I., Moschovakis, G. L. & Förster, R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu. Rev. Immunol. 34, 1–40 (2015).
Sokol, C. L. & Luster, A. D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 7, a016303 (2015).
Zuena, A. R., Casolini, P., Lattanzi, R. & Maftei, D. Chemokines in Alzheimer’s disease: new insights Into prokineticins, chemokine-like proteins. Front. Pharm. 10, 622 (2019).
Galimberti, D. et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 63, 538–543 (2006).
Xia, M. Q., Bacskai, B. J., Knowles, R. B., Qin, S. X. & Hyman, B. T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer’s disease. J. Neuroimmunol. 108, 227–235 (2000).
Wojcieszak, J., Kuczyńska, K. & Zawilska, J. B. Role of chemokines in the development and progression of Alzheimer’s disease. J. Mol. Neurosci. 72, 1929–1951 (2022).
Zaheer, S. et al. Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer’s disease mice. Neurochem. Res. 38, 218–225 (2013).
Duan, R.-S. et al. Decreased fractalkine and increased IP-10 expression in aged brain of APPswe transgenic mice. Neurochem. Res. 33, 1085–1089 (2008).
Groom, J. R. & Luster, A. D. CXCR3 in T cell function. Exp. Cell Res. 317, 620–631 (2011).
Kurachi, M. et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 208, 1605–1620 (2011).
Krummel, M. F., Bartumeus, F. & Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Maurice, N. J., McElrath, M. J., Andersen-Nissen, E., Frahm, N. & Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat. Commun. 10, 4987 (2019).
Krauthausen, M. et al. CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer’s disease model. J. Clin. Invest. 125, 365–378 (2015).
Ozga, A. J. et al. CXCL10 chemokine regulates heterogeneity of the CD8+ T cell response and viral set point during chronic infection. Immunity 55, 82–97.e8 (2021).
Gericke, C. et al. Early β‐amyloid accumulation in the brain is associated with peripheral T cell alterations. Alzheimer’s Dement. https://doi.org/10.1002/alz.13136 (2023).
Piehl, N. et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 185, 5028–5039 (2022).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Hao, Y. et al. Dictionary learning for integrative, multimodal, and scalable single-cell analysis. Preprint at Nat. Biotech. https://doi.org/10.1038/s41587-023-01767-y (2023).
Acknowledgements
This work was supported by the Cure Alzheimer’s Fund and the JPB Foundation. All microfabrication procedures of the PiChip platform were conducted at the BioMEMS Resource center. All illustrations, except the 3D microfluidic platform, were created with Biorender (https://www.biorender.com). The scRNA-seq computations in this paper were, in part, run on the FASRC Cannon cluster supported by the FAS Division of Science Research Computing Group at Harvard University.
Author information
Authors and Affiliations
Contributions
M.J., J.P., DY.K. and R.E.T. conceived and designed the present study. M.J. and J.P. conceptualized and M.J. designed and microfabricated the PiChip system. M.J., J.P. and C.K.H. conducted the PiChip experiments and collected and analyzed data. J.P. collected and analyzed the data from neurodegeneration and scRNA-seq experiments. C.C.L. and M.C. conducted animal studies. J.M.K. developed a pipeline to quantify image-based cellular damage. B.K. helped with microglia differentiation. Y.C. and D.P. helped with scRNA-seq alignment and QC. D.M. performed bioinformatic analysis for cytokines. M.J., J.P., D.I., D.Y.K. and R.E.T. discussed the results and interpreted the data. M.J. wrote the manuscript with input from all authors. R.E.T. supervised and directed the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Julia TCW and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the PiChip model.
a, 3D illustration of three different layers of the microfluidic-based PiChip system. Layer I consists of an orthogonal array of 400 microgrooves of 10 μm height and width and 500 μm length. Layer II consists of BRAIN and four PERIPHERAL compartments of 100 μm height. Layer III consists of a cylindrical chamber with 6000 μm length and radius and 2500 μm open hollow. The top and bottom panels show low and high magnification of each layer, respectively. b, Representative immunofluorescence staining of 3D-differentiated neurons (MAP-2; blue) and astrocytes (GFAP; red) in AD and CTRL cultures after four weeks of differentiation. Scale bar, 100 μm. c, d, Quantification of MAP-2 (c) and GFAP+ (d) expressing surface area in AD and CTRL cultures (n = 4 independent ROIs for each MAP2+ and GFAP+ CTRL, n = 5 independent ROIs for each MAP2+ and GFAP+ AD; P-values from unpaired, two-sided, t-test). e–h, Quantification of soluble Aβ38 (e), Aβ40 (f), Aβ42 (g) and Aβ42/40 ratio (h) in AD and CTRL cultures after four weeks of differentiation (n = 4 cell cultures from two independent experiments; P-values from unpaired, two-sided, t-test with Welch’s correction). i, Representative immunofluorescence staining of p-Tau (PHF-1; blue) in neuronal cell bodies and neurites (GFP; green) in AD and CTRL cultures. Scale bar, 50 μm. j, Quantification of PHF-1+ expressing surface area in AD and CTRL cultures (n=6 ROIs from at least 5 independent experiments; P-values from nonparametric, two-sided, Mann-Whitney test). k, UMAP visualization of a total of 20153 single cells from AD and CTRL cultures by single-cell transcriptome profiles. Distinct cell types are depicted with different colors. l, Volcano plot of differentially expressed genes in astrocytes in AD Neu/AC/iMGL versus AD cultures lacking iMGL (P-values from Wilcoxon Rank Sum test). Significantly upregulated (P < 0.05 and FC > 0.1) and downregulated (P < 0.05 and FC < 0.1) genes are shown in red and blue dots, respectively. m, Violin plot of highly enriched C3 gene in astrocytes in AD Neu/AC/iMGL compared to CTRL (n = 5 independent scRNA-seq data; P-values from nonparametric, two-sided, Mann-Whitney test). n, Vioin plots of selected significantly enriched genes associated with reactive astrocytes (VIM and GFAP) in AD Neu/AC/iMGL compared to AD cultures lacking iMGL (n = 5 independent single cell RNAseq data; P-values from nonparametric, two-sided, Mann-Whitney test). o, Violin plots of highly enriched genes associated with reactive astrocytes and interferon-γ in astrocytes in AD Neu/AC/iMGL compared to AD cultures lacking iMGL (n = 5 independent scRNA-seq data; P-values from nonparametric, two-sided, Mann-Whitney test). (c–h, j) In boxplots, the center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend to minimum and maximum values. (m, n) White circles show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; polygons represent density estimates of data and extend to extreme values.
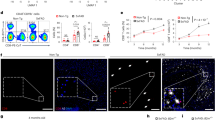
Extended Data Fig. 2 Increasing number of extravascular CD4+ and CD8+ T cells in 5XFAD brains.
a, Representative flow cytometry plots illustrating the gating of CD45+CD11b- cells, CD45_FITC negative CD4+ and CD8+ T cells. b, Quantification of the percentages and absolute numbers of CD45+CD11b- non-myeloid cells. FITC, Fluorescein isothiocyanate (n = 12 each for WT and 5XFAD mice; P-values from nonparametric, two-sided, Mann-Whitney test). c, Representative flow cytometry plots illustrating the gating of CD4+ and CD8+ T cells among all CD45+ extravascular non-myeloid cells. d, Quantification of the percentages of CD4+ and CD8+ T cells among all CD45+ extravascular non-myeloid cells in 5XFAD mice (n = 9 WT and 5XFAD mice each; P-values from nonparametric, two-sided, Mann-Whitney test). (b, d) In boxplots, the center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend to minimum and maximum values.
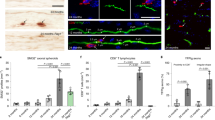
Extended Data Fig. 3 Infiltrating T cells exacerbate neuronal damage in the presence of microglia in AD.
a, Time-lapse confocal imaging showing neurite cleavage in AD cultures. Scale bar, 100 μm. The circled area shows depletion of cells (GFP+ cells) in the presence of microglia and T cells in AD condition. b, Box plots represent quantification of neuronal (MAP2+ expressing surface area) damage in AD Neu/AC (+/−) iMGL conditions in the presence of CD4+ or CD8+ T cells (n = 10 independent ROIs from 4 independent experiments; P-values from two-way ANOVA with Tukey multiple comparisons tests). c, Box plots represent quantification of neuronal damage using ELISA assay for Tuj1 in AD Neu/AC (+/−) iMGL conditions in the presence of CD3+ T cells (n = 4 independent experiments; P-values from nonparametric, Kruskal-Wallis test with Dunn’s multiple comparisons test). d, Box plots represent quantification of calcium dynamics in AD cultures in the presence of T cells compared to CTRL, as monitored using Cal-520 AM, a Ca2+ indicator (n = 251 independent ROIs, CTRL and n = 222, AD from 5 independent experiments; P-values from nonparametric, two-sided, Mann-Whitney test). (b–d) In the boxplots, the center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend to minimum and maximum values.
Extended Data Fig. 4 Summary of scRNA-seq data, selected identity gene expression and gene ontology of significantly enriched genes.
a, UMAP visualization of the cell-type composition of the PiChip cultures by single-cell transcriptome profiles. Distinct cell types are depicted with different colors. b, Dot plot of identity genes for distinct cell types. Color scale (a.u.) indicates the average of expression of identity genes in each cell population, and dot size is proportional to the percentage of cells expressing the identity genes. c, Number of significantly upregulated (FDR < 0.2 and FC > 0) and downregulated (FDR < 0.2 and FC < 0) genes across distinct cell types. Significantly upregulated genes are depicted in red, while downregulated are in blue. d, Volcano plots of differentially expressed genes in all cell types in AD cultures versus CTRL. Significantly upregulated (P < 0.05 and FC > 0.1) and downregulated (P < 0.05 and FC < 0.1) genes are shown in red and blue dots, respectively (P-values from Wilcoxon Rank Sum test). e, Gene ontology of significantly upregulated pathways in all cell types in AD versus CTRL. From left to right, the order of conditions is the same as in d. Color scale indicates the adjusted P-value for significantly enriched pathways in each cell population. f, Gene ontology/network analysis of significantly upregulated genes (P < 0.05 and FC > 0.1). Top five category was visualized for associated genes and pathways. g, Gene ontology of significantly downregulated pathways in all cell types in AD versus CTRL. From left to right, the order of conditions is the same as in d. Color scale indicates the adjusted P-value for significantly enriched pathways in each cell population. h, Gene ontology/network analysis of significantly downregulated genes (P < 0.05 and FC < 0.1). Top five category was visualized for associated genes and pathways (e-h, P-values were calculated from a Fisher exact test and the adjusted p-values given by Benjamini & Hochberg method).
Extended Data Fig. 5 Altered proportions of neurons, astrocytes and microglia in AD cultures and upon infiltration of T cells.
a, UMAP visualization of the cell-type composition of the PiChip cultures by single-cell transcriptome profiles. Distinct cell types are depicted with different colors. b, Dot plot of identity genes for distinct cell types. Color scale (a.u.) indicates the average of expression of identity genes in each cell population, and dot size is proportional to the percentage of cells expressing the identity genes. c, Compositional changes of neurons, astrocytes, microglia in AD Neu/AC/iMGL and CTRL cultures (P-values were calculated from the Ward test and the FDR indicates the adjusted p-values given by Benjamini & Hochberg method). d, Compositional changes of neurons, astrocytes, microglia and T cells in AD Neu/AC/iMGL (+/−) T cell and CTRL conditions (P-values were calculated from the Ward test and the FDR indicates the adjusted p-values given by Benjamini & Hochberg method). e, UMAP visualization of T cell-type composition of the PiChip cultures by single-cell transcriptome profiles. Distinct subtypes of microglia (homeostatic, DAM, MHCII, interferon and proliferative) are depicted with different colors. f, Compositional changes of homeostatic, DAM, MHCII, interferon and proliferative microglia in AD vs CTRL conditions (P-values were calculated from the Ward test and the FDR indicates the adjusted p-values given by Benjamini & Hochberg method).
Extended Data Fig. 6 Interferon-associated pathways are enriched in glial cells following infiltration of T cells in AD.
a, Volcano plot of differentially expressed genes in microglia in the presence of infiltrating T cells in AD cultures compared to AD cultures lacking T cells. Significantly upregulated (P < 0.05 and FC > 0.1) and downregulated (P < 0.05 and FC < 0.1) genes are shown in red and blue dots, respectively (P-values from Wilcoxon Rank Sum test). b, Violin plots of highly enriched genes associated with cytokines (ARF1, CXCL9, CXCL10, CXCL16, CXCL8, CXCL14, CXCL5, IL24, IL32, IL17RA, IL1B, IL10, IL21R, IL18, IL6R, IL7R) and interferon-associated genes (NR1H3, CDC37, OTOP1, HCK, HPX, IRGM, IFNG, IFNGR1, IFNGR2, IRF1, JAK1, JAK2, ARG1, PARP14, PPARG, MED1, PTPN2, SP100, STAT1, TP53, TXK, NR1H2, PARP9, NLRC5, SOCS1, NMI) in microglia in the presence of infiltrating T cells in AD compared to AD cultures lacking T cells (P-values from nonparametric, two-sided, Mann-Whitney test). c, d, Gene ontology of significantly upregulated (c) and downregulated (d) pathways in microglia in the presence of infiltrating T cells in AD cultures vs AD cultures lacking T cells. Color scale indicates the adjusted P-value for significantly enriched pathways in microglia, and dot size is proportional to the count of genes (P-values were calculated from a Fisher exact test and the adjusted p-values given by Benjamini & Hochberg method). e, Volcano plot of differentially expressed genes in astrocytes in the presence of microglia and infiltrating T cells in AD compared to AD cultures lacking T cells. Significantly upregulated (P < 0.05 and FC > 0.1) and downregulated (P < 0.05 and FC < 0.1) genes are shown in red and blue dots, respectively. f, Violin plots of highly enriched genes associated with reactive astrocytes (GFAP, ALDOC, FABP7, TSPO, CRYAB, HSPB1, C3, CHI3L1, NTRK2, S100B, SOX9, STAT3) and interferon-associated genes (NR1H3, CDC37, OTOP1, HCK, HPX, IRGM, IFNG, IFNGR1, IFNGR2, IRF1, JAK1, JAK2, ARG1, PARP14, PPARG, MED1, PTPN2, SP100, STAT1, TP53, TXK, NR1H2, PARP9, NLRC5, SOCS1, NMI) in astrocytes in the presence of microglia and infiltrating T cells in AD compared to AD cultures lacking T cells (P-values from nonparametric, two-sided, Mann-Whitney test). g, h, Gene ontology of significantly upregulated (g) and downregulated (h) pathways in astrocytes in the presence of microglia and infiltrating T cells in AD compared to AD cultures lacking T cells. Color scale indicates the adjusted P-value for significantly enriched pathways in microglia, and dot size is proportional to the count of genes (P-values were calculated from a Fisher exact test and the adjusted p-values given by Benjamini & Hochberg method).
Extended Data Fig. 7 NicheNet analysis of upstream ligand-receptor pairs inducing the DE genes of neurons, astrocytes, and microglia upon T-cell infiltration.
a, Dot plot of identity genes for distinct cell types. Color scale (a.u.) indicates the average expression of identity genes in each cell population, and the dot size is proportional to the percentage of cells expressing the identity genes. b, NicheNet’s ligand–target analysis represents potential upstream receptors expressed by neurons, astrocytes, microglia, and T cells associated with the top 20 potential ligands. c, Predicted target genes of the top 20 of potential ligands in AD Neu/AC/iMGL/T-cell cultures vs CTRL.
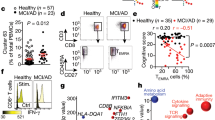
Extended Data Fig. 8 Increased level of pro-inflammatory cytokines in the presence of CD8+ T cells and microglia in AD cultures.
a, Quantification of granzyme B, IL-15, IL-2, TFN-α, and CXCL10 in the AD cultures (+/−) CD8+ T cells and iMGL, as measured by MSD assay (n = 6–30 biological replicates from n = 7–10 independent experiments; P-value from two-way ANOVA with Tukey multiple comparisons test). b, Enrichment of major cytotoxic genes (GZMA, GZMB, GZMH, GZMM, and GZMK) in CD4+ naïve, CD4+ memory, and CD8+ cytotoxic T cells in the AD cultures in the presence of microglia. Color scale (a.u.) indicates the expression of identity genes in each cell population. c, Quantification of INF-γ levels in the AD Neu/AC (+/−) iMGL and CD4+ or CD8+ T cells using ELISA assay (n=8 biological replicates; P-value from two-way ANOVA with Tukey multiple comparisons test). (a, c) In boxplots, the center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Video
Supplementary Video 1 Infiltration of CD8+ T cells into the PiChip system with AD neural/glial culture. Time-lapse confocal video showing infiltration of CD8+ T cells (nuclei stained, magenta) through microchannels over time toward the BRAIN compartment of the PiChip system containing AD cultures (Neu/AC, yellow; iMGL, blue). Scale bar, 100 μm.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jorfi, M., Park, J., Hall, C.K. et al. Infiltrating CD8+ T cells exacerbate Alzheimer’s disease pathology in a 3D human neuroimmune axis model. Nat Neurosci 26, 1489–1504 (2023). https://doi.org/10.1038/s41593-023-01415-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01415-3
This article is cited by
-
CD4 T-cell aging exacerbates neuroinflammation in a late-onset mouse model of amyotrophic lateral sclerosis
Journal of Neuroinflammation (2024)
-
Switch of innate to adaptative immune responses in the brain of patients with Alzheimer’s disease correlates with tauopathy progression
npj Aging (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
T cell exhaustion is associated with cognitive status and amyloid accumulation in Alzheimer’s disease
Scientific Reports (2023)