Abstract
Iron is critical during host–microorganism interactions1,2,3,4. Restriction of available iron by the host during infection is an important defence strategy, described as nutritional immunity5. However, this poses a conundrum for externally facing, absorptive tissues such as the gut epithelium or the plant root epidermis that generate environments that favour iron bioavailability. For example, plant roots acquire iron mostly from the soil and, when iron deficient, increase iron availability through mechanisms that include rhizosphere acidification and secretion of iron chelators6,7,8,9. Yet, the elevated iron bioavailability would also be beneficial for the growth of bacteria that threaten plant health. Here we report that microorganism-associated molecular patterns such as flagellin lead to suppression of root iron acquisition through a localized degradation of the systemic iron-deficiency signalling peptide Iron Man 1 (IMA1) in Arabidopsis thaliana. This response is also elicited when bacteria enter root tissues, but not when they dwell on the outer root surface. IMA1 itself has a role in modulating immunity in root and shoot, affecting the levels of root colonization and the resistance to a bacterial foliar pathogen. Our findings reveal an adaptive molecular mechanism of nutritional immunity that affects iron bioavailability and uptake, as well as immune responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw RNA-seq data have been uploaded to the NCBI Gene Expression Omnibus (GSE213557). Uncropped gel and blot source data are provided in Supplementary Fig. 1. Gene sequences for RNA-seq read mapping were obtained from the TAIR10 reference genome. Source data are provided with this paper.
Code availability
Scripts for imaging quantification in Fiji and code for RNA-seq analysis in R are available at GitHub (https://github.com/cm010713/immunity-iron-project). All bioinformatic tools used in this study are cited in the Methods.
References
Yilmaz, B. & Li, H. Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals https://doi.org/10.3390/ph11040098 (2018).
Seyoum, Y., Baye, K. & Humblot, C. Iron homeostasis in host and gut bacteria—a complex interrelationship. Gut Microbes 13, 1–19 (2021).
Harbort, C. J. et al. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe 28, 825–837 (2020).
Stringlis, I. A. et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl Acad. Sci. USA 115, E5213–E5222 (2018).
Ganz, T. & Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 15, 500–510 (2015).
Robinson, N. J., Procter, C. M., Connolly, E. L. & Guerinot, M. L. A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697 (1999).
Eide, D., Broderius, M., Fett, J. & Guerinot, M. L. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl Acad. Sci. USA 93, 5624–5628 (1996).
Vert, G. G. et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233 (2002).
Schmidt, W., Michalke, W. & Schikora, A. Proton pumping by tomato roots. Effect of Fe deficiency and hormones on the activity and distribution of plasma membrane H+-ATPase in rhizodermal cells. Plant Cell Environ. 26, 361–370 (2003).
Hentze, M. W., Muckenthaler, M. U., Galy, B. & Camaschella, C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010).
Brown, J. C. Iron chlorosis in plants. Adv. Agron. 13, 329–369 (1961).
Romera, F. J. et al. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 10, 287 (2019).
Zamioudis, C. et al. Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J. 84, 309–322 (2015).
Aznar, A., Patrit, O., Berger, A. & Dellagi, A. Alterations of iron distribution in Arabidopsis tissues infected by Dickeya dadantii. Mol. Plant Pathol. 16, 521–528 (2015).
Xing, Y. et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell 33, 2015–2031 (2021).
Nobori, T. et al. Transcriptome landscape of a bacterial pathogen under plant immunity. Proc. Natl Acad. Sci. USA 115, E3055–E3064 (2018).
Gu, S. et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 5, 1002–1010 (2020).
Meziane, H., I, V. D. S., LC, V. A. N. L., Höfte, M. & Bakker, P. A. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 6, 177–185 (2005).
Verbon, E. H. et al. Iron and immunity. Ann. Rev. Phytopathol. 55, 355–375 (2017).
Platre, M. P. et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nat. Commun. 13, 4445 (2022).
Colangelo, E. P. & Guerinot, M. L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412 (2004).
Grillet, L., Lan, P., Li, W., Mokkapati, G. & Schmidt, W. IRON MAN is a ubiquitous family of peptides that control iron transport in plants. Nat. Plants 4, 953–963 (2018).
Kroh, G. E. & Pilon, M. Connecting the negatives and positives of plant iron homeostasis. N. Phytol. 223, 1052–1055 (2019).
Ngou, B. P. M., Ahn, H.-K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Dubeaux, G., Neveu, J., Zelazny, E. & Vert, G. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 69, 953–964 (2018).
Martín-Barranco, A., Spielmann, J., Dubeaux, G., Vert, G. & Zelazny, E. Dynamic control of the high-affinity iron uptake complex in root epidermal cells. Plant Physiol. 184, 1236–1250 (2020).
Faulkner, C. et al. LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proc. Natl Acad. Sci. USA 110, 9166–9170 (2013).
Vatén, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Li, Y. et al. IRON MAN interacts with BRUTUS to maintain iron homeostasis in Arabidopsis. Proc. Natl Acad. Sci. USA 118, e2109063118 (2021).
Rodríguez-Celma, J. et al. Arabidopsis BRUTUS-LIKE E3 ligases negatively regulate iron uptake by targeting transcription factor FIT for recycling. Proc. Natl Acad. Sci. USA 116, 17584–17591 (2019).
Liu, L. et al. Extracellular pH sensing by plant cell-surface peptide-receptor complexes. Cell 185, 3341–3355 (2022).
Yu, K. et al. Rhizosphere-associated pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 29, 3913–3920 (2019).
Liu, Y. et al. Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nat. Microbiol. https://doi.org/10.1038/s41564-023-01402-1 (2023).
Verbon, E. H. et al. Rhizobacteria-mediated activation of the Fe deficiency response in arabidopsis roots: impact on Fe status and signaling. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.00909 (2019).
Zhou, F. et al. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 180, 440–453 (2020).
Sanchez, K. K. et al. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell 175, 146–158 (2018).
Hiruma, K. et al. Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165, 464–474 (2016).
Castrillo, G. et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 543, 513–518 (2017).
Dindas, J. et al. Direct inhibition of phosphate transport by immune signaling in Arabidopsis. Curr. Biol. 32, 488–495 (2022).
Gruber, B. D., Giehl, R. F. H., Friedel, S. & von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 163, 161–179 (2013).
Wyrsch, I., Domínguez-Ferreras, A., Geldner, N. & Boller, T. Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. N. Phytol. 206, 774–784 (2015).
Cao, M. et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243 (2019).
Gautam, C. K., Tsai, H.-H. & Schmidt, W. A quick method to quantify iron in Arabidopsis seedlings. Bio Protoc. 12, e4342 (2022).
Bayle, V., Platre, M. P. & Jaillais, Y. Automatic quantification of the number of intracellular compartments in Arabidopsis thaliana root cells. Bio Protoc. https://doi.org/10.21769/BioProtoc.2145 (2017).
Santi, S. & Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. N. Phytol. 183, 1072–1084 (2009).
Gujas, B., Alonso-Blanco, C. & Hardtke, C. S. Natural Arabidopsis brx loss-of-function alleles confer root adaptation to acidic soil. Curr. Biol. 22, 1962–1968 (2012).
Berardini, T. Z. et al. The Arabidopsis information resource: making and mining the “gold standard” annotated reference plant genome. Genesis 53, 474–485 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Acknowledgements
We thank J. Balk for providing genetic materials (btsl1,2 (btsl1-1 btsl2-2), pBTSL1::GFP and pBTSL2::GFP); L. Grillet and W. Schmidt for providing genetic materials (ima8x, IMA1ox and pIMA1::EYFP-IMA1;ima8x); C. Keel for providing bacteria materials (CHA0-gfp2 and CHA0-mCherry); the members of the Salk Peptide Synthesis Core for synthesizing flg22 and flg20 peptides; the members of the Salk Next Generation Sequencing Core Facility for NGS library preparation and sequencing; and S. Lee, C. Miller and N. Gibbs for comments on the manuscript. This study was funded by the National Institute of General Medical Sciences of the National Institutes of Health (grant number R01GM127759 to W.B.), start-up funds from the Salk Institute for Biological Studies (to W.B.), funds from the Hess Chair in Plant Science (to W.B.), a long-term postdoctoral fellowship (LT000340/2019 L) by the Human Frontier Science Program Organization to M.P.P., funds from the Taiwan’s Ministry of Science and Technology (grant number 111-2917-I-564-021 to H.-H.T.), funds from the Human Frontiers Science Program (HFSP) Long-term Fellowship (grant number LT000661/2020-L to T.N.). This study was supported by the NGS Core Facility of the Salk Institute with funding from NIH-NCI CCSG: P30 014195, the Chapman Foundation and the Helmsley Charitable Trust. J.R.E. is an investigator of the Howard Hughes Medical Institute. L.A. is supported by a Maria Zambrano postdoctoral fellowship by de Ministerio de Universidades and the European Union—NextGenerationEU. Research at CRAG was supported by grant MCIN/AEI/PID2019-108595RB-I00 funded by MCIN/AEI/10.13039/501100011033, grant TED2021-131457B-I00 funded by MCIN/AEI/10.13039/501100011033 and by the ‘European Union NextGenerationEU/PRTR’, through the ‘Severo Ochoa Programme for Centres of Excellence in R&D’ (CEX2019-000917 funded by MCIN/AEI/10.13039/501100011033), and by the CERCA Pro-gram/Generalitat de Catalunya (N.S.C.).
Author information
Authors and Affiliations
Contributions
M.C. and W.B. conceived the project. M.C., M.P.P. and W.B. conceived and designed experiments. M.C. conceived and conducted most of the experiments. M.P.P. conducted most of the confocal microscopy experiments and all of the imaging quantification. H.-H.T., T.N. and L.A. provided protocols and conducted the bacterial inoculation experiments. L.Z. performed RNA-seq analysis. Y.C. conducted IMA1 imaging experiments in the shoot. W.H. drew schematics for the manuscript. L.B. participated in the generation of transgenic plants. W.B., N.G., J.R.E. and N.S.C. supervised work and provided funds and resources. M.C. and W.B. wrote and revised the manuscript with input from M.P.P., H.-H.T. and Y.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Flg22 represses iron uptake through FLS2.
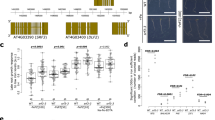
a, Phenotype of 15-day-old Arabidopsis seedling leaves response to different iron concentrations (shown in figures) with or without low levels of flg22 (10 nM) treatment. Scale bar 1 cm. b,c, Phenotype of 15-day-old Arabidopsis seedling leaves response to sufficient iron (50 µM) or low iron (10 µM) with or without low levels of flg20 or flg22 (10 nM) treatment. b, Shoots; scale bar 1 cm. c, Total chlorophyll concentration of Col-0 shoots. Bar chart centres show means of 3 biological replicates. Error bar: s.e.m. The numbers correspond to P-values analysed by two-tailed Student t-test. d, Quantitative analysis of ferric chelate reductase activities in Col-0 roots grown for 7 days under +Fe conditions and transferred to -Fe, -Fe with flg20 and -Fe with flg22 liquid medium for 2 days. The bar chart centres show means of 5 biological replicates. Error bar: s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). e, Promoter activity of IRT1 in the root of pIRT1::NLS-2xYpet seedlings in response to -Fe, -Fe with flg20 and -Fe with flg22 treatment. Seedlings were grown on the +Fe medium and after 5 days transferred to the different liquid media for 24 h treatment. Green: nuclear localized Ypet; red: propidium iodide (PI) cell wall stain. For each treatment, a representative single confocal section (single image, GFP/PI), maximum intensity Z-projection (Z-max, GFP only), a single optical section of the transverse view, and the Z-projection of the transverse section are shown. Scale bar, 50 µm. f–h, Western blots showing IRT1 protein levels in Col-0 and irt1-1 roots grown in +Fe and -Fe (f) or Col-0 under +Fe, -Fe and -Fe with different concentrations of flg22 treatment (g) or Col-0 under +Fe, -Fe and -Fe with flg20 or flg22 treatment (h). Arrow indicates the IRT1 protein band. The asterisk indicates non-specific band. Tubulin protein was blotted as an internal control. i–k, Phenotype of 15-day-old Arabidopsis Col-0 and fls2 seedling leaves in response to sufficient iron (50 µM) or low iron (10 µM) with or without low levels of flg22 (10 nM) treatment. The numbers correspond to p-values that were analysed by two-tailed Student t-test. i, Shoots; scale bar 1 cm; j, total chlorophyll concentration of Col-0 shoots; k, iron concentration of Col-0 seedlings; bar chart centres show means of 3 biological replicates. Error bar: s.e.m. l, Quantitative analysis of ferric chelate reductase activities in Col-0 and fls2 roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centres show means of 5 biological replicates. Error bar: s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). m, Western blots showing IRT1 protein levels in Col-0 and fls2 roots in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. The tubulin protein was blotted as an internal control.
Extended Data Fig. 2 MAMPs regulate iron uptake through distinct mechanisms.
a,b, Promoter activity of FRK1 in roots of pFRK1::NLS-3xmVenus seedlings in response to -Fe and -Fe with flg22 treatment (a) or promoter activity of MYB51 in roots of pMYB51::NLS-3xmVenus seedlings in response to -Fe and -Fe with elf18 treatment (b). 7-day-old seedlings are treated with -Fe and -Fe with 100 nM flg22 (a) or -Fe and -Fe with 100 nM elf18 (b) for 24 h in liquid media. Green: nuclear localized mVenus signals; red: propidium iodide (PI) cell wall stain. In each treatment, a representative single confocal section (single image, GFP/PI) and Maximum Intensity Z-Projection (Z-max, GFP only) is shown. Scale bar, 50 µm. c, Promoter activity of CYP71A12 in roots of pCYP71A12::GUS seedlings in response to -Fe and -Fe with chitin treatment. 7-day-old seedlings are treated with -Fe and -Fe with 1 mg/mL chitin for 24 h in liquid media. Scale bar, 0.5 cm. d, Quantitative analysis of ferric chelate reductase activities in Col-0 roots grown for 7 days under +Fe conditions and transferred to -Fe, -Fe only or -Fe with flg22, elf18 and chitin liquid medium for 2 days. The bar chart centres show means of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). e, Western blots showing IRT1 protein levels in Col-0 roots grown +Fe, -Fe only or -Fe with flg22, elf18 and chitin treatment. Tubulin protein was blotted as an internal control. f, Promoter activity of IRT1 in the root of pIRT1::NLS-2xYpet seedlings in response to -Fe only or -Fe with flg22, elf18 and chitin treatment. Green: nuclear localized Ypet signals; red: propidium iodide (PI) cell wall stain. In each treatment, the Z-stack scan is processed to single confocal section (single image, GFP/PI), maximal Z-projection (Z-max, GFP only). Scale bar, 50 µm. g, Western blots showing IRT1 protein levels in 35S::3xHA-FIT roots in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. HA-tagged FIT protein was blotted using anti-HA antibody. Tubulin protein was blotted as an internal control.
Extended Data Fig. 3 Flg22 antagonistically regulates the iron deficiency transcriptional landscape through FLS2.
a, Venn diagram of up/downregulated genes of -Fe versus +Fe, +Fe+flg22 versus +Fe, and -Fe+flg22 versus -Fe, respectively. The statistical analysis of p-value was calculated by hypergeometric test (one-sided). b, Heat map of mean-centred Z-scores for 1290 differentially expressed genes identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering. Box plot indicates the relative expression level based on median centred Z-score in different clusters. The GO terms analysis was performed using PANTHER17.0 (Fisher’s Exact test, p-value < 0.05) and indicated on the right side of the heatmap. c,d, Heat map of mean-centred Z-scores for differentially expressed genes (cluster 5 (c) and cluster 1 (d) in WT and fls2) identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering. e, Heat map of mean-centred Z-scores (normalized by Col-0 +Fe) for well-known iron responsive genes in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22 in Col-0 and fls2 roots. f, Gene expression analysis in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in Col-0 and fls2 roots by quantitative RT-PCR. The gene expression level is normalized to ACT2 internal control. The bar chart centres show means of 3 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05).
Extended Data Fig. 4 Flg22 antagonistically regulates the iron deficiency transcriptional landscape through IMA1.
a,b, Venn diagram of the iron deficiency upregulated genes in Col-0 versus downregulated genes of -Fe+flg22 versus -Fe in Col-0 versus down-regulated genes of -Fe+flg22 versus -Fe in UBQ10::mCitrine-IMA1 (a). Venn diagram of the iron deficiency upregulated genes in Col-0 versus upregulated genes in UBQ10::mCitrine-IMA1 in +Fe versus Col-0 +Fe (b). The statistical analysis of p-value was calculated by hypergeometric test. c,d, Heat map of mean-centred Z-scores for differentially expressed genes (cluster 5 and cluster 1 refer to Extended Data Fig. 3b in WT and UBQ10::mCitine-IMA1) identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering. e, Heat map of mean-centred Z-scores (normalized by Col-0 +Fe) for well-known PTI components in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22, respectively.
Extended Data Fig. 5 Flg22 spatially regulates IMA1 protein level in the ground tissue of the root through FLS2.
a, Western blots showing IRT1 protein levels in Col-0, ima8x and pIMA1::EYFP-IMA1;ima8x in different treatment conditions. Seedlings were treated with +Fe and -Fe (Col-0 and ima8x) and +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment (pIMA1::EYFP-IMA1;ima8x). Tubulin protein was blotted as an internal control. b, Quantitative analysis of ferric chelate reductase activities in pIMA1::EYFP-IMA1;ima8x roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centres show mean of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). c, IMA1 distribution in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root. 5-day-old pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 seedlings were grown on the +Fe medium and then transferred to different liquid media for 24 h treatment. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with propidium iodide (PI, cell wall staining, red channel). For each treatment, a representative single confocal section (single image, EYFP/PI), a maximal Z-projection of the Z-stack (Z-max, EYFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 µm. d,e, Quantification of IMA1 fluorescence signal intensity (d) and normalized IMA1 signal diameter (e) in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 (n = 15 biologically independent seedlings from 3 biological repeats). The same dataset from Fig. 3d,f of pIMA1::EYFP-IMA1;ima8x were used here as the images for quantification were taken at the same time. Different letters indicate statistically significant differences between different conditions analysed by Kruskal Wallis/two tailed test followed by Multiple pairwise comparisons using the Steel-Dwass-Critchlow-Fligner procedure/two-tailed test (p < 0.05). f, Western blots showing IMA1 protein levels in the roots of pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. Tubulin protein was blotted as an internal control. g, Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 roots. The seedlings were pre-treated with -Fe for 36 h, then treated with -Fe+flg22 (1 µm flg22) for 0, 3 and 6 h. Tubulin protein was blotted as an internal control. h, Representative image of EYFP-IMA1 signal intensity profile in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of root in pIMA1::EYFP-IMA1;ima8x (left panel) and pIMA1::EYFP-IMA1;fls2 (right panel). The white line in YFP channel indicates the line for signal quantification. The red dashed lines indicate the boundary between the ground tissue and the stele. Scale bar, 50 µm.
Extended Data Fig. 6 Flg22 does not fully repress IMA1 transcription in the ground tissue and flg22 dependent callose deposition is not required for IRT1 repression.
a, Promoter activity of IMA1 in the root of pIMA1::mCitrine-NLS-mCitrine seedlings in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. 5-day-old seedlings were grown on the +Fe medium and then transferred to the different liquid media for 24 h treatment. The nuclear localized mCitrine signals (Yellow channel) are visualized with propidium iodide (PI, red channel). For each treatment, a representative single confocal section (single image, GFP/PI), maximum intensity Z-projection (Z-max, GFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 µm. b,c, Normalized IMA1 promoter activity quantification in all cell layers (b) or in epidermis-cortex cell layers (c) in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22 treatments (n = 9, 3 repeats). Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Fisher’s LSD test(two-sided, p < 0.05). d, Western blots showing IRT1 protein levels in Col-0 roots is in response to +Fe, +Fe with flg22, -Fe, -Fe with flg22 treatment and -Fe with flg22 and DDG treatment. Tubulin protein was blotted as an internal control. e, Representative images of cell-layer specific IMA1 expression transgenic plants. 3 individual lines are shown under +Fe condition. Scale bar, 100 μm.
Extended Data Fig. 7 IMA1 is not regulated through cell-to-cell movement but regulated through protein level in the epidermis-cortex under -Fe by flg22.
a, Representative images of roots of plants with different tissue-specific promoters driving IMA1 expression. 5-day-old transgenic seedlings were grown on +Fe solid medium and then transferred to +Fe or +Fe+flg22 liquid medium for 24 h treatment. For each treatment, the cytosolic and nuclear-localized mCitrine-IMA1 signals (yellow channel) are visualized with longitudinal section and transverse section. Scale bar, 50 µm. b, Representative images of roots of plants with pIMA1, pELTP or pPGP4 promoters driving IMA1 expression in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22. For each treatment, mCitrine-IMA1 signals (yellow channel) and bright field are visualized with longitudinal section. Scale bar, 50 µm. c, Quantification of normalized IMA1 signal diameter in different treatment conditions in differentiation zones of the roots. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Fisher’s LSD test (two-sided, p < 0.05). d, Quantification of normalized IMA1 signal intensity of pPGP4::mCitrine-IMA1 in different treatment conditions. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Fisher’s LSD test (two-sided, p < 0.05).
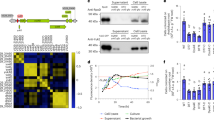
Extended Data Fig. 8 Flg22 dependent IMA1 degradation in the ground tissue is regulated by BTSL1 and BTSL2 but not by BTS.
a, Western blots showing IMA1 protein is degraded through ubiquitin-dependent proteasome mechanism under -Fe condition. The pIMA1::EYFP-IMA1;ima8x was pre-treated with -Fe with DMSO only or -Fe with 10 μM MG132 for 36 h, subsequently with 100 μM Cycloheximide (CHX) for the indicated time period. Tubulin protein was blotted as an internal control. b, Western blots showing IRT1 protein levels in Col-0 and bts-1 roots in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. Tubulin protein was blotted as an internal control. c, Quantitative analysis of Ferric Chelate Reductase activities in Col-0 and bts-1 roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centres show mean of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). d, IMA1 distribution in bts-1 in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root. 5-day-old pIMA1::EYFP-IMA1;bts-1 seedlings were grown on the +Fe medium and then transferred to different liquid medium for 24 h treatment. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with propidium iodide (PI, red channel). For each treatment, a representative single confocal section (single image, EYFP/PI), a maximal Z-projection of the Z-stack (Z-max, EYFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 µm. e, Western blots showing IMA1 protein levels in the roots of pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2 in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. Tubulin protein was blotted as an internal control. f, Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2 roots. The seedlings were pretreated with -Fe for 36 h, then treated with -Fe+flg22 (1 µm flg22) for 0, 3 and 6 h. Tubulin protein was blotted as an internal control. g, Representative image of EYFP-IMA1 signal intensity profile in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of roots in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2. The white line in YFP channel indicates the line for signal quantification. The red dashed lines indicate the boundary between the ground tissue and the stele. Scale bar, 50 µm. h–j, Quantification of normalized total IMA1 signal intensity (h), normalized IMA1 signal intensity in epidermis and cortex (i) and IMA1 signal diameter (j) in differentiation zone of roots in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2. The seedlings were pretreated with +Fe/-Fe 36 h with/without flg22 and treated with 100 μM CHX for 2 h before imaging. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Fisher’s LSD test (two-sided, p < 0.05). k, Schematic of flg22-mediated IMA1 depletion in the outer cell layers (epidermis, cortex, and endodermis) and IRT1 repression in epidermis in response to +Fe, -Fe and -Fe with flg22 treatment respectively. By contrast, IMA1 is not fully degraded in btsl1,2 in the outer cell layers upon flg22 treatment, resulting in IRT1 level maintenance in epidermis.
Extended Data Fig. 9 IMA1 mediates defence responses to different pathogens in the shoot.
a, Confocal images of pIMA1::EYFP-IMA1;ima8x responses to +Fe, +Fe with flg22, -Fe and -Fe with flg22 in the shoot. Three different zones were imaged: epidermal cells, mesophyll cells and vasculature. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with bright field (bright field channel). For each treatment, a representative single confocal section is shown. Scale bar, 50 µm. b, Gene ontology analysis of upregulated genes in IMA1ox compared with Col-0 in the shoot. GO term analysis is performed by using PANTHER17.0 (p-value < 0.05). X axis, fold enrichment. c, Western blots showing MAPK phosphorylation by flg22 in Col-0, ima8x and UBQ10::mCitrine-IMA1 shoot in response to flg22. The shoot parts were treated with 1 µM flg22 for 0, 5 and 10 min. Tubulin protein was blotted as an internal control. d, Gene expression analysis in response to +Fe and +Fe with flg22 by quantitative RT-PCR. The gene expression level is normalized to ACT2 internal control. The shoot parts were treated with 1 µM flg22 for 1 h. The bar chart centres show means of 3 biological replicates and error bars show s.e.m. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Tukey’s test (p < 0.05). e, Growth of Pseudomonas syringae pv. tomato DC3000 in the leaves of Col-0 and UBQ10::mCitrine-IMA1. Bacteria were syringe-infiltrated at OD600 = 0.001, and bacterial colony forming units (CFUs) were counted 0 and 48 h after inoculation (hpi). n = 22-24 biological replicates from three independent experiments. Different letters indicate statistically significant differences (adjusted P < 0.01; two-tailed Student’s t-test followed by Benjamini–Hochberg method). Results are shown as box plots with boxes displaying the 25th–75th percentiles, the centre line indicating the median and whiskers extending to the minimum and maximum values no further than 1.5× interquartile range.
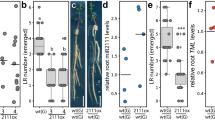
Extended Data Fig. 10 IRT1 and IMA1 accumulation is distinctly regulated by CHA0 or flg22 peptide.
a, Western blots showing IRT1 protein levels in Col-0 roots in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 treatments. The inoculation of CHA0-gfp2 was detected by anti-GFP western blot. Tubulin protein was blotted as an internal control. b, Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x roots in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 treatment. The EYFP-IMA1 and the inoculation of CHA0-gfp2 were detected by anti-GFP western blot. Tubulin protein was blotted as an internal control. c, The promoter activity of FRK1 in pFRK1::NLS-3xmVenus roots in responses to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 in differentiation zone or at lateral root primordia. Scale bar, 50 µm. d, Quantification of the total signal intensity of the promoter activity of FRK1 in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 in differentiation zone or at lateral root primordia. Different letters indicate statistically significant differences between different conditions analysed by one-way ANOVA and Fisher’s LSD test(p < 0.05). e, Graphical summary of main findings. Red colour gradient indicates IMA1 abundance. Left and middle columns: IMA1 and IRT expression in the root under +Fe (upper left), -Fe (lower left), +Fe + flg22 (upper middle), -Fe + flg22 (lower middle). In +Fe IMA1 and IRT1 are expressed at very low levels in the early differentiation zone of the root. In -Fe, IMA1 highly accumulates in the ground tissue of the root under; roots actively lower the rhizosphere pH and increase iron availability, but this decreases PTI responsiveness. In -Fe and flg22 treatment, IMA1 is degraded in a BTSL dependent manner. The rhizosphere pH is not decreased. Right column: IMA1 accumulation in relation to relation to root and shoot host-microbe interactions. Upper right: In +Fe conditions IMA1 levels are low and don’t respond to commensal bacteria CHA0. Lower right: high levels IMA1 enhances a subset of PTI in the shoot. In -Fe IMA1 is accumulated in the root. In the early differentiation zone, surface dwelling CHA0 bacteria don’t affect IMA1 levels; high IMA1 levels increase bacterial colonization on the surface. When CHA0 colonizes inner root tissues at lateral root primordia cracks, IMA1 decreases in cells adjacent to this colonization. IMA1 accumulation is distinctly regulated by CHA0 in early differentiation zone compared to emerging LR region. The differences in IMA1 signal intensity between the depictions in the panels relating to CHA0 are due to different experiment settings (half sucrose concentration and different laser power for real bacteria experiment; see methods section).
Supplementary information
Supplementary Figure 1
The uncropped blots and gel images.
Supplementary Table 1
Primers used in this study.
Supplementary Table 2
Raw RNA-seq data (CPM).
Supplementary Table 3
DEGs and Venn gene list in Col-0 samples.
Supplementary Table 4
Gene list for 5 clusters.
Supplementary Table 5
Gene list for Venn diagram analysis.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, M., Platre, M.P., Tsai, HH. et al. Spatial IMA1 regulation restricts root iron acquisition on MAMP perception. Nature 625, 750–759 (2024). https://doi.org/10.1038/s41586-023-06891-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06891-y
This article is cited by
-
IRON MAN is a jack of all trades
Nature Plants (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.