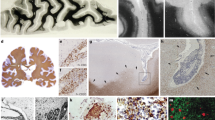
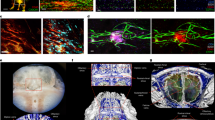
Abstract
The meninges, comprising the leptomeninges (pia and arachnoid layers) and the pachymeninx (dura layer), participate in central nervous system (CNS) autoimmunity, but their relative contributions remain unclear. Here we report on findings in animal models of CNS autoimmunity and in patients with multiple sclerosis, where, in acute and chronic disease, the leptomeninges were highly inflamed and showed structural changes, while the dura mater was only marginally affected. Although dural vessels were leakier than leptomeningeal vessels, effector T cells adhered more weakly to the dural endothelium. Furthermore, local antigen-presenting cells presented myelin and neuronal autoantigens less efficiently, and the activation of autoreactive T cells was lower in dural than leptomeningeal layers, preventing local inflammatory processes. Direct antigen application was required to evoke a local inflammatory response in the dura. Together, our data demonstrate an uneven involvement of the meningeal layers in CNS autoimmunity, in which effector T cell trafficking and activation are functionally confined to the leptomeninges, while the dura remains largely excluded from CNS autoimmune processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA sequencing datasets have been deposited online in the Gene Expression Omnibus (GSE203046). Source data are provided with this paper. No custom software code was used. Paraffin blocks used in the study will be made available upon reasonable request. Source data are provided with this paper.
References
Bartholomäus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
Schläger, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Korn, T. & Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 17, 179–194 (2017).
Rua, R. & McGavern, D. B. Advances in meningeal immunity. Trends Mol. Med. 24, 542–559 (2018).
Lodygin, D. et al. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat. Med. 19, 784–790 (2013).
Lodygin, D. et al. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature 566, 503–508 (2019).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Flügel, A. et al. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity 14, 547–560 (2001).
Odoardi, F. et al. T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679 (2012).
Flügel, A., Willem, M., Berkowicz, T. & Wekerle, H. Gene transfer into CD4+ T lymphocytes: green fluorescent protein-engineered, encephalitogenic T cells illuminate brain autoimmune responses. Nat. Med. 5, 843–847 (1999).
Zarbock, A., Ley, K., McEver, R. P. & Hidalgo, A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751 (2011).
van Zwam, M. et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J. Pathol. 217, 543–551 (2009).
Jung, E. et al. Development and characterization of a novel Prox1-EGFP lymphatic and Schlemm’s canal reporter rat. Sci. Rep. 7, 5577 (2017).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016 (2021).
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667 (2017).
Howell, O. W. et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011).
Weller, R. O. Microscopic morphology and histology of the human meninges. Morphologie 89, 22–34 (2005).
Schafflick, D. et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 24, 1225–1234 (2021).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
McMenamin, P. G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 405, 553–562 (1999).
Fitzpatrick, Z. et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587, 472–476 (2020).
Mazzitelli, J. A. et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat. Neurosci. 25, 555–560 (2022).
Pulous, F. E. et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat. Neurosci. 25, 567–576 (2022).
Phillips, M. J., Needham, M. & Weller, R. O. Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J. Pathol. 182, 457–464 (1997).
Odoardi, F. et al. Instant effect of soluble antigen on effector T cells in peripheral immune organs during immunotherapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 104, 920–925 (2007).
Kawakami, N. et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 199, 185–197 (2004).
Schnell, A. et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298 (2021).
Wang, J. et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183, 1264–1281 (2020).
Miyauchi, E. et al. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature 585, 102–106 (2020).
Magliozzi, R. et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 68, 477–493 (2010).
Bergsland, N. et al. Leptomeningeal contrast enhancement is related to focal cortical thinning in relapsing-remitting multiple sclerosis: a cross-sectional MRI study. AJNR Am. J. Neuroradiol. 40, 620–625 (2019).
Luchetti, S. et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 135, 511–528 (2018).
Machado-Santos, J. et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082 (2018).
Absinta, M. et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474–1483 (2019).
McMahon, E. J., Bailey, S. L., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Planas, A. M. et al. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 188, 2156–2163 (2012).
Warren, S. A. et al. Traumatic injury and multiple sclerosis: a systematic review and meta-analysis. Can. J. Neurol. Sci. 40, 168–176 (2013).
Locatelli, G. et al. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat. Neurosci. 15, 543–550 (2012).
Traka, M., Podojil, J. R., McCarthy, D. P., Miller, S. D. & Popko, B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci. 19, 65–74 (2016).
Reali, C. et al. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 30, 779–793 (2020).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Määttä, J. A., Coffey, E. T., Hermonen, J. A., Salmi, A. A. & Hinkkanen, A. E. Detection of myelin basic protein isoforms by organic concentration. Biochem. Biophys. Res. Commun. 238, 498–502 (1997).
Flach, A. C. et al. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc. Natl. Acad. Sci. USA 113, 3323–3328 (2016).
Hosang, L. The lung microbiome regulates brain autoimmunity. Nature 603, 138–144 (2022).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 25, 402–408 (2001).
Cabeza, R. et al. An RNA sequencing transcriptome analysis reveals novel insights into molecular aspects of the nitrate impact on the nodule activity of Medicago truncatula. Plant Physiol. 164, 400–411 (2014).
Schläger, C., Litke, T., Flügel, A. & Odoardi, F. In vivo visualization of (auto)immune processes in the central nervous system of rodents. Methods Mol. Biol. 1304, 117–129 (2016).
Manglani, M. & McGavern, D. B. Intravital imaging of neuroimmune interactions through a thinned skull. Curr. Protoc. Immunol. 120, 24.2.1–24.2.12 (2018).
Akerman, S., Holland, P. R. & Hoffmann, J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia 33, 577–592 (2013).
Roth, T. L. et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228 (2014).
McDowell, K. P., Berthiaume, A. A., Tieu, T., Hartmann, D. A. & Shih, A. Y. VasoMetrics: unbiased spatiotemporal analysis of microvascular diameter in multi-photon imaging applications. Quant. Imaging Med. Surg. 11, 969–982 (2021).
Zudaire, E., Gambardella, L., Kurcz, C. & Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 6, e27385 (2011).
Allan, C. et al. OMERO: flexible, model-driven data management for experimental biology. Nat. Methods 9, 245–253 (2012).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Radzun, H. J. et al. Detection of a monocyte/macrophage differentiation antigen in routinely processed paraffin-embedded tissues by monoclonal antibody Ki-M1P. Lab Invest. 65, 306–315 (1991).
Acknowledgements
The authors thank S. Hamann, B. Curdt and E. Kretzschmar for excellent technical assistance. We thank T. Michaelis for performing MRI imaging. We thank G. Salinas for support in performing the transcriptome analyses and M. Gößwein for support with the artwork. We thank Y.-K. Hong for providing Prox-1-eGFP transgenic rats and T. Issekutz for providing anti-VLA4 antibodies. We are grateful to C. Ludwig for text editing. This work was supported by the Deutsche Forschungsgemeinschaft (OD 87/1-1, OD 87/3-1 and SFB TRR 274/1 2020 – 408885537 and RK-Grant FL 377/3-1 to A.F. and F.O. and STA 1389/5-1 and EXC 2067/1-390729940 to C.S.). N.G. was supported by the medMS program of the Gemeinnützige Hertie Foundation. J.F. is member of the CIDBN, funded by the Ministry for Science and Education of Lower Saxony and the Volkswagen Foundation through the program Niedersächsisches Vorab.
Author information
Authors and Affiliations
Contributions
A.M. performed most of the experimental and imaging studies. M.H. contributed to the vascular transcriptome analyses and performed most transcriptome analyses. J.S. performed the mouse EAE work. L.H. contributed with molecular analyses. N.G. and J.F. contributed with the analyses of human MS specimen. D.C. contributed to animal experiments. D.C. and K.A. developed the VEGF-C/D trap AAVs and control AAVs. C.F.-K. contributed with morphological analyses and evaluations. C.S. provided human MS samples and contributed with their evaluations. A.F., together with F.O., designed the study, coordinated the experimental work and wrote the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks David Hafler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 T cell infiltration in the SC meninges.
a, Clinical scores in rat EAE induced by transfer of TMBP cells (MBP-tEAE) or TbSYN cells (bSYN-tEAE). Representative data (n = 4) of 3 (MBP-tEAE) and 4 (bSYN- tEAE) independent experiments including at least 3 animals/group. b, Distribution of αβTCR+ CD4+ and CD8+ T cells in the indicated brain compartments in naive 6-8 week old rats. Number of T cells/g and absolute T cell number. Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons. Representative data (n = 4) of 3 independent experiments. c,d, Effector T cells preferentially infiltrate the SC leptomeninges during white matter and gray matter autoimmunity and in immune surveillance. c, Histological analysis depicting the infiltration pattern of TbSYN and TMBP cells in the SC at the indicated time points after T cell transfer. Overview images and magnified areas. Yellow, white and green arrow heads points to antigen specific T cells located in the dura, leptomeninges and parenchyma, respectively. d, Flow cytometry quantification of TbSYN, TMBP and TOVA cells in the indicated SC compartments at the indicated time points after transfer. Number of T cells/g and absolute T cell number. Gray background: clinical EAE phase. Mean + s.d. One-way ANOVA with post-hoc correction for multiple comparisons. Magenta asterisks: comparison between dura and leptomeninges; gray asterisks: comparison between dura and parenchyma. Representative data of 4 (TbSYN cells), 3 (TMBP cells) and 4 (TOVA cells) independent experiments including at least 3 animals/group/time point. e, Effector T cells preferentially infiltrate the leptomeninges regardless of antigen specificity. Number of extravascular TbSYN, TMBP and TOVA cells in the brain meninges quantified from TPLSM images on day 3.5 after transfer. Mean + s.e.m. Unpaired two-tailed t-test. Cumulative data of 4 (TbSYN cells), 5 (TMBP cells) and 5 (TOVA cells) independent experiments. f, The leptomeninges are the main target of meningeal T cell infiltration also in mouse MOG active EAE (MOG-aEAE). Clinical score and cytofluorimetric quantification of CD4+, CD8+ T cells and CD11b+ CD45high infiltrating monocytes/ macrophages in the indicated compartments during the preclinical phase, at the onset and peak of the disease. Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons. Cumulative data of 2 experiments. The number of analyzed samples is indicated. b, d, e, f: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P< 0.0001. SC, spinal cord.
Extended Data Fig. 2 The leptomeninges represent the preferential target of T cell infiltration both in rat and mouse EAE models.
a, Lewis rats were transferred with naive TbSYN cells. Four hours later, active EAE (aEAE) was induced by immunization with the cognate antigen. Plots: quantification of TbSYN cells and infiltrating CD4+ T cells (T cells/g and absolute number) at the peak of EAE in the indicated brain compartments. Flow cytometry. n = 13 from 5 independent experiments. b, Lewis rats were transferred with naive TMBP cells. Four hours later, aEAE was induced by immunization with the cognate antigen. Plots: T cell quantification in brain and SC as in a. n = 6 from 2 independent experiments. Panel: representative confocal images depicting the pattern of infiltration of TMBP cells (green) and CD43+ lymphocytes (red) in SC and brain at the peak of MBP-aEAE. Overview images and magnified areas. Yellow, white and green arrowheads point to antigen specific T cells located in the dura, leptomeninges and parenchyma, respectively. c, C57Bl/6 mice were transferred with 2D2 T cells. Two days later, aEAE was induced by immunization with MOG peptide. T cell quantification in brain and SC as in a. Gating strategies are depicted. n = 8 from 2 independent experiments. d, EAE was induced in C57Bl/6 mice by transfer of MOG-reactive Nur77-GFP-T cells (TMOG-Nur77-GFP). T cell quantification in brain and SC as in a. n = 7 from 2 independent experiments. a-d, Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons. a-d: *P< 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. SC, spinal cord.
Extended Data Fig. 3 T-cell adhesiveness to dural vessel is low in basal conditions but can increase upon dural inflammation.
a, The differential motility pattern of T cells in the hemispheric dural and leptomeningeal vessels is not due to differences in vessel caliber. Vessel diameter in the indicated compartments calculated from areas imaged by intravital TPLSM. Each dot represents one vessel. Median is indicated. Cumulative data from 14 independent experiments. b, Expression pattern of chemokine receptors, integrins and adhesion molecules in TbSYN cells. Normalized expression values from RNA-Seq data. Blood, day 3.5 p.t. n = 4 from 4 independent experiments. c, Tight junction proteins, CXCR3 and integrin ligands are expressed to a higher level in the leptomeninges than the dural vessels. Relative expression of the indicated molecules in dural and leptomeningeal endothelial cells of naive animals. Quantitative PCR. House-keeping gene, β-actin. Mean + s.e.m. Unpaired two-tailed t-test. Cumulative data from 2 independent experiments. The number of samples is indicated. d, Alpha 4-integrin antibodies are less efficient in blocking T cell adhesion to dural than leptomeningeal vessels. Panel: representative 30-minute TPLSM time projections of TOVA cells (3 days p.t.) in the indicated meningeal vessels recorded before and 60 minutes after i.v. injection of blocking anti-VLA4 antibody. Turquoise: TOVA cells; red: 70-kDa dextran labelled vessels. Left graph: percentage reduction of intravascular TOVA cells in hemispheric dural or leptomeningeal vessels after anti-VLA4 antibody treatment over time. Data from 30-minute TPLSM videos. Mean ± s.d. Right graph: percentage reduction of intravascular TOVA cells in the indicated compartments 30-90 minutes after treatment. Two-tailed t-test. Mean + s.e.m. n = 6 (brLepto) and n = 12 (brDura) recordings from 2 independent experiments. e-f, Dural vessels become permissive to T cell adhesion and transmigration under inflammatory conditions. IFNγ and TNFα were applied on the thinned skull in animals transferred 3 days before with TOVA cells. e, Panel: representative TPLSM images and 30-minute time projections depicting TOVA cells rapidly accumulating in the dural vessels and extravasating in the surrounding tissue at the indicated time points after cytokine administration. Yellow and white arrows point to intravascular or extravascular TOVA cells, respectively. Graph: corresponding quantification of TOVA cell locomotion behavior in the dural vessels before and 210 minutes after cytokine administration. Mean + s.d. Chi square. Cumulative data of 2 independent experiments. The number of analyzed T cells is indicated. f, Number of TOVA cells in the dural vessels and in the extravascular milieu at the indicated time point after cytokine application. TOVA cell numbers were calculated from 30-minute TPLSM videos. Mean + s.d. Ratio paired two-tailed t-test. Cumulative data of 2 independent experiments. The number of analyzed recordings is indicated. c-f:, *P < 0.05 and **P < 0.01.
Extended Data Fig. 4 CNS-reactive T cells are less activated in the dura than in the leptomeninges.
a-b, The brain dura only marginally contributes to inflammation during T cell mediated gray matter autoimmunity. a, Cytokine and chemokine expression in the indicated brain compartments measured at the indicated time points after TbSYN cell transfer. Quantitative PCR. House-keeping gene, β-actin. Mean + SEM. Two-tailed t-test was applied for comparison within the same compartment (colored asterisk), one-way ANOVA with post-hoc correction for multiple comparisons for comparison between compartments (black asterisks). n = 6 from 2 independent experiments (day 0 p.t.) and n = 8 from 3 independent experiments (day 3 p.t.). b, Protein expression of IFNγ and IL17 in TbSYN cells upon stimulation with PMA and ionomycin. Experimental set-up and statistical analysis as in Fig. 3b. n = 8 from 2 independent experiments. c, The SC dura is marginally involved during T cell mediated white matter autoimmunity. Expression of pro-inflammatory cytokines in sorted TMBP cells and in the corresponding SC tissues on day 3.5 p.t. Quantitative PCR. House-keeping gene, β-actin. Mean + s.e.m. Two-tailed t-test. Cumulative data of 2 independent experiments including 10 animals. The number of analyzed samples is indicated. d-g, T cells move unrestrained in the dura and establish contact with local macrophages. d, Representative 3D lateral view of the meningeal layers visualized by TPLSM depicting the experimental set-up. Dural and leptomeningeal macrophages could be distinguished by i.v. injection of 3-kDa dextran (orange) and by i.c.m. injection of SeTau647-anti-CD11b antibody (green), respectively. Turquoise: TOVA cells. e, Representative TPLSM snapshots and 30-minute time projections depicting TOVA cell motility (turquoise) in leptomeninges and overlying hemispheric dura 3.5 days p.t. Red: 70-kDa dextran labelled vessels and dural macrophages; green: leptomeningeal macrophages; turquoise: TOVA cells; blue: collagen. f, Cell tracks (superimposed after normalizing their starting coordinates to the origin), mean square displacement (± s.e.m.) and mean velocity (line at mean) of extravascular TOVA cells in the leptomeningeal (129 tracks) and hemispheric dural (68 tracks) milieu. Cumulative data of 4 independent experiments. Note that the mean square displacement progressively increases over time, consistent with persistent movement in both the analyzed compartments. g, Sequential TPLSM images depicting a representative TOVA cell (red) establishing serial contacts (asterisk) with local dural macrophages (white) during 20-minute observation time. Blue: collagen; yellow: TOVA cell track; green: additional TOVA cells appearing in the observation field during the recording time. a-c, f: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Extended Data Fig. 5 Properties of the dural milieu in basal and inflammatory conditions.
a, Dural-derived TbSYN cells are not anergic. TbSYN cells, isolated from dura or leptomeninges on day 3.5 p.t., were stimulated in vitro with the cognate antigen (SYN). As control, an irrelevant antigen (OVA) or PBS were added to the culture (values pooled together). Left: expression of Ifng and Il17a in TbSYN cells 24 hours after antigen stimulation. Quantitative PCR. House-keeping gene, β-actin. Right: MFI of CD134 and IL2R in TbSYN cells 48 h after antigen stimulation. Flow cytometry. Mean + s.e.m. Two-tailed t-test. Cumulative data of 4 independent experiments including 14 animals. Number of analyzed samples are indicated. b, No differences in regulatory T cells are observed between the meningeal compartments. Percentage and absolute numbers of FoxP3+ T cells in the indicated T cell populations measured in brain parenchyma, leptomeninges and dura on day 3.5 after TbSYN cell transfer. Flow cytometry. Mean + s.e.m. One way-ANOVA with post-hoc correction for multiple comparisons. n = 6 from 2 independent experiments. c, Dural and leptomeningeal myeloid cells display comparable MHC class II expression. Percentage of MHCIIhigh CD11b+CD45high myeloid cells in brain parenchyma, leptomeninges and dura. Mean + s.e.m. Representative data of 3 experiments including 3 animals/group. d-e, Dural APCs do not spontaneously present CNS antigens in vitro. MHC class II+ cells were isolated from the indicated compartments of naive animals and cultured with bSYN- or MBP-reactive T cells in the presence or in the absence of the cognate antigen as in Fig. 4a-b. MFI of CD134 (OX40) and IL2R quantified by flow cytometry in TbSYN cells (d) and TMBP cells (e) 48 hours after antigen encounter. Flow-cytometry. Notably, in absence of exogenously added antigen, the leptomeningeal but not the dural MHC class II+ cells were able to activate the antigen specific T cells. Cumulative data of 2 independent experiments including 20 (d) and 24 (e) animals. The number of analyzed samples is indicated. Mean + s.e.m. Statistical analysis as in Fig. 4a-b. f, TPLSM snapshots depicting NFAT translocation from cytosol to nucleus (indicative of T cell activation) in a TOVA-NFAT-YFP H2B-mCherry cell. Images were acquired 120 minutes after transcranial OVA peptide application. Timing of the translocation (minutes) is indicated. Representative of 2 independent experiments. g, Quantification of pro-inflammatory cytokines in the dural tissue and underlying leptomeninges 3 hours after transcranial application of OVA peptide or PBS performed 3.5 days after TOVA cell transfer. Quantitative PCR. House-keeping gene, β-actin. Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons. n = 6 (OVA) and n = 5 (PBS) from 5 independent experiments a-e, g: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. MFI, median fluorescent intensity; NFAT, nuclear factor of activated T cells.
Extended Data Fig. 6 Role of antigen drainage from the CNS in physiological and diseased conditions.
a, Solutes injected in the cisterna magna accumulate rapidly in the dura lymphatics and dura tissue. TPLSM time-lapse pictures of a lymphatic vessel (green) in the transverse sinus of a Prox1-eGFP rat i.c.m. injected with 70-kDa dextran (10 µl, red). b, Dura and dcLN derived APCs can present i.c.v. injected antigens ex vivo. Top: experimental setup. Bottom: IFNγ production by TOVA cells cultured with cells isolated from the indicated compartments of rats i.c.v. injected with either PBS, 10 µg or 25 µg of OVA 8 hours prior. ELISA. + Ag: IFNγ in TOVA cells cultured with cells from the inguinal LNs (ingLNs) in the presence OVA. Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons was applied for comparison within the same compartment (colored asterisk) and between compartments (black asterisks). Cumulative data (n = 4) of 2 independent experiments. b, i.c.m. injection of OVA induces TOVA cells accumulation in the leptomeninges but not in the dura or dcLNs. Top: experimental set-up. Bottom: fold increase (normalized on PBS) in the number of TOVA cells in the indicated compartments 12 hours upon i.c.m. injection of 10 µg OVA or PBS in animals transferred 3 days before with TOVA cells. Flow cytometry. Mean + SEM. Two-tailed t-test was applied for comparison within same compartment (colored asterisks). Representative data of 2 independent experiments (PBS: n = 3; OVA: n = 4). d,e, TOVA cells accumulate in the leptomeninges but not in the dura or dcLNs after i.c.v. OVA administration. d, Top: experimental set-up. Bottom: representative TPLSM images of TOVA cells (green) in brain dura and leptomeninges 12 hours after i.c.v. injection of 25 µg OVA or PBS in animals transferred 3 days before with TOVA cells. Arrowheads: TOVA cells in the dura. Representative of 3 independent experiments. e, Fold increase (normalized on PBS) in the number of TOVA cells in the indicated compartments 12 hours upon i.c.v. injection of 10 µg (upper plot) or 25 µg of OVA (lower plot). Flow cytometry. Animals i.c.v. injected with PBS were used as control. Mean + s.e.m. Two-tailed t-test. Cumulative data of 2 (10 µg, n = 6) and 3 (10 µg, n = 6) independent experiments. f,g, Antigen delivered in the CSF is mainly processed by leptomeningeal phagocytes. f, DQ-OVA was injected i.c.v. or i.c.m. Quantification (T cell/g, absolute number and percentage) of DQ-OVA+ CD11b+ and DQ-OVA+CD11b− cells in the indicated compartments 8 hours after injection. brDura (hem) and brDura (sin) indicate cells isolated from the hemispheric cranial dura and dural sinuses, respectively. Flow cytometry. Mean + s.e.m. One way-ANOVA with post-hoc correction for multiple comparisons. Representative data of 2 independent experiments including at least 3 animals/group/procedure. g, Corresponding percentage of DQ-OVA+ CD11b+ and DQ-OVA+CD11b− cells. Flow cytometry. Mean + s.e.m. Representative data of 2 independent experiments including at least 3 animals/group/procedure. b-g: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Extended Data Fig. 7 Role of the dural lymphatics and dcLNs in EAE.
a, T cells do not preferentially accumulate in the dcLNs during EAE. Experimental set-up as in Extended Data Fig. 2. Quantification of CNS reactive T cells (T cell/g and absolute number) in the indicated peripheral compartments at the peak of T cell infiltration in the indicated rat and mouse EAE models. Flow cytometry. Mean + s.e.m. One way-ANOVA with post-hoc correction for multiple comparisons. Cumulative data of 5 (bSYN-aEAE, n = 13), 2 (MBP-aEAE, n = 6), 2 (MOG-aEAE, n = 9) and 2 (MOG-tEAE, n = 7) independent experiments, medLNs: n = 5 from 2 independent experiments. b-c, TbSYN cells in the blood and LNs uniformly display a Th1/17 phenotype. b, Heat map of hierarchically clustered values of genes related to Th lineage. Experimental set-up as in Fig. 5d. c, Validation of the NGS data by quantitative PCR. House-keeping gene, β-actin. Cumulative data of 3 independent experiments (n = 11 animals). The number of analyzed samples is indicated. d, Effectors T cells are not activated in the dcLNs. EAEs were induced as in Extended Data Fig. 2. Flow cytometry quantification of the indicated surface activation markers in CNS-reactive T cells in bSYN-, MBP- and MOG-aEAE and percentage of Nur77+ TMOG-Nur77-GFP cells in MOG-tEAE. Peak of the disease. Mean + s.e.m. One way-ANOVA with post-hoc correction for multiple comparisons. Cumulative data of 5 (bSYN-aEAE, n = 13), 2 (MBP-aEAE, n = 6), 2 (MOG-aEAE, n = 9) and 2 (MOG-tEAE, n = 7) independent experiments. n.s.: not significant. e-h, AAV ablation of the dural lymphatics does not affect T cell mediated CNS inflammation. e, Panel: whole-mount of the dura in Prox1-eGFP rats depicting the effective ablation of the dural lymphatics by the AAV-rVEGFR3 at the confluence of sinuses (COS) and at the skull basis 3 months after AAV treatment. Overviews images and magnified areas. Graphs: corresponding quantification of the effect of AAV-rVEGFR3 and control AAV (AAV-Sham) on the lymphatic vessel area-percentage in the indicated regions. Mean + s.e.m. Two-tailed t-test. Each dot represents an area of interest (COS: AAV-Sham n = 19; AAV-rVEGFR3 n = 19; Skull base: AAV-Sham n = 12; AAV-rVEGFR3 n = 23) from 2 animals/group. f, Lymphatic ablation prevents the drainage of Evans blue to the dcLNS. Rats were injected i.c.m. with Evans blue 3 months after AAV treatment. Representative photos depicting the lack of dye uptake in the dcLNs of AAV-rVEGFR3 lymphatic depleted rats compared to controls (AAV-Sham), 2 hours after dye injection. Arrowheads point to the dcLNs. Representative data of two independent experiments. g, T2-weighted coronal MRI sections performed 3 months after ablation of the lymphatic vessels. No enlargement of the lateral ventricles (arrowhead) suggestive of intracranial hypertension is observed. Representative data of 3-5 animals/group. h, Number of CD11b+ MHCIIlow and CD11b+ MHCIIhigh myeloid cells at the peak of the disease in the indicated brain compartments as in Fig. 5e. Mean + s.e.m. n = 7 from 2 independent experiments. a,c,d,h: *P < 0.05, **P < 0.01, ***P < 0.001 and ****p < 0.0001. MRI, magnetic resonance imaging; MFI, median fluorescence intensity.
Extended Data Fig. 8 Involvement of peri-sinus dural areas in CNS autoimmunity.
CNS-reactive T cells accumulate in higher numbers in the vicinity of the dural sinuses than in the dural hemispheres. a, Quantification of the CNS reactive T cells (T cell/g and absolute number) in the indicated compartments at the peak of T cell infiltration in the indicated transfer and active rat EAE models. Flow cytometry. Mean + s.e.m. Two-tailed t-test was applied for comparison within the dural compartments, one-way ANOVA with post-hoc correction for multiple comparisons for comparison between compartments. Representative data of 2 (bSYN-tEAE, n = 4) experiments and cumulative data of 5 (bSYN-aEAE, n = 13) and 2 (MBP-aEAE, n = 6) independent experiments. b, Top: distribution of TbSYN cells (red) at the COS, in the hemispheric dura and in the leptomeninges. Overview images (fluorescence microscopy) and magnified areas (confocal microscopy) of dural whole-mount and leptomeninges from the same animal. TS: transverse sinus. SSS: superior sagittal sinus. Blue: DAPI. Bottom: distribution of TbSYN cells in the area around the SSS. MMA: middle meningeal artery. Overviews and magnified areas. Fluorescence microscopy. c,d, Intravascular and extravascular T cell motility behavior in sinus and hemispheric dural vessels is comparable. c, Representative TPLSM 15-minute time-projection depicting the intravascular behavior of TOVA cells in the SSS on day 3 after T cell transfer. Note that the attenuation of laser intensity by erythrocytes limits the detection of T cells in the central part of the sinus. Turquoise: TOVA cells; red: 70-kDa dextran labelled vessels. Graph: corresponding quantification of the percentage of TOVA or TbSYN cells crawling, rolling or fast rolling within a 30-minute recording time in the indicated compartments on day 3 p.t. Mean + s.e.m. One-way ANOVA with post-hoc correction for multiple comparisons. Each dot represents one recording. TOVA cells: cumulative data from 28 (leptomeninges), 45 (dura hemisphere) and 6 (dura sinus) recordings. TbSYN cells: cumulative data from 8 (leptomeninges and dura hemisphere) and 6 (dura sinus) recordings. d. Mean velocity (line at mean), straightness (line at mean) and mean square displacement (± s.e.m.) of TbSYN cells recorded by TPLSM on day 3.5 p.t. in leptomeninges, peri-sinus areas and in hemispheric dura. Each dot represents one cell. Kruskall Wallis test with post-hoc correction for multiple comparisons. Cumulative data from 9 (leptomeninges), 14 (dura hemispheres) and 6 (dura sinus) recordings. e, Brain autoantigens are not spontaneously presented in the sinus adjacent areas. e, Experimental set-up as in Fig. 4a. MFI of CD134 and IL2R in TbSYN cells cultured for 48 h with cell suspension from the indicated compartments of naive animals in presence of the cognate antigen. As control (Ct), an irrelevant antigen (OVA) or no antigen were added to the culture (values pooled together). Flow cytometry. Mean + s.e.m. Statistical evaluation as in Fig. 4a. Representative data of 2 independent experiments. The number of analyzed samples is indicated. f-h, CNS-reactive T cells in peri-sinus areas are not activated. f, Expression of pro-inflammatory cytokines in TbSYN cells isolated from the indicated compartments on day 3.5 p.t. Quantitative PCR. House-keeping gene, β-actin. Mean + s.e.m. Representative data of 2 independent experiments. n = 4 (brLepto, brDura sinuses), n = 3 (brDura hemispheres) g, Animals were co-injected with TbSYN and TOVA cells. Relative expression of Il17a in TbSYN cells vs TOVA cells on day 3.5 p.t (ΔΔCt). Quantitative PCR. House-keeping gene, β-actin. Mean + SEM. Representative data of 2 independent experiments. n = 5 (brLepto, brDura hemispheres), n = 4 (brDura sinuses). h, Fluorescent intensity (FI) of CD134 in TbSYN or T MBP cells from the indicated compartments in actively immunized rats at the peak of T cell infiltration. Flow cytometry. Cumulative data of 5 (bSYN-aEAE) and 2 (MBP-aEAE) independent experiments. Medians (continuous line), quartiles (dotted lines) and number of analyzed T cells. e-g, One way-ANOVA with post-hoc correction for multiple comparisons. h, Kruskall Wallis test with post-hoc correction for multiple comparisons. a, c-h: *P < 0.05, **P < 0.01, ***P < 0.01 and ****P < 0.0001. MFI, median fluorescence intensity.
Extended Data Fig. 9 The leptomeninges but not the dura are affected in chronic brain autoimmunity.
a, Experimental set-up. Single or repeated inflammatory bouts were induced in tg-bSYN rats by 1- or 4-time transfer of TbSYN cells. Animals injected 1 or 4 times with TOVA cells were used as control. Analysis was performed 3 months upon the 1st or 4th bout. Clinical score (bars) and body weight change (lines). b, CNS inflammation persists in the leptomeninges but not in the dura after one inflammatory attack. Experimental set-up as in a. Infiltration pattern of CD43+ T cells and CD68+ myeloid cells in the leptomeninges (left panel) and dura (hemispheres and areas around the SSS, right panel) after single T cell transfer. Overviews images and magnified areas. Fluorescence microscopy. Green: Iba1+ microglia. SSS: superior sagittal sinus c, MHC class II is over-expressed in the leptomeninges upon a single inflammatory bout and it further increases upon repeated bouts. Overview pictures and magnified areas of the leptomeninges 3 months after 1x or 4x transfer of TOVA or TbSYN cells. Green: Iba1+ microglia. d, Repeated inflammatory bouts do not affect the dura at an ultrastructural level. Representative scanning micrographs of the dura after 4x transfer of TbSYN or TOVA cells. In the control (4x TOVA), to be seen is the neuroepithelium consisting of flat regular cells and the deeper extracellular layer identified as collagen bundles following the same direction (top). The other image of the same animal shows the superficial layer of thinner collagen fibers that are interlaced, forming the typical disorganized network. Similar findings are seen in the 4x TbSYN cell injected animal with no signs of destruction of the neuroepithelium or changes of the extracellular network. e, No spontaneous antigen presentation in the dura in chronic autoimmunity. IFNγ production from TbSYN cells cultured for 48 hours with APCs isolated from the indicated compartments in presence of cognate antigen 3 months after the 4th inflammatory bout. As control, an irrelevant antigen (OVA) or no antigen (NA) were added to the culture (values pooled together). ELISA. Each value represents a pool of 3 animals. Representative data of 2 independent experiments.
Extended Data Fig. 10 Inflammation of the dura is virtually absent in MS.
No inflammation foci are observed in the dura in MS. Histological characterization of the immune cell subpopulations present in the dura (upper panel) and leptomeninges (lower panel) in controls and MS patients. Extensive perivascular infiltrates are detected in leptomeningeal sections of MS patients stained for HE (arrows). These infiltrates are composed of CD3+ and CD8+ T cells and LFB-PAS+ leukocytes. No immune infiltrates are present in the MS patients’ dura that appears indistinguishable from the control dura. b-c, Immune cell distribution in the dura is sparse during MS but dramatically increases in pachymeningitis. b, Nearest neighbor distance for CD8+ cells in different areas of the dura and in the leptomeninges. Horizontal line: Mean nearest neighbor distance in one case of pachymeningitis. Data are represented as boxes containing the 25th to 75th percentiles of the dataset and whiskers extending to the minimum and maximum value. The line in the middle of the box is at the median. n = 13. One way-ANOVA with post-hoc correction for multiple comparisons. **P < 0.01 c, The dura presents an intense immune infiltration during pachymeningitis. Representative pictures revealing a dense infiltrate composed by CD138+ /Ig+ plasma cells and CD68 (KiM1P)+ myeloid cells. LFB-PAS, Luxol fast blue-periodic acid Schiff; Ig, immunoglobulin.
Supplementary information
Supplementary Information
Supplementary Videos 1–12, Supplementary Fig. 1 and Supplementary Tables 1 and 2.
Supplementary Video 1
Differential permeability of brain meningeal vessels to solutes. First part of the video: experimental setup allowing the simultaneous in vivo visualization of pial and leptomeningeal vessels through a thinned-skull window. Second part of the video: assessment of dural and pial vessel permeability to solutes. Intravital TPLSM recording was performed immediately after i.v. injection of 3-kDa dextran Texas Red (in magenta). The dye leaked out from the dural vessels already 2 minutes after injection and, after 40 minutes, was taken up by local phagocytes (white open arrows in the video). No leakage was observed from the leptomeningeal vessels during the observation time. Green: blood vessels labeled by i.v. injection of 70-kDA FITC-dextran.
Supplementary Video 2
Locomotion behavior of intravascular TbSYN, TMBP and TOVA cells in hemispheric dural and leptomeningeal vessels of the brain. Intravital TPLSM recordings performed on day 3 p.t. 30-minute time-lapse videos and corresponding time projections. Turquoise: antigen-specific T cells; red: blood vessels.
Supplementary Video 3
Effect of VLA-4 blockade on T cell adhesion to meningeal vessels. Intravital TPLSM recordings depicting the intravascular locomotion behavior of TOVA cells (day 3 p.t.) in leptomeningeal and hemispheric dural vessels before and 60 minutes after i.v. injection of VLA-4 monoclonal antibodies. 30-minute time-lapse recordings and corresponding time projections. Turquoise: TOVA cells; red: blood vessels.
Supplementary Video 4
Changes in T cell motility upon induction of dural inflammation. Motility behavior of TOVA cells (day 3 p.t.) recorded by intravital TPLSM in the brain hemispheric dura before (steady state), 150 minutes and 210 minutes after transcranial application of IFNγ and TNFα. 30-minute time-lapse videos and corresponding time projections. Red: TOVA cells; white: blood vessels; blue: collagen.
Supplementary Video 5
In vivo TPLSM visualization of T cell locomotion in the distinct brain meningeal layers. Single plane, maximal projection and 3D reconstruction of the dural and leptomeningeal layers. Turquoise: TOVA cells (day 3.5 p.t.); green and orange: leptomeningeal and dural phagocytes, respectively.
Supplementary Video 6
Locomotion behavior of TOVA cells in the leptomeninges and dura. Intravital TPLSM recordings on day 3.5 after transfer of TOVA cells in the hemispheric leptomeninges and overlying dura. 30-minute time-lapse videos. Turquoise: antigen-specific T cells; green and red: leptomeningeal and dura macrophages, respectively; yellow: tracks of extravascular TOVA cells.
Supplementary Video 7
Interactions between T cells and dural phagocytes. Depicted is a TOVA cell (false color: turquoise) establishing serial contacts (false color: yellow) with local phagocytes (red) during 30-minute intravital TPLSM recording performed on day 3.5 p.t. Green: other TOVA cells in dura.
Supplementary Video 8
T cell motility in the dura upon local Ag application. Motility behavior of TOVA cells recorded on day 3.5 p.t. in the brain dura before (steady state) and 120 minutes after transcranial application of OVA peptide. Yellow arrows indicate stationary T cells. 30-minute TPLSM time-lapse videos. Turquoise: TOVA cells; red: dura macrophages; blue: collagen.
Supplementary Video 9
Real-time detection of T cell activation in the dura upon local application of the cognate antigen. Intravital TPLSM recording performed on brain dura 3.5 days p.t. of OVA-reactive T cells expressing NFAT biosensor. Before transcranial application of OVA peptide, in the circled T cell, NFAT is located in the cytosol that appears green. 120 minutes upon Ag application, in the circled OVA-reactive T cell, NFAT translocates from the cytosol to the nucleus, indicated by the red nucleus turning yellow. Overview 30-minute TPLSM time-lapse videos and magnified areas of interest. Green: NFAT-YFP; red: histone H2B-mCherry labeling the nucleus; blue: collagen.
Supplementary Video 10
T cell trafficking in the dura lymphatic vessels. Intravital TPLSM recording in a Prox1-eGFP rat depicting a TbSYN cell (yellow circle) located inside a lymphatic vessel on day 3.5 p.t. Red: TbSYN cells; green: lymphatic vessels; blue: collagen. 30-minute time-lapse videos.
Supplementary Video 11
T cell trafficking in the sinus. Motility behavior of TOVA and TbSYN cells in the sagittal sinus on day 3 p.t. 16-minute TPLSM time-lapse videos and corresponding time projections. Attenuation of laser intensity by blood cells limits T cell detection in the central part of the sinus. Turquoise: TOVA/TbSYN cells; red: blood vessels.
Supplementary Video 12
Extravascular locomotion behavior of TbSYN cells in the leptomeninges, hemispheric dura and sagittal sinus. Note the less straight and more confined movement of TbSYN cells in the leptomeninges. 25-minute time-lapse intravital TPLSM recordings on day 3.5 p.t. Turquoise: TbSYN cells, red: leptomeningeal and dural macrophages; yellow: tracks of extravascular TbSYN cells.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Source Data Extended Data Fig. 10
Statistical Source Data
Rights and permissions
About this article
Cite this article
Merlini, A., Haberl, M., Strauß, J. et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat Neurosci 25, 887–899 (2022). https://doi.org/10.1038/s41593-022-01108-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01108-3
This article is cited by
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage
Nature (2024)
-
Imaging of brain barrier inflammation and brain fluid drainage in human neurological diseases
Cellular and Molecular Life Sciences (2024)
-
Meningeal immunity and neurological diseases: new approaches, new insights
Journal of Neuroinflammation (2023)
-
Lateralized response of skull bone marrow via osteopontin signaling in mice after ischemia reperfusion
Journal of Neuroinflammation (2023)