Abstract
Preserving cells in a functional, non-senescent state is a major goal for extending human healthspans. Model organisms reveal that longevity and senescence are genetically controlled, but how genes control longevity in different mammalian tissues is unknown. Here, we report a new human genetic disease that causes cell senescence, liver and immune dysfunction, and early mortality that results from deficiency of GIMAP5, an evolutionarily conserved GTPase selectively expressed in lymphocytes and endothelial cells. We show that GIMAP5 restricts the pathological accumulation of long-chain ceramides (CERs), thereby regulating longevity. GIMAP5 controls CER abundance by interacting with protein kinase CK2 (CK2), attenuating its ability to activate CER synthases. Inhibition of CK2 and CER synthase rescues GIMAP5-deficient T cells by preventing CER overaccumulation and cell deterioration. Thus, GIMAP5 controls longevity assurance pathways crucial for immune function and healthspan in mammals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The human exome and genome sequencing data reported in this paper are deposited in dbGaP as phs002816.v1.p1. The coordinates and structure factors for the crystal structure have been deposited at the PDB with the accession code 6Z3E (the validation report can be found online). Database/datasets used are PDB 2XTP, 2XTO and 3ZJC and 10x Genomics, 3k PBMCs from a healthy donor (www.10xgenomics.com/resources/datasets/3-k-pbm-cs-from-a-healthy-donor-1-standard-1-1-0). Source data are provided with this paper.
Change history
12 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41590-024-01779-z
References
Ohtsuka, H., Shimasaki, T. & Aiba, H. Genes affecting the extension of chronological lifespan in Schizosaccharomyces pombe (fission yeast). Mol. Microbiol. 115, 623–642 (2021).
Jiang, J. C., Kirchman, P. A., Allen, M. & Jazwinski, S. M. Suppressor analysis points to the subtle role of the LAG1 ceramide synthase gene in determining yeast longevity. Exp. Gerontol. 39, 999–1009 (2004).
Jiang, J. C., Kirchman, P. A., Zagulski, M., Hunt, J. & Jazwinski, S. M. Homologs of the yeast longevity gene LAG1 in Caenorhabditis elegans and human. Genome Res. 8, 1259–1272 (1998).
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 (2008).
Bikman, B. T. & Summers, S. A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 121, 4222–4230 (2011).
Kurz, J., Parnham, M. J., Geisslinger, G. & Schiffmann, S. Ceramides as novel disease biomarkers. Trends Mol. Med. 25, 20–32 (2019).
Tonnetti, L., Veri, M. C., Bonvini, E. & D’Adamio, L. A role for neutral sphingomyelinase-mediated ceramide production in T cell receptor-induced apoptosis and mitogen-activated protein kinase-mediated signal transduction. J. Exp. Med. 189, 1581–1589 (1999).
MacMurray, A. J. et al. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 12, 1029–1039 (2002).
Drzewiecki, K. et al. GIMAP5 maintains liver endothelial cell homeostasis and prevents portal hypertension. J. Exp. Med. 218, e20201745 (2021).
Gasper, R., Meyer, S., Gotthardt, K., Sirajuddin, M. & Wittinghofer, A. It takes two to tango: regulation of G proteins by dimerization. Nat. Rev. Mol. Cell Biol. 10, 423–429 (2009).
Schwefel, D. et al. Structural insights into the mechanism of GTPase activation in the GIMAP family. Structure 21, 550–559 (2013).
Wong, V. W. et al. The autoimmunity-related GIMAP5 GTPase is a lysosome-associated protein. Self Nonself 1, 259–268 (2010).
Cousins, L. et al. Eosinophilic bowel disease controlled by the BB rat-derived lymphopenia/Gimap5 gene. Gastroenterology 131, 1475–1485 (2006).
Schulteis, R. D. et al. Impaired survival of peripheral T cells, disrupted NK/NKT cell development, and liver failure in mice lacking Gimap5. Blood 112, 4905–4914 (2008).
Barnes, M. J. et al. Loss of T cell and B cell quiescence precedes the onset of microbial flora-dependent wasting disease and intestinal inflammation in Gimap5-deficient mice. J. Immunol. 184, 3743–3754 (2010).
Yano, K. et al. Gimap3 and Gimap5 cooperate to maintain T-cell numbers in the mouse. Eur. J. Immunol. 44, 561–572 (2014).
Kupfer, R. et al. Loss of a gimap/ian gene leads to activation of NF-κB through a MAPK-dependent pathway. Mol. Immunol. 44, 479–487 (2007).
Nitta, T. et al. IAN family critically regulates survival and development of T lymphocytes. PLoS Biol. 4, e103 (2006).
Chen, X. L. et al. GIMAP5 deficiency is associated with increased AKT activity in T lymphocytes. PLoS ONE 10, e0139019 (2015).
Serrano, D. et al. GTPase of the immune-associated nucleotide protein 5 regulates the lysosomal calcium compartment in T lymphocytes. Front. Immunol. 8, 94 (2017).
Patterson, A. R. et al. Gimap5-dependent inactivation of GSK3β is required for CD4(+) T cell homeostasis and prevention of immune pathology. Nat. Commun. 9, 430 (2018).
Heinonen, M. T. et al. GIMAP GTPase family genes: potential modifiers in autoimmune diabetes, asthma, and allergy. J. Immunol. 194, 5885–5894 (2015).
Hellquist, A. et al. The human GIMAP5 gene has a common polyadenylation polymorphism increasing risk to systemic lupus erythematosus. J. Med. Genet. 44, 314–321 (2007).
Lee, Y. J. et al. Genome-wide association study identifies GIMAP as a novel susceptibility locus for Behcet’s disease. Ann. Rheum. Dis. 72, 1510–1516 (2013).
Schwefel, D. et al. Structural basis of oligomerization in septin-like GTPase of immunity-associated protein 2 (GIMAP2). Proc. Natl Acad. Sci. USA 107, 20299–20304 (2010).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Leipold, M. D. & Maecker, H. T. Phenotyping of live human PBMC using CyTOF mass cytometry. Bio Protoc. 5, 1382 (2015).
Shearer, W. T. et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 112, 973–980 (2003).
Pawelec, G. Is there a positive side to T cell exhaustion? Front. Immunol. 10, 111 (2019).
Seki, A. & Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 215, 985–997 (2018).
Gire, V. & Dulic, V. Senescence from G2 arrest, revisited. Cell Cycle 14, 297–304 (2015).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Mullen, T. D. & Obeid, L. M. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 12, 340–363 (2012).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Trayssac, M., Hannun, Y. A. & Obeid, L. M. Role of sphingolipids in senescence: implication in aging and age-related diseases. J. Clin. Invest. 128, 2702–2712 (2018).
Ahmed, K., Gerber, D. A. & Cochet, C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 12, 226–CK230 (2002).
Pinna, L. A. & Meggio, F. Protein kinase CK2 (‘casein kinase-2’) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 3, 77–97 (1997).
von Mering, C. et al. STRING: known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res. 33, D433–D437 (2005).
Sassa, T., Hirayama, T. & Kihara, A. Enzyme activities of the ceramide synthases CERS2-6 are regulated by phosphorylation in the C-terminal region. J. Biol. Chem. 291, 7477–7487 (2016).
Hernandez-Corbacho, M. J. et al. Tumor necrosis factor-alpha (TNFα)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J. Biol. Chem. 290, 25356–25373 (2015).
Mouton, R. E. & Venable, M. E. Ceramide induces expression of the senescence histochemical marker, β-galactosidase, in human fibroblasts. Mech. Ageing Dev. 113, 169–181 (2000).
Limoges, M. A., Cloutier, M., Nandi, M., Ilangumaran, S. & Ramanathan, S. The GIMAP family proteins: an incomplete puzzle. Front. Immunol. 12, 679739 (2021).
Prudent, R. & Cochet, C. New protein kinase CK2 inhibitors: jumping out of the catalytic box. Chem. Biol. 16, 112–120 (2009).
Chen, Y. et al. Strategies of targeting CK2 in drug discovery: challenges, opportunities, and emerging prospects. J. Med. Chem. 66, 2257–2281 (2023).
DeLeve, L. D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61, 1740–1746 (2015).
Cogolludo, A., Villamor, E., Perez-Vizcaino, F. & Moreno, L. Ceramide and regulation of vascular tone. Int. J. Mol. Sci. 20, 411 (2019).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
O’Driscoll, M. et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590, 140–145 (2021).
Mellacheruvu, D. et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat. Methods 10, 730–736 (2013).
Vella, A., Teague, T. K., Ihle, J., Kappler, J. & Marrack, P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J. Exp. Med. 186, 325–330 (1997).
Kishimoto, H. & Sprent, J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J. Immunol. 163, 1817–1826 (1999).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Pascall, J. C. et al. The immune system GTPase GIMAP6 interacts with the Atg8 homologue GABARAPL2 and is recruited to autophagosomes. PLoS ONE 8, e77782 (2013).
Yao, Y. et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell 185, 1172–1188 (2022).
Cook, S. A. et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 369, 202–207 (2020).
Lucas, C. L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 (2014).
Lo, B. et al. Autoimmune disease. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349, 436–440 (2015).
Acknowledgements
The authors thank the physicians, patients and families participating in this study; University of Washington Center for Mendelian Genomics for Geno2MP; L. Folio for preparation of clinical data; the late Professor I. Barlan for helping initiate the study; NIAID Biological Imaging Section; C. Schwartz (Electron Microscopy Unit, Research Technologies Section, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases) for EM analysis; R. Kissinger (Visual Medical Arts, Research Technologies Branch, Rocky Mountain Laboratories, NIAID) for illustrations; D. E. Anderson (Advanced Mass Spectrometry Core, National Institute of Diabetes and Digestive and Kidney Diseases); F. Meylan (National Institute of Arthritis and Musculoskeletal and Skin Diseases); and H. Bulut for help in the thermal unfolding assays. We thank R. Perlmutter and J. McElwee for advice and support and acknowledge generous support from Merck. We thank NIAID Central Sequencing Initiative for patient referral and advice. Finally, we thank R. N. Germain, L. M. Staudt and A. L. Snow for critically reading the manuscript.
This work was supported by the Division of Intramural Research, NIAID, NIH and Merck and Co.; German Research Foundation SFB958, project A12 (O.D.); NIH-NIDDK, K08 DK113109 and R01 DK131033 (S.V.); Biotechnology and Biological Sciences Research Council BBS/E/B/000C0407 and BBS/E/B/000C0427 (G.B. and J.P.); The Scientific and Technological Research Council of Turkey 318S202 (S.B.); Recurrent institutional funding from INSERM, CEA, Ligue Nationale contre le Cancer, University Grenoble Alpes (C.C. and O.F.); and TUBITAK-1003 Project, project ID 315S125 (A.I., S.H. and F.D.).
Figures 2g, 4a and 6e and Extended Data Fig. 6b were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of A.Y.P., M.L.-G., M.L., J.Q.G., X.X., B.C., H.C.S. and M.J.L. Data curation was the responsibility of A.Y.P., M.L.-G., M.L., J.Q.G., X.X., B.S., L.Z., A.B., H.H., B.C., J.C., Y.Y., S.K., J.M.C., A.R.M., P.J., J.C.R., Y.H.C., A.G., A.F., S.G., J.A., J.E.-H., E.B., H.O., H.M., C.C., O.F. and D.S. Formal analysis was carried out by A.Y.P., M.L.-G., M.L., J.Q.G., X.X., B.S., L.Z., A.B., B.C., Y.Z., A.R.M., S.A.C., Y.H.C., S.D.C., J.A., J.E.-H., C.C., O.F. and D.S. Funding acquisition was the responsibility of G.W.B., J.P., F.D., C.C., O.F., O.D., S.H., A.I., S.V., S.B., H.C.S. and M.J.L. Investigation was conducted by A.Y.P., M.L.-G., M.L., J.Q.G., X.X., B.S., L.Z., A.B., B.C., J.E.-H., C.C., O.F., D.S., H.C.S. and M.J.L. Methodology was the responsibility of A.Y.P., M.L.-G., M.L., J.Q.G., X.X., B.S., L.Z., A.B., B.C., Y.Z., H.M., A.M.P., L.X. and D.S. Resources were the responsibility of H.F.M., C.T., G.W.B., J.P., E.K.-A., A.K., İ.T., S.E., R.J.H., D.A., S.T., A.K., Z.K., E.U., T.K., F.D., E.K., T.P.A., C.A., G.E.Ş., A.Ü.A., G.U., V.K.R., S.S., B.D., K.B., D.C., S.H., A.I., S.V., S.B. and A.O.; Supervision was carried out by C.M.B., M.M.D., R.P.L., E.L.P., O.D., H.C.S. and M.J.L. Writing of the original draft was carried out by A.Y.P., M.L.-G. and M.J.L. Review and editing was carried out by A.Y.P., M.L.-G., M.L. J.Q.G., B.S., L.Z., A.B., S.A.C., J.R.E.B., O.D., D.S., H.C.S. and M.J.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Takeshi Nitta, Claudio Mauro, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 GIMAP5 mutations cause protein destabilization in patients’ T cells.
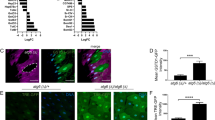
a, Computerized tomography scan of P7.1 showing liver nodules (left, magenta arrowheads) and esophageal varices (right, cyan arrowheads) suggesting liver failure. b, Absolute lymphocyte counts versus patient age (years, yrs), shaded area representing 95% confidence interval for normal range. c, Patient amino acid substitutions on GIMAP5 protein schematic (top). GTPase (G) domain: green; switch I (SwI) and switch II (SwII): blue; conserved box (CB): cyan; alpha helices (α) 6 and 7: orange; hydrophobic segment: maroon. Amino acid alignment in GIMAP5 orthologues and 7 human paralogues (bottom). Mutations marked in purple and boxed. d, Omit Fo-Fc density map for GDP and the Mg2+ ion bound to GIMAP51-276, contoured at 3σ (green). The refined GDP•Mg2+ molecules shown in ball-and-stick. e, Comparison of GIMAP51-276-GDP (left), GIMAP21-260-GDP (middle, PDB code 2xto), and GIMAP7 L100Q-GMPPNP (right, PDB code 3zjc). G domains: green; switch I (SwI) and switch II (SwII): purple; P-loops (PL): red; conserved box (CB): cyan; nucleotides: space-filling spheres; secondary structure elements differing from the core G domain of H-Ras, helices α3* and the C-terminal helices α6 and α7 (C-terminal extension, ext.): orange; unstructured loops; dotted lines. Root mean square deviation of aligned Cα atoms: 1.4 Å (GIMAP5 vs. GIMAP2) and 2.0 Å (GIMAP5 vs. GIMAP7). f, Thermal denaturation profiles. GST- WT GIMAP51-276 displayed two temperature transitions - GIMAP51-276 unfolding (red arrow) and GST unfolding (blue arrow). GST-GIMAP51-276 variants (left: I47T, L204P and N221S; right: L223F) showed a single transition, representing GST unfolding, with no WT GIMAP51-276 transition. Left and right experiments were conducted in separate laboratories. g, Single-cell GIMAP5 RNA expression in human peripheral blood mononuclear cells (PBMCs). h, Immunoblots of GIMAP5 in primary human CD19+ B cells, CD14+ monocytes, CD56+ NK cells, and T cells with β-actin loading control (n = 3). i, Immunoblots of GIMAP5 from patients (P), family, normal control (NC) or shipping control (TC) T cell lysates with a b-actin or an HSP90 loading control (n = 3). j, GIMAP5 RNA expression by RT-qPCR in patients (n = 6) and controls (n = 6) normalized to 18S. Samples were examined over at least 3 independent experiments (mean ± s.d.).
Extended Data Fig. 2 GIMAP5 deficiency induces senescence in human T cells.
a, CyTOF mass cytometry from patient (P1.3, P1.4) or control (n = 8) PBMCs. NK = natural killer; TEM = T effector memory; TEMRA = Terminally differentiated effector memory; TCM = T central memory. b, Percent of patient (red, n = 11) and control (black, CD4+: n = 16, CD8+: n = 17) naive T cells against age in years. Simple linear regression of control data (black line). c, Phenotyping CyTOF, depicting relative CD57 levels as in (a). d, Percent of CD4+CD57+ T cells. Controls (n = 42), Patients (n = 11). e, MFI of SA-β-gal activity in CD4+ T cells. Controls (n= 16), patients (n = 8). f-i, Percent of KLRG1+ (f, controls: n = 24, patients: n = 11), CD27+ (g, controls: n = 30, patients: n = 11), CD28+ (h, controls: n = 28, patients: n = 11), and PD1+ (i, controls: n = 32, patients: n = 11) in T cells. j, Telomere length in kilobases (kb) against age in years (yrs) for P1.3 and P1.4 lymphocytes. Colored lines indicate percentile isobars. k, l, Percent of CD25 (k) or CD69 (l) on T cells 24 hours post-activation. Controls (n = 15), patients (n = 7). m, Proliferation via CellTrace Violet dilution in T cells (3 days post-activation, left two panels). Normal controls (NC), Shipping controls (TC). % divide quantified (right two panels). Controls (n = 15), patients (n = 6). n, DNA content in Ctrl or G5 KO human T cells on day 16 post-electroporation (upper). Percent of Ctrl and G5 KO T cells in the indicated phases (bottom). Red arrowhead indicates G2/M population (n = 5). o, Percent of viable G5 KO or Ctrl human T cells over time post-electroporation (n = 6 per group). p, Percent of CD25 (left) and CD69 (right) on G5 KO or Ctrl human naïve T cells 24 hours after CD3/CD28 Dynabeads activation, 5 days post-electroporation (n = 5 per group). All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (b,f). n.s. = not significant. p < 0.0001: in n, p = 2.11 × 10−5.
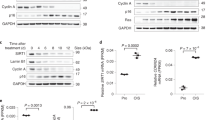
Extended Data Fig. 3 Gimap5 deficiency in mice induces a partial senescence phenotype in lung endothelial cells (ECs).
a, Immunoblots of heart, kidney, and brain tissue lysates from sphinx (Gimap5sph/sph), Het (Gimap5+/sph) and WT (wild-type) mice for Gimap5, p21 and Hsp90. Representative immunoblots from two independent experiments are shown. Littermate controls (n = 8), sphinx (n = 7). Note that bands detected in Gimap5 brain tissue immunoblot are unaffected by sphinx mutation, implying background signals. b, MFI of p21, p53, p16 and SA-β-gal activity in CD45-CD31+ lung ECs from young mice (littermate controls), sphinx, and aged mice. CD45-CD31+ p21: young mice (n = 14), sphinx (n = 11), aged mice (n = 10); CD45-CD31+ p53: young mice (n = 15), sphinx (n = 10), aged mice (n = 10); CD45-CD31+ p16: young mice (n = 21), sphinx (n = 11), aged mice (n = 10); CD45-CD31+ SA-β-gal: young mice (n = 8), sphinx (n = 3), aged mice (n = 10). 7- to 12-week-old (young, sphinx) or 16 to 19-month-old (aged) male and female mice were used in each experiment. All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (b). n.s. = not significant. p < 0.0001: in b (left to right), p = 3.49 × 10−5, p = 1.89 × 10−5, p = 4.23 × 10−6.
Extended Data Fig. 4 GIMAP5 controls the levels of CER and TAG.
a, Thy1.1 levels in murine T cells post-electroporation (left). Immunoblot of indicated proteins from left (day 3 post-electroporation, right)(n = 5). b, Volcano plot of lipids in G5 KO murine T cells vs. Ctrl, day 3 post-electroporation. Triacylglyceride (TAG) species, red (n = 5). c, Viable CD4+ T cell numbers from WT or Gimap5sph/sph (sphinx) mice with IL-7 (unstimulated) or stimulated with anti-CD3/CD28 antibodies, relative to unstimulated WT (n = 4). d, SPL heat map of 24-hour samples from (c), key changes in cyan or yellow boxes (FDR ≤ 0.05) (n = 5). e, Immunoblots of NIH-3T3 cells following indicated transductions (left)(n = 3 per group). f, Percent of GFP+CD4+ T cells, day 2 post-transduction in Gimap5sph/sph CD4+ T cells (n = 3 per group). g, Relative viable cell numbers in GFP+CD4+ T cells from (f) over time (n = 3 per group). h, SPL heat map of Ctrl and G5 KO murine T cells from Fig.4f, day 3 post-electroporation. Only significant SPLs from Fig. 4e are displayed (p ≤ 0.05) (n = 5). i, Murine T cell Cers relative mRNA expression (RT-qPCR, normalized to Gapdh)(n = 4 per group). j, Immunoblots of indicated proteins in indicated murine KO T cells, day 3 post-electroporation (n = 3). k, Relative mRNA expression of Cers1-6, in Ctrl and Cers2 KO murine T cells, day 3 post-electroporation (RT-qPCR, normalized to Ctrl KO)(Cers1: n = 5 per group, Cers2-6: n = 7 per group). l, SPL heat map of Ctrl and Cers2 KO murine T cells, p values < 0.1, all samples normalized to Ctrl (n = 3). m, Relative viable cell numbers of murine T cell blasts from (j) (Ctrl, G5 KO, Cers2 KO: n = 4, G5 KO + Cers2 KO: n = 3). All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (b,h,g,k,l,m) or by two-way ANOVA with Bonferroni post-test (c,e,i). n.s. = not significant. p < 0.0001: in c, p = 7.33 × 10−6, in e (left to right), p = 3.42 × 10−5, p = 3.39 × 10−5, in k, p = 1.19 × 10−7.
Extended Data Fig. 5 GIMAP5 controls the levels of CER, thereby regulating cell survival or cell senescence.
a, Relative viable cell number of Ctrl and G5 KO murine T cell blasts treated with 200 μM conduritol-β-epoxide (CBE), starting on day 1 post-electroporation, measured on day 3 post-electroporation (n = 3 per group). b, Heat map of SPLs detected in (a) using the z scale indicated. Only SPLs found to be significantly affected in Fig. 4e are displayed (p ≤ 0.05) (n = 3). c, Relative viable cell number of Ctrl and G5 KO murine T cell blasts treated with zVAD (20 μM), necrostatin-1 (Nec-1, 20 μM), ferrostatin-1 (Fer-1, 3 μM) or liproxstatin-1 (Lip-1, 3 μM) on day 1 and quantified on day 3 post-electroporation (n = 3 per group). d, Heat map of SPLs detected in P1.4, healthy family members, normal controls (NC) or shipping controls (TC). Hits on the heatmaps are >1 standard deviation from the mean (>1 z-score) (n = 3). e, Immunoblots of Ctrl or G5 KO human T cell lysates treated with FB1 (10 μM, 24 hr) for proteins shown (left). WCLs were collected as in Fig. 2i. Quantified left panel bands with ImageJ (right). Bands normalized to HSP90, and relative intensities presented as Ctrl vs. each sample (n = 3 per group). f, Immunoblot analysis of p21 and Gimap5, with Hsp90 loading control, in Ctrl or G5 KO murine T cells on day 2, 3, and 4 post-electroporation (left). Quantification of p21 and Gimap5 band intensities from left panel using ImageJ (center and right). Bands of interest, normalized to Hsp90, show relative intensities compared to Ctrl (day 2 post-electroporation) for each sample (n = 3 per group). All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (a,b,f) or by two-way ANOVA with Bonferroni post-test (c,e). n.s. = not significant. p < 0.0001: in c (left to right), p = 4.35 × 10−11, p = 4.35 × 10−11, p = 2.25 × 10−9, p = 2.25 × 10−9, in e (left to right), p = 5.46 × 10−5, p = 1.89 × 10−5.
Extended Data Fig. 6 GIMAP5 interacts with CK2 and LCK.
a, HEK293T cells were transfected with tagged plasmids expressing GFP-Flag, GIMAP5-Flag, CK2A1-HA, CK2A2-HA or CK2B-Myc, as indicated by a plus sign. Cell lysates were prepared 24 hours post transfection (Input), immunoprecipitated (IP) for Flag tag, and immunoblotted with the indicated antibodies (n = 3). b, Schematic diagram illustrating GIMAP5 full-length (FL), GIMAP5 N-terminal 219-amino-acid (G domain) truncated mutant (GIMAP5 NΔ), and GIMAP5 C-terminal 30-amino-acid (hydrophobic domain) truncated mutant (GIMAP5 CΔ). c, HEK293T cells were transiently transfected with Flag, Flag-GIMAP5 FL, Flag-GIMAP5 NΔ, Flag-GIMAP5 CΔ, or HA-CK2A1, as indicated by a plus sign. Cell lysates were prepared 24 hours post transfection (Input), immunoprecipitated (IP) for Flag tag, and immunoblotted with Flag and HA antibodies (n = 3). d, Immunoblots of p21, pERK (phospho-ERK1/2), and GIMAP5 with an HSP90 loading control. Cell lysates were collected from human T cell blasts with or without CERS inhibitor, FB1 (20 μM) pretreatment (1 hr) followed by TCR stimulation for the indicated times in minutes (mins) (n = 3). Note that the variable GIMAP5 expression levels were not detected in other repeats. e, Confocal microscopy of GIMAP5 + CK2α (top) in PLA after TCR stimulation as in Fig.5b. PLA signals were quantified by flow cytometry (bottom left) and by the number of PLA dots per cell (bottom right) (3 biological replicates; Isotype: n = 381 from 14 images; 0: n = 390 from 14 images; 15 mins: n = 395 from 17 images; 30 mins: n = 487 from 17 images; 45 mins: n = 491 from 14 images). f, Immunoprecipitation (IP) with anti-GIMAP5 or isotype control in human T cell blasts after TCR stimulation for multiple timepoints measured in minutes (mins)(n = 6). Two-tailed, unpaired Student’s t-test (f; mean ± s.d.). n.s. = not significant. p < 0.0001: in e, p = 4.75 × 10−6.
Extended Data Fig. 7 GIMAP5 colocalizes with CK2α and LCK after TCR stimulation at the late endosome/lysosome.
a, Immunofluorescence staining with LAMP1, Rab7, LAMTOR4, Calnexin or MitoTracker (magenta) and GIMAP5 (green) in human T cell blasts (left). Colocalization of indicated markers by Pearson′s correlation coefficient (PCC) (right). n (left to right): 485, 907, 566, 507, 412. b, Immunofluorescence staining with anti-LCK (green), GIMAP5 (magenta), and Rab7 (yellow) in human T cell blasts before and after TCR stimulation (60 mins) (left). Colocalization of indicated markers before and after TCR stimulation by PCC (right). n (left to right): 2664, 3071, 2751, 2388, 1419, 1570, 1411, 1181, 1419, 1570, 1411, 1181. c, Colocalization of CK2α with LCK, GIMAP5, Calnexin, Rab7, and LAMTOR4 and Rab7 with Calnexin before and after TCR stimulation by PCC. n (left to right): 2226, 2382, 2191, 2042, 1426, 1716, 1521, 1377, 2502, 2387, 2484, 2139, 1865, 1599, 2118, 1625, 1653, 2000, 2091, 1501, 1630, 1473, 1219, 1361. d, Immunofluorescence staining with anti-CK2α (yellow), Calnexin (green), and Rab7 (magenta) in human T cell blasts before and after TCR stimulation (60 mins). e, Immunofluorescence staining with anti-CK2α (yellow), LAMTOR4 (green), and Calnexin (magenta) in human T cell blasts after TCR stimulation (60 mins). Representative of at least three biological replicates (a,b,d,e). All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (a,c) or by two-way ANOVA with Bonferroni post-test (b). n.s. = not significant. p < 0.0001: in a (left to right), p = 8.41 × 10−5, p = 5.62 × 10−6, in b, p = 1.68 × 10−6.
Extended Data Fig. 8 GIMAP5 controls the levels of CER and TAG through its association with CK2 without affecting the level of CK2.
a, Immunoprecipitation with anti-Ck2α or isotype control in control or G5 KO murine T cell blasts after TCR stimulation (n = 3). b, Immunoblots of T cell lysates from patients, family members, and healthy donors (NC = normal control, TC = shipping control) for the indicated proteins (left). Whole cell lysates (WCL) were collected between 7 to 15 days post-activation. Quantified left panels with ImageJ (right, Ctrl: n = 12; patient: n = 6). Bands normalized to HSP90. c, Immunoblots of Ctrl or G5 KO human T cells for the indicated proteins (left). WCLs from human T cell blasts were collected 16 days post-activation. Quantified left bands with ImageJ (right). Bands normalized to HSP90 (n = 4 per group). d, Immunoblots of Ctrl or G5 KO murine T cells for the indicated proteins (left). WCLs from murine T cell blasts were collected on days 2, 3, and 4 post-electroporation. Quantified bands from 3 days post-electroporation samples using ImageJ (right). Bands normalized to Hsp90 (n = 6 per group). e, Signal intensity of TAG species in Ctrl and G5 KO murine T cell blasts, 3 days post-electroporation, treated with 2.5 µM CK2 inhibitor CX-4945 on day 1 post-electroporation. Only significant TAGs from Extended Data Fig. 4b are shown (p ≤ 0.05). Key changes in yellow boxes. Signal levels reflect the total sum normalized and auto-scaled (n = 5). f, Quantified Fig. 5j bands with ImageJ, normalized to HSP90 bands, and presented relative intensities compared to Ctrl (untreated) for each sample (n = 4 per group). g, Immunoblots of cell lysates for proteins shown. WCLs were collected after CX-4945 or dimethylsulfoxide (DMSO) vehicle pretreatment (1 hr) followed by TCR stimulation. (n = 4). All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (b-e) or by two-way ANOVA with Bonferroni post-test (f). n.s. = not significant. p < 0.0001: in c, p = 5.99 × 10−6, in d, p = 3.64 × 10−5, in f (left to right), p = 1.11 × 10−8, p = 5.10 × 10−8.
Extended Data Fig. 9 Lack of senescence induction in T cells by C6-ceramide.
a-d, Quantification of intracellular staining of SA-β-gal activity (CellEvent senescence green probe)(a, n = 3 per each group), p21 (b, day 3: n= 6, day 7: n = 3, day 10: n = 3), p53 (c, day 3: n = 6, day 7: n = 3, day 10: n = 3), and p16 (d, day 3: n= 6, day 7: n = 3, day 10: n = 3) in human T cell blasts with or without C6-Ceramide (C6-CER) treatment at days 3, 7, and 10 post-treatment. Mean and standard deviation of mean fluorescence intensity (MFI) is shown. e, Flow cytometry analysis of live/dead staining on human T cell blasts with or without C6-CER treatment for 3 days. f, Quantification of the live percentage by gating on the negative population of the live/dead staining as in (e). T cell blasts from healthy donors were treated for 3 days (n = 6), 7 days (n = 3), and 10 days (n = 3) at 0, 1, 3, and 10 mM (for 3 days) of C6-CER. All data are mean ± s.d. and were analyzed by two-tailed, unpaired Student’s t-test (a-d, f). n.s. = not significant.
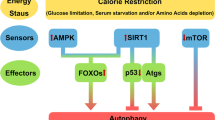
Extended Data Fig. 10 A model of GIMAP5 regulating CER levels through CK2 following TCR stimulation.
Upon TCR stimulation, the interaction of GIMAP5, CK2, and LCK rapidly increases. CK2 then dissociates from GIMAP5 and LCK when LCK-containing endosomes are transported to the plasma membrane. Following dissociation from GIMAP5 and LCK, CK2 interacts with and activates CERS thereby generating CER following TCR stimulation (left). GIMAP5 deficiency in T cells results in constitutive and sustained CK2-CERS association leading to the accumulation of CER which induces T cell death or T cell senescence through the upregulation of p21 and p53 (right).
Supplementary information
Supplementary Information
Supplementary Fig. 1, Supplementary Tables 1–6, Supplementary Text, Supplementary Clinical Descriptions and Supplementary Descriptions for Fig. 1f.
Supplementary Data 1
Sequences of oligonucleotides.
Supplementary Data 2
P values for Fig. 4e and Extended Data Figs. 4h, 4l, 5b and 8e.
Source data
Source Data Fig. 1
Unprocessed immunoblots.
Source Data Fig. 2
Unprocessed immunoblots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Unprocessed immunoblots.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Unprocessed immunoblots.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Unprocessed immunoblots.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Unprocessed immunoblots.
Source Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 1
Unprocessed immunoblots.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig.e 3
Unprocessed immunoblots.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Unprocessed immunoblots.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Unprocessed immunoblots.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Unprocessed immunoblots.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Unprocessed immunoblots.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Park, A.Y., Leney-Greene, M., Lynberg, M. et al. GIMAP5 deficiency reveals a mammalian ceramide-driven longevity assurance pathway. Nat Immunol 25, 282–293 (2024). https://doi.org/10.1038/s41590-023-01691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01691-y
This article is cited by
-
When ceramides meet immune senescence, a GIMAP5 connection
Nature Immunology (2024)