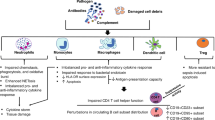
Abstract
Sepsis remains a major cause of morbidity and mortality in both low- and high-income countries. Antibiotic therapy and supportive care have significantly improved survival following sepsis in the twentieth century, but further progress has been challenging. Immunotherapy trials for sepsis, mainly aimed at suppressing the immune response, from the 1990s and 2000s, have largely failed, in part owing to unresolved patient heterogeneity in the underlying immune disbalance. The past decade has brought the promise to break this blockade through technological developments based on omics-based technologies and systems medicine that can provide a much larger data space to describe in greater detail the immune endotypes in sepsis. Patient stratification opens new avenues towards precision medicine approaches that aim to apply immunotherapies to sepsis, on the basis of precise biomarkers and molecular mechanisms defining specific immune endotypes. This approach has the potential to lead to the establishment of immunotherapy as a successful pillar in the treatment of sepsis for future generations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 315, 801–810 (2016).
Kaukonen, K. M., Bailey, M., Suzuki, S., Pilcher, D. & Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311, 1308–1316 (2014).
Shankar-Hari, M. et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 775–787 (2016).
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101, 1644–1655 (1992).
Levy, M. M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 29, 530–538 (2003).
Gaieski, D. F. et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 41, 1167–1174 (2013).
Martin, G. S. et al. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Rhee, C. et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 318, 1241–1249 (2017).
Bauer, M. et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—results from a systematic review and meta-analysis. Crit. Care 24, 239 (2020).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020).
Fleischmann, C. et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 (2016).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
Prescott, H. C. et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am. J. Respir. Crit. Care Med. 200, 972–981 (2019).
Vincent, J. L. et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. J. Am. Med. Assoc. 323, 1478–1487 (2020).
van der Poll T. & Wiersinga, W. J. in Principles and Practice of Infectious Diseases 9th edn (eds Mandell, D. & Bennett, J. E.) Ch. 73 (Saunders, 2019).
Finfer, S. et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 30, 589–596 (2004).
van Vught, L. A. et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 315, 1469–1479 (2016).
Leligdowicz, A. et al. Association between source of infection and hospital mortality in patients who have septic shock. Am. J. Respir. Crit. Care Med. 189, 1204–1213 (2014).
van der Poll, T. et al. The immunology of sepsis. Immunity 54, 2450–2464 (2021).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017).
Merle, N. S. et al. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
Iba, T. et al. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Semin. Thromb. Hemost. 46, 89–95 (2020).
Torres, L. K. et al. Sepsis-induced immunosuppression. Annu. Rev. Physiol. 84, 157–181 (2022).
Boomer, J. S. et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011).
Stienstra, R. et al. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 26, 142–156 (2017).
Cheng, S. C. et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 17, 406–413 (2016).
Wu, D. et al. Epigenetic mechanisms of Immune remodeling in sepsis: targeting histone modification. Cell Death Dis. 14, 112 (2023).
Reyes, M. et al. An immune-cell signature of bacterial sepsis. Nat. Med. 26, 333–340 (2020).
Wakeley, M. E. et al. Check point inhibitors and their role in immunosuppression in sepsis. Crit. Care Clin. 36, 69–88 (2020).
Adelborg, K. et al. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br. J. Haematol. 192, 803–818 (2021).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Blanco, J. et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit. Care 12, R158 (2008).
Brun-Buisson, C. et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 30, 580–588 (2004).
Ranieri, V. M. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–2533 (2012).
Matthay, M. A. et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 5, 18 (2019).
Alipanah, N. et al. Phenotyping in acute respiratory distress syndrome: state of the art and clinical implications. Curr. Opin. Crit. Care 28, 1–8 (2022).
Antonucci, E. et al. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J. Crit. Care 29, 500–511 (2014).
Takasu, O. et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 187, 509–517 (2013).
Peerapornratana, S. et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96, 1083–1099 (2019).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative (ADQI) workgroup. Nat. Rev. Nephrol. 19, 401–417 (2023).
Seymour, C. W. et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321, 2003–2017 (2019).
Kaukonen, K. M. et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med 372, 1629–1638 (2015).
Abe, T. et al. Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J. Intensive Care 8, 44 (2020).
Shankar-Hari, M. et al. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br. J. Anaesth. 119, 626–636 (2017).
Engoren, M. et al. A comparison of Sepsis-2 (systemic inflammatory response syndrome based) to Sepsis-3 (sequential organ failure assessment based) definitions—a multicenter retrospective study. Crit. Care Med. 48, 1258–1264 (2020).
Vermassen, J. et al. Characteristics of Sepsis-2 septic shock patients failing to satisfy the Sepsis-3 septic shock definition: an analysis of real-time collected data. Ann. Intensive Care 11, 154 (2021).
Litell, J. M. et al. Most emergency department patients meeting sepsis criteria are not diagnosed with sepsis at discharge. Acad. Emerg. Med. 28, 745–752 (2021).
Wright, S. W. et al. Enhanced bedside mortality prediction combining point-of-care lactate and the quick Sequential Organ Failure Assessment (qSOFA) score in patients hospitalised with suspected infection in southeast Asia: a cohort study. Lancet Glob. Health 10, e1281–e1288 (2022).
Park, J. E. et al. Complementary use of presepsin with the Sepsis-3 criteria improved identification of high-risk patients with suspected sepsis. Biomedicines 9, 1076 (2021).
Giamarellos-Bourboulis, E. J. et al. Validation of the new Sepsis-3 definitions: proposal for improvement in early risk identification. Clin. Microbiol. Infect. 23, 104–109 (2017).
Huang, Q. et al. The diagnostic and prognostic value of suPAR in patients with sepsis: a systematic review and meta-analysis. Shock 53, 416–425 (2020).
Armstrong, G. L. et al. Trends in infectious disease mortality in the United States during the 20th century. JAMA 281, 61–66 (1999).
Luhr, R. et al. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002–2016. Crit. Care 23, 241 (2019).
Imaeda, T. et al. Trends in the incidence and outcome of sepsis using data from a Japanese nationwide medical claims database—the Japan Sepsis Alliance (JaSA) study group. Crit. Care 25, 338 (2021).
Iwashyna, T. J. et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304, 1787–1794 (2010).
Fleischmann-Struzek, C. et al. Epidemiology and costs of postsepsis morbidity, nursing care dependency, and mortality in Germany, 2013 to 2017. JAMA Netw. Open 4, e2134290 (2021).
Kosinski, S. et al. What is post-intensive care syndrome (PICS)? Am. J. Respir. Crit. Care Med. 201, P15–P16 (2020).
Rivers, E. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345, 1368–1377 (2001).
Rowan, K. M. et al. Early, goal-directed therapy for septic shock—a patient-level meta-analysis. N. Engl. J. Med. 376, 2223–2234 (2017).
Mouncey, P. R. et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 372, 1301–1311 (2015).
ARISE Investigatorset al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 371, 1496–1506 (2014).
Guntupalli, K. et al. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit. Care Med. 41, 706–716 (2013).
Vincent, J. L. et al. Talactoferrin in severe sepsis: results from the phase II/III Oral tAlactoferrin in Severe sepsIS trial. Crit. Care Med. 43, 1832–1838 (2015).
Bernard, G. R. et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study. Crit. Care Med. 42, 504–511 (2014).
Karnad, D. R. et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 40, 830–838 (2014).
Sehgal, I. S. et al. A randomized trial of Mycobacterium w in severe sepsis. J. Crit. Care 30, 85–89 (2015).
Sehgal, I. S. et al. A randomized trial of Mycobacterium w in severe presumed Gram-negative sepsis. Chest 160, 1282–1291 (2021).
Giamarellos-Bourboulis, E. J. et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 46, 1157–1164 (2008).
Giamarellos-Bourboulis, E. J. et al. Effect of clarithromycin in patients with suspected Gram-negative sepsis: results of a randomized controlled trial. J. Antimicrob. Chemother. 69, 1111–1118 (2014).
Karakike, E. et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: a randomized clinical trial. Crit. Care 26, 183 (2022).
Wu, J. et al. The Efficacy of Thymosin Alpha 1 for Severe Sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit. Care 17, R8 (2013).
Singh, R. K. et al. The effects of atorvastatin on inflammatory responses and mortality in septic shock: a single-center, randomized controlled trial. Indian J. Crit. Care Med. 21, 646–654 (2017).
Francois, B. et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3, e98960 (2018).
Daix, T. et al. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: a double-blind, randomized, placebo-controlled trial. Ann. Intensive Care 13, 17 (2023).
Hotchkiss, R. S. et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit. Care Med. 47, 632–642 (2019).
Hotchkiss, R. S. et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 45, 1360–1371 (2019).
Meisel, C. et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 180, 640–648 (2009).
Kalvelage, C. et al. Personalized medicine with IgGAM compared with standard of care for treatment of peritonitis after infectious source control (the PEPPER trial): study protocol for a randomized controlled trial. Trials 20, 156 (2019).
Leventogiannis, K. et al. Toward personalized immunotherapy in sepsis: the PROVIDE randomized clinical trial. Cell Rep. Med. 3, 100817 (2022).
Kotsaki, A. et al. ImmunoSep (Personalised Immunotherapy in Sepsis) international double-blind, double-dummy, placebo-controlled randomised clinical trial: study protocol. BMJ Open 12, e067251 (2022).
Karakike, E. et al. Coronavirus disease 2019 as cause of viral sepsis: a systematic review and meta-analysis. Crit. Care Med. 49, 2042–2057 (2021).
Horby, P. et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 384, 693–704 (2021).
Gordon, A. C. et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N. Engl. J. Med. 384, 1491–1502 (2021).
Renieris, G. et al. IL-1 Mediates Tissue-Specific Inflammation and Severe Respiratory Failure in COVID-19. J. Innate Immun. 14, 643–656 (2022).
Kyriazopoulou, E. et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 27, 1752–1760 (2021).
Marshall, J. C. Why have clinical trials in sepsis failed? Trends Mol. Med 20, 195–203 (2014).
Stanski, N. L. et al. Prognostic and predictive enrichment in sepsis. Nat. Rev. Nephrol. 16, 20–31 (2020).
Collins, F. S. et al. A new initiative on precision medicine. N. Engl. J. Med 372, 793–795 (2015).
Sinha, P. et al. Biological phenotyping in sepsis and acute respiratory distress syndrome. Annu Rev. Med. 74, 457–471 (2023).
DeMerle, K. M. et al. Sepsis subclasses: a framework for development and interpretation. Crit. Care Med. 49, 748–759 (2021).
Kyriazopoulou, E. et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 15, 172 (2017).
Bodinier, M. et al. Monocyte trajectories endotypes are associated with worsening in septic patients. Front. Immunol. 12, 795052 (2021).
Shakoory, B. et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44, 275–281 (2016).
Coupet, C. A. et al. Intravenous injection of a novel viral immunotherapy encoding human interleukin-7 in nonhuman primates is safe and increases absolute lymphocyte count. Hum. Vaccin. Immunother. 18, 2133914 (2022).
Davenport, E. E. et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 4, 259–271 (2016).
Antcliffe, D. B. et al. Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am. J. Respir. Crit. Care Med. 199, 980–986 (2019).
Scicluna, B. P. et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 5, 816–826 (2017).
Sweeney, T. E. et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care Med. 46, 915–925 (2018).
Baghela, A. et al. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. EBioMedicine 75, 103776 (2022).
Kwok, A. J. et al. Neutrophils and emergency hematopoiesis drive immune suppression and an extreme response endotype during sepsis. Nat. Immunol. 24, 767–779 (2023).
Acknowledgements
The authors are partly supported by the ImmunoSep grant (no. 847422) and HDM-FUN grant from the Horizon 2020 program of the European Union. M.G.N. was partly supported by an ERC Advanced Grant (no. 833247) and a Spinoza Grant of the Netherlands Organization for Scientific Research. A.C.A., J.L.S. and M.G.N. are members of the excellence cluster ImmunoSensation (EXC 2151), funded by the German Research Foundation (DFG) under grant agreement no. 390873048. S.W. is currently funded by the Deutsche Forschungsgemeinschaft, DFG, project no. WE 4971/6-1, the Excellence Cluster Balance of the Microverse (EXC 2051; 390713860) and the Federal Ministry of Education and Research (BMBF) project no. 01EN2001.
Author information
Authors and Affiliations
Contributions
E.J.G.-B., M.B., C.B., T.C., I.G.-V., E.K., M.L., G.M., J.L.S., A.C.A., T.v.d.P, P.P., F.L.v.d.V., A.P.J.V., S.W., W.J.W., M.G.N. designed the manuscript, wrote the manuscript and corrected subsequent versions.
Corresponding author
Ethics declarations
Competing interests
E.J.G.-B. has received honoraria from Abbott, bioMérieux, Brahms, GSK, InflaRx, Sobi and XBiotech, and independent educational grants from Abbott, AxisShield, bioMérieux, InflaRx, Johnson & Johnson, MSD, Sobi and XBiotech. A.V. receives consulting fees from Inflarx paid to Amsterdam UMC. M.B. is cofounder and holds shares of SmartDyeLivery, Jena. M.G.N. is a scientific founder and holds shares of TTxD, BioTRIP and Lemba. All other authors have no conflicts of interest.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jamie D. K. Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
Supplementary Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giamarellos-Bourboulis, E.J., Aschenbrenner, A.C., Bauer, M. et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol 25, 19–28 (2024). https://doi.org/10.1038/s41590-023-01660-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01660-5