Abstract
Development of immunocompetent T cells in the thymus is required for effective defence against all types of pathogens, including viruses, bacteria and fungi. To this end, T cells undergo a very strict educational program in the thymus, during which both non-functional and self-reactive T cell clones are eliminated by means of positive and negative selection1.Thymic epithelial cells (TECs) have an indispensable role in these processes, and previous studies have shown the notable heterogeneity of these cells2,3,4,5,6,7. Here, using multiomic analysis, we provide further insights into the functional and developmental diversity of TECs in mice, and reveal a detailed atlas of the TEC compartment according to cell transcriptional states and chromatin landscapes. Our analysis highlights unconventional TEC subsets that are similar to functionally well-defined parenchymal populations, including endocrine cells, microfold cells and myocytes. By focusing on the endocrine and microfold TEC populations, we show that endocrine TECs require Insm1 for their development and are crucial to maintaining thymus cellularity in a ghrelin-dependent manner; by contrast, microfold TECs require Spib for their development and are essential for the generation of thymic IgA+ plasma cells. Collectively, our study reveals that medullary TECs have the potential to differentiate into various types of molecularly distinct and functionally defined cells, which not only contribute to the induction of central tolerance, but also regulate the homeostasis of other thymus-resident populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Bulk RNA-seq, scRNA-seq and scATAC-seq datasets appear in the series GSE236075 in the NCBI’s Gene Expression Omnibus (GEO). Source data are provided with this paper.
Change history
30 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41586-023-06881-0
References
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Bornstein, C. et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626 (2018).
Baran-Gale, J. et al. Ageing compromises mouse thymus function and remodels epithelial cell differentiation. eLife 9, e56221 (2020).
Bautista, J. L. et al. Single-cell transcriptional profiling of human thymic stroma uncovers novel cellular heterogeneity in the thymic medulla. Nat. Commun. 12, 1096 (2021).
Dhalla, F. et al. Biologically indeterminate yet ordered promiscuous gene expression in single medullary thymic epithelial cells. EMBO J. 39, e101828 (2020).
Park, J. E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Michelson, D. A., Hase, K., Kaisho, T., Benoist, C. & Mathis, D. Thymic epithelial cells co-opt lineage-defining transcription factors to eliminate autoreactive T cells. Cell 185, 2542–2558 (2022).
Abramson, J. & Anderson, G. Thymic epithelial cells. Annu. Rev. Immunol. 35, 85–118 (2017).
Sansom, S. N. et al. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 24, 1918–1931 (2014).
Anderson, M. S. et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science 298, 1395–1401 (2002).
Metzger, T. C. et al. Lineage tracing and cell ablation identifiy a post-Aire expressing thymic epithelial cell population. Cell Rep. 5, 166–179 (2013).
Miller, C. N. et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631 (2018).
Miragaia, R. J. et al. Single-cell RNA-sequencing resolves self-antigen expression during mTEC development. Sci. Rep. 8, 685 (2018).
Miyao, T. et al. Integrative analysis of scRNA-seq and scATAC-seq revealed transit-amplifying thymic epithelial cells expressing autoimmune regulator. eLife 11, e73998 (2022).
Wang, X. et al. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Front. Immunol. 3, 19 (2012).
Goldstein, J. D. et al. IL-36 signaling in keratinocytes controls early IL-23 production in psoriasis-like dermatitis. Life Sci. Alliance 3, e202000688 (2020).
Wang, W., Yu, X., Wu, C. & Jin, H. IL-36γ inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int. J. Med. Sci. 14, 1002–1007 (2017).
Mabbott, N. A., Donaldson, D. S., Ohno, H., Williams, I. R. & Mahajan, A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6, 666–677 (2013).
Onder, L. et al. Alternative NF-κB signaling regulates mTEC differentiation from podoplanin-expressing precursors in the cortico-medullary junction. Eur. J. Immunol. 45, 2218–2231 (2015).
Wells, K. L. et al. Combined transient ablation and single-cell RNA sequencing reveals the development of medullary thymic epithelial cells. eLife 9, e60188 (2020).
Goldfarb, Y. et al. Mechanistic dissection of dominant AIRE mutations in mouse models reveals AIRE autoregulation. J. Exp. Med. 218, e20201076 (2021).
Borromeo, M. D. et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 16, 1259–1272 (2016).
Osipovich, A. B. et al. Insm1 promotes endocrine cell differentiation by modulating the expression of a network of genes that includes Neurog3 and Ripply3. Development 141, 2939–2949 (2014).
Jia, S. et al. Insm1 cooperates with Neurod1 and Foxa2 to maintain mature pancreatic β-cell function. EMBO J. 34, 1417–1433 (2015).
Henry, C., Close, A.-F. & Buteau, J. A critical role for the neural zinc factor ST18 in pancreatic β-cell apoptosis. J. Biol. Chem. 289, 8413–8419 (2014).
Guo, T. et al. ISL1 promotes pancreatic islet cell proliferation. PLoS One 6, e22387 (2011).
Gehart, H. et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell 176, 1158–1173 (2019).
Fothergill, L. J. et al. Distribution and co-expression patterns of specific cell markers of enteroendocrine cells in pig gastric epithelium. Cell Tissue Res. 378, 457–469 (2019).
Jiang, W., Anderson, M. S., Bronson, R., Mathis, D. & Benoist, C. Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202, 805–815 (2005).
Tuncel, J., Benoist, C. & Mathis, D. T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33. J. Exp. Med. 216, 1328–1344 (2019).
Dixit, V. D. et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 114, 57–66 (2004).
Dixit, V. D. et al. Ghrelin promotes thymopoiesis during aging. J. Clin. Invest. 117, 2778–2790 (2007).
Kobayashi, N., Takahashi, D., Takano, S., Kimura, S. & Hase, K. The roles of Peyer’s patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front. Immunol. 10, 2345 (2019).
Kanaya, T. et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat. Immunol. 13, 729–736 (2012).
Akiyama, N. et al. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J. Exp. Med. 211, 2425 (2014).
Kimura, S. et al. Osteoprotegerin-dependent M cell self-regulation balances gut infection and immunity. Nat. Commun. 11, 234 (2020).
McCarthy, N. I. et al. Osteoprotegerin-mediated homeostasis of Rank+ thymic epithelial cells does not limit Foxp3+ regulatory t cell development. J. Immunol. 195, 2675–2682 (2015).
Dillon, A. & Lo, D. D. M cells: intelligent engineering of mucosal immune surveillance. Front. Immunol. 10, 1499 (2019).
Wang, J., Gusti, V., Saraswati, A. & Lo, D. D. Convergent and divergent development among M cell lineages in mouse mucosal epithelium. J. Immunol. 187, 5277–5285 (2011).
Komban, R. J. et al. Activated Peyer’s patch B cells sample antigen directly from M cells in the subepithelial dome. Nat. Commun. 10, 2423 (2019).
Sakhon, O. S. et al. M cell-derived vesicles suggest a unique pathway for trans-epithelial antigen delivery. Tissue Barriers 3, e1004975 (2015).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
López-Fraga, M., Fernández, R., Albar, J. P. & Hahne, M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2, 945–951 (2001).
de Lau, W. et al. Peyer’s patch M cells derived from Lgr5+ stem cells require SpiB and are induced by RankL in cultured ‘miniguts’. Mol. Cell. Biol. 32, 3639–3647 (2012).
Meredith, M., Zemmour, D., Mathis, D. & Benoist, C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat. Immunol. 16, 942–949 (2015).
Lucas, B. et al. Diversity in medullary thymic epithelial cells controls the activity and availability of iNKT cells. Nat. Commun. 11, 2198 (2020).
Rios, D. et al. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 9, 907–916 (2016).
Kim, Y.-I. et al. CX3CR1+ macrophages and CD8+ T cells control intestinal IgA production. J. Immunol. 201, 1287–1294 (2018).
Reboldi, A. et al. Mucosal immunology: IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822 (2016).
Vobořil, M. et al. A model of preferential pairing between epithelial and dendritic cells in thymic antigen transfer. eLife 11, e71578 (2022).
Vollmann, E. H. et al. Specialized transendothelial dendritic cells mediate thymic T-cell selection against blood-borne macromolecules. Nat. Commun. 12, 6230 (2021).
Gardner, J. M. et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843 (2008).
Su, G. H. et al. Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 16, 7118–7129 (1997).
Pacary, E. et al. Proneural transcription factors regulate different steps of cortical neuron migration through rnd-mediated inhibition of RhoA signaling. Neuron 69, 1069–1084 (2011).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Xu, H. et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res 25, 1147–1157 (2015).
Concordet, J. P. & Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46, W242–W245 (2018).
Jaitin, D. A. et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 343, 776–779 (2014).
Kohen, R. et al. UTAP: user-friendly transcriptome analysis pipeline. BMC Bioinformatics 20, 154 (2019).
Stuart, T. et al. Comprehensive integration of Single-cell data. Cell 177, 1888–1902 (2019).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Zhang, Y. et al. Model-based Analysis of ChIP–Seq (MACS). Genome Biol. 9, R137 (2008).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Khan, A. et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 (2018).
Boyle, E. I. et al. GO::TermFinder—open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 20, 3710–3715 (2004).
Kachitvichyanukul, V. & Schmeiser, B. Computer generation of hypergeometric random variates. J. Stat. Comput. Sim. 22, 127–145 (2007).
Benjaminit, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Stoeckius, M. et al. Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224 (2018).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Acknowledgements
We thank current and past members of the Abramson group for their feedback and help; the staff of the animal and transgenic facilities at WIS for their continuous support; M. Cohen and L. Prichislov for their dedication and expertise; and K. Katzav, T. Bigdary and I. Sher for help with figures and with creating the graphical summary in Extended Data Fig. 9. Research in the J.A. laboratory is supported by the European Research Council (ERC-2016-CoG-724821), Chan Zuckerberg Initiative, the Binational Science Foundation (2019289), the Israel Science Foundation (1919/21), the Bill and Marika Glied and Family Fund, the Sy Syms Foundation, the David E. Stone and Sheri Hirschfield Stone 75th Anniversary Fund and the Eugene and Marcia Applebaum Professorial Chair.
Author information
Authors and Affiliations
Contributions
T. Givony, Y. Goldfarb and J.A. conceived and designed the project. T. Givony and Y. Goldfarb performed most of the experiments. D.D.C., T. Givony, Y. Gruper and Y.A. performed and analysed the immunofluorescence experiments. O.G. performed image analysis. S.N., N.K. and J.D. helped with bulk RNA-seq library preparation. O.B.-N. helped with RNA extraction and qPCR experiments. R.K. helped with slot-blot experiments. S.B.-D. designed CRISPR mice. R.H.-K. created CRISPR mice. D.L. performed all 10x multiome bioinformatics analyses (scRNA-seq and scATAC-seq) with help from B.D. and T. Gome. Z.P. performed ImageStream analysis. O.B. scored all histopathology. S.N., M.K. and H.K.-S. helped with 10x Genomics library preparation and experiment design. J.D., D.L., D.D.C. and D.D.L. critically evaluated the manuscript. T. Givony, Y. Goldfarb and J.A. wrote the manuscript. D.D.L., Y. Goldfarb and J.A. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Single-cell multiomics reveals multiple TEC subsets with features of functionally defined parenchymal cells.
a, Flow cytometry plots of the gating strategy used to sort 60,000 EpCAM+CD45– TECs followed by enrichment with 6,800 EpCAM+CD45–IA-IEloITGB4lo/neg TECs from four pooled thymi of five-week-old B6.Adig mice for 10x Chromium Single Cell Multiome ATAC + Gene Expression assay. b, Frequency of 19 different subsets in the mouse thymic epithelial compartment as determined by scRNA-seq. Numbers above each bar indicate absolute counts out of 9,155 nuclei. c, UMAPs of selected gene expression per TEC subset as assayed by scRNA-seq. Each dot represents one nucleus. d, UMAPs of selected genes whose expression is enriched in hetreopost TEC subset as assayed by scRNA-seq. Each dot represents one nucleus.
Extended Data Fig. 2 Single-cell ATAC-seq reveals transcription factor activity in mimetic TEC subsets in correlation with parenchymal analogues.
a, Transcription factor motif enrichment of the indicated motifs as analysed by scATAC-seq data (macspeaks). The motif sequence for each factor is located beneath it. b, Transcription factor motif enrichment for Cebpb (left) and Foxa2 (right) in accessible regions highlighted by scATAC-seq. The motif DNA sequence and P value for each factor are located beneath it. c, Heat map of normalized transcription factor expression as assayed by scRNA-seq (scaled data).
Extended Data Fig. 3 Development of an isolation strategy for the individual mimetic TECs.
a,b, Representative flow cytometry plots showing the sorting strategy for Hetero-post TEC, mTEC-II, mTEC-IV, endoTEC, corneoTEC and microfoldTEC (a), and myoTEC and cilTEC (b). c, Representative flow cytometry plots of AIRE expression in mTEC-II (top) and Hetero-post TEC (bottom). d, Frequency (top) and median fluorescent intensity (MFI, bottom) of Aire-expressing cells in mTEC-II and Hetero-post TEC as assayed in c (n = 5 mice per group). Data shown as mean ± s.e.m. Data were analysed using unpaired one-tailed t-tests. e, Volcano plot of bulk RNA-seq of sorted Hetero-post TEC and mTEC-II with 10 Hetero-post representative genes highlighted in red; Aire-dependent TRA genes downregulated 10-fold in Aire–/– mice9 are highlighted in blue. f–k, Volcano plots of bulk RNA-seq of sorted hetero-post cells (f), corneoTEC (g), endoTEC (h), microfoldTEC (i) from a, versus all other cells sorted in a, as well as, myoTEC (j) and cilTEC (k) from b, versus all other cells sorted in b. For each volcano plot, 10 genes representative of the individual mimetic signature from the scRNA-seq in Fig. 1 are highlighted in red and all genes are in grey.
Extended Data Fig. 4 CSNB+MHCIIhi mTECs have the potential to differentiate into most mTEC mimetics.
a, UMAP of scATAC-seq data (macspeaks) nuclei ordered in pseudotime as predicted by Monocle3 and coloured in a gradient from purple (earliest) to yellow (latest). Origin set from PDPN+ TECs (left) or proliferating TECs (right). b, UMAP of Csn2 gene expression as assayed by scRNA-seq. Each dot represents one nucleus. c, Representative flow cytometry histograms of Csnb.Cre+Rosa26tdTomato reporter mice showing tdTomato expression in each cell subtype indicated in addition to a Csnb.Cre– control lacking tdTomato expression. d, Representative flow cytometry plots of TECs (EpCAM+CD45–) from Csnb.Cre+Rosa26tdTomato mice (n = 3 biologically independent mice) (bottom left) and their Rosa26tdTomato counterparts (top left) indicating the cell populations sorted according to their expression of tdTomato and MHC-II levels. Numbers within gates indicate frequency from the parent TEC gate.Gates are numbered 1–4 and specific gene expression assayed by qPCR, representative of several mTEC subsets, is shown for each sorted population (four top and bottom panels from the right). Data are representative of at leasttwo independent experiments. Data shown as mean ± s.e.m. e, Representative flow cytometry plots from five-week-old B6.Aire+/+ (top) and Aire–/– mice (bottom) showing the gating strategy for isolating the Hetero-post TEC (light blue gate), endoTEC (purple gate), microfoldTEC (pink gate) and corneoTEC (dark blue gate) subsets. Numbers within/beside gates indicate the frequency of the gate from the parent population. f, Frequencies of mTEChi (left panel) and mTEClo (right panel) from B6.Aire+/+ and Aire–/– mice, as assayed in c (n ≥ 7). g, Representative flow cytometry plots from five-week-old B6.Aire+/+ (left) and Aire–/– mice (right) showing the gating strategy for isolating myoTEC and cilTEC. Numbers within/beside gates indicate the frequency of the gate from the parent population. h,i, Frequencies (h) and absolute counts (i) of indicated mTEC subsets from TECs as assayed by flow cytometry in Aire+/+ and Aire–/– mice (n ≥ 4). e, n refers to the number of biologically independent mice per genotype/group. Data shown as mean ± s.e.m. Data were analysed using unpaired two-tailed t-tests or unpaired one-tailed t-tests.
Extended Data Fig. 5 Endocrine TECs require INSM1 for their development.
a, Sequencing tracks of chromatin accessibility near selected upregulated transcription factor genes by TEC subsets. The range of normalized tag densities is indicated in parentheses in each panel. b, Insm1 (left), Ascl1 (middle) and Isl1 (right) transcription factor motif enrichment as analysed by scATAC-seq. The motif logo for each factor is located beneath it. c, UMAP of Insm1 expression per TEC subset as assayed by scRNA-seq. Each dot represents one nucleus. d, Total cellularity (left) and frequencies of endoTEC from all TECs (right) from Foxn1.Cre-Isl1fl/fl mice or Isl1fl/fl littermates (n = 5). e, Frequencies of endoTECs from all TECs (left) and SCG5+ endoTECs from all TECs (right) in Foxn1.Cre-Ascl1fl/fl mice or Ascl1fl/fl littermates (n = 9). f, Frequencies of mTEC-IV from all TECs (left) and mTEC-I from all TECs (right) in Foxn1.Cre-Ascl1fl/fl mice or Ascl1fl/fl littermates (n = 3). g, Representative flow cytometry plots from five-week-old B6 mice showing the gating strategy for isolating pooled CD177+, GP2+ and Ly6A+ TEC subsets. EndoTEC (gate highlighted in purple), microfoldTEC (gate highlighted in pink) and corneoTEC (gate highlighted in dark blue). Numbers within or beside gates indicate the frequency of the gate from the parent population. h, Volcano plot of bulk RNA-seq of sorted EpCAM+CD45–Ly51–IA-IElo/negL1CAM–ITGB4–CD177+GP2+Ly6A+ TECs from Foxn1.Cre-Insm1fl/fl mice and Insm1fl/fl littermates. The top 50 endoTEC gene signature as assayed in Fig. 2a is highlighted in red, all genes are in grey. i,j, Volcano plots of bulk RNA-seq of sorted EpCAM+CD45–Ly51–IA-IElo/negL1CAM–ITGB4–CD177+GP2+Ly6A+ TECs from Foxn1.Cre-Insm1fl/fl mice and Insm1fl/fl littermates. The top 50 corneoTEC (i), and top 50 microfoldTEC (j) gene signature as assayed in Fig. 2a is highlighted in red, all genes are in grey. k, Total cellularity from four-week-old Foxn1.Cre-Insm1fl/fl mice or Insm1fl/fl littermates (n ≥ 6). l, Frequencies of mTEC-I from all TECs (left) and mTEC-IV from all TECs (right) in Foxn1.Cre-Insm1fl/fl mice or Insm1fl/fl littermates (n = 4). m, Frequency from all TECs (left) and absolute count (right) of mTEC-II cells in Foxn1.Cre-Insm1fl/fl mice or Insm1fl/fl littermates (n = 3). n, Volcano plot of bulk RNA-seq of sorted endoTEC vs all other cells sorted in Fig. 2a, endoTEC-specific signature highlighted in red, and Aire-dependent TRA genes downregulated 10-fold in Aire–/– mice9 are highlighted in blue. d–f,k–m, n refers to the number of biologically independent mice per genotype/group. Data shown as mean ± s.e.m. Data were analysed using unpaired two-tailed t-tests. e,k, Data were examined over two independent experiments and calculated as a percentage of the average frequency or count of all wild-type mice within a given experiment.
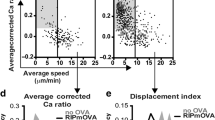
Extended Data Fig. 6 Loss of endoTECs results in the breakdown of self-tolerance to some endocrine tissues and accelerated age-dependent thymus involution9.
a, Volcano plot of bulk RNA-seq of sorted EpCAM+CD45–Ly51–IA-IElo/negL1CAM–ITGB4–CD177+GP2+Ly6A+ TECs from Aire+/+ mice and Aire–/– littermates. The top 50 endoTEC-specific gene signature as assayed in Fig. 2a is highlighted in red, all genes are in grey. b, Frequency of nuceli from Fig. 1a expressing the indicated gene within the mTEC-II (green circles) or endoTEC (purple squares) subsets. c,d, Representative ImageStream snapshots of endoTECs from wild-type mice co-expressing CD177 and SCG5 (c), or CD177 and CHGA (d). e, Frequency of cells from the endoTEC population expressing SCG5 (n = 4) and CHGA (n = 2) as assayed by flow cytometry. f–k, Representative slot-blot analysis of stomach (f), stomach corpus (g), stomach antrum (h), stomach fundus (i), thyroid (j) and eye (k) probed with serum from aged (>20 weeks) Foxn1.Cre-Insm1fl/fl mice and Insm1fl/fl littermates. l, Representative H&E images of thyroid glands (left) and stomachs (right) of aged (>20 weeks) Foxn1.Cre-Insm1fl/fl (bottom) and Insm1fl/fl (top). Scale bars, 200 µm and 500 µm, respectively. m, Representative H&E images of gastric inflammation (left), liver (middle) and eyes (right) in anti-PD-1-treated Foxn1.Cre-Insm1fl/fl (bottom) and Insm1fl/fl (top) mice. Scale bars, 200 µm, 1,000 µm and 500 µm, respectively. n, Representative ImageStream snapshots of endoTEC from wild-type mice demonstrating their granular morphology. o,p, Relative mRNA expression of Grhl (o) and Iapp (p) from the indicated sorted thymic cell populations as assayed by qPCR (n = 3). q, Absolute counts of endoTECs at the specified ages (n ≥ 5). r, Grhl (left), Iapp (middle) and Cd177 (right) relative abundance in the thymic mTEC-III compartment of mice of the indicated ages (n ≥ 2). s, Absolute counts of CD4+CD8+DP thymocytes from 15-week-old Foxn1.Cre-Insm1fl/fl mice or Insm1fl/fl littermates (n = 4). e,o–q,s) n refers to the number of biologically independent mice per genotype/group. Data shown as mean ± s.e.m. Data were analysed using unpaired one-tailed t-tests (s), or one-way ANOVA (o,p) followed by Tukey’s multiple comparisons.
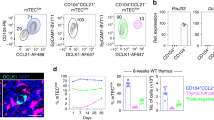
Extended Data Fig. 7 MicrofoldTECs require SpiB for their development and regulate mTEC cellularity.
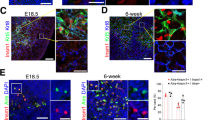
a, Sequencing tracks of chromatin accessibility near Spib by TEC subset. The range of normalized tag densities is indicated by the numbers in parentheses at the bottom left corner. UMAP highlighting Spib motif enrichment analysis of scATAC-seq data from Fig. 1c. The microfoldTEC subset is indicated by a black arrow. Each dot represents one nucleus. The Spib motif DNA sequence and P value are located to the left of the UMAP. b, Representative flow cytometry plots from four-week-old Spib+/– (top) and Spib–/– mice (bottom) showing the gating strategy for isolating Hetero-post TEC, mTEC-II, mTEC-I, mTEC-IV, endoTEC and corneoTEC. Numbers within or beside gates indicate the frequency of the gate from the parent population. c, Absolute counts of mTEC-II cells in Spib+/– mice or Spib-/– littermates (n = 4). d, Frequencies of corneoTECs from all TECs (left), endoTECs from all TEC (right) and mTEC-IV cells from all TECs (bottom) in Spib+/– mice or Spib–/– littermates (n = 4). e,f, Volcano plot of bulk RNA-seq of sorted EpCAM+CD45–Ly51–IA-IElo/negL1CAM–ITGB4–CD177+GP2+Ly6A+ TECs from Spib+/– mice and Spib–/– littermates. The top 50 corneoTEC (e) and top 50 endoTEC (f) gene signature as assayed in Fig. 2a is highlighted in red, all genes are in grey. g, Relative mRNA expression by qPCR of the indicated genes after ex vivo stimulation of sorted mTEC-II cells with recombinant RANKL (n = 3). h, Normalized UMI count of Tnfrsf11b (encoding OPG) in the indicated cell populations sorted in Fig. 2a (n = 3). i, Frequencies of microfoldTECs from total TECs at the specified ages (n = 4). j, Representative flow cytometry plots showing the gating strategy for identifying PGRP-S-dsRed+ thymic APC populations. Numbers within or beside gates indicate the frequency of the gate from the parent population. k, Frequencies of PGRP-S-dsRed+ thymic APC populations (n = 3). l,m, Volcano plot of bulk RNA-seq of sorted EpCAM+CD45–Ly51–IA-IElo/negL1CAM–ITGB4–CD177+GP2+Ly6A+ TECs from either IgM+/+ mice and their IgM–/– littermates (l), or Ccr6+/+ mice and Ccr6–/– littermates (m). The top 50 microfoldTEC gene signature as assayed in Fig. 2a is highlighted in red, all genes are in grey. n, Confocal microscopy of frozen sections of Ccr6+/+ mice (left) and their Ccr6–/– littermates (right) highlighting GP2+ microfoldTECs (green) and DCLK1+ mTEC-IV cells (purple) in relation to B220+ B cells (red). Scale bars, 200 µm. o, Confocal microscopy of frozen sections of PGRP-S-dsRed+ mice highlighting microfoldTECs (red), CD31+ endothelium (purple) and CX3CR1+ APCs (green). Scale bar, 200 µm. p, Representative three-dimensional reconstruction of confocal microscopy of frozen sections of PGRP-S-dsRed+ mice highlighting microfoldTECs (magenta), CD31+ endothelium (cyan) and CX3CR1+ APCs (green). Scale bar, 4 µm. c,d,g–i,k, n refers to the number of biologically independent mice. Data shown as mean ± s.e.m. Data were analysed using unpaired two-tailed t-tests (c,d), unpaired one-tailed t-tests (g) or one-way ANOVA (i) followed by Tukey’s multiple comparisons.
Extended Data Fig. 8 MicrofoldTECs are essential for the generation of thymic IgA+ PCs.
a, Generating chimeric mice by transferring CD45.1 wild-type bone marrow into irradiated CD45.2 Spib+/– or Spib–/– recipients. Scheme generated using BioRender.com. b, Flow cytometry plots of gating strategy used to sort CD45.1+CD3–(CD4/CD8DP)–MHCIImid-high cells from perfused Spib–/– and Spib+/– chimeric mice for 10x Genomics scRNA-seq. c, Representative flow cytometry plots from Spib+/– chimeric mice (top) and Spib–/– chimeric mice (bottom) showing the gating strategy of thymic PCs and IgA+ PCs, IgM+ PCs and IgG+ PCs. Numbers within or beside gates indicate the frequency of the gate from the parent population. d, Absolute counts (bottom) and frequencies (top) of thymic PCs (left), IgG+ PCs (middle) and IgM+PCs (right) from Spib–/– (n = 11) and Spib+/– (n = 8) chimeric mice. e, Frequencies (top) and absolute counts (bottom) of thymic IgA+ B cells (left), IgG+ B cells (middle) and IgM+ B cells (right) from Spib–/– (n = 11) and Spib+/– (n = 8) chimeric mice. f, Circle plot visualizing cell–cell interactions in which microfoldTECs are the source. Line thickness indicates interaction strength. g, Circle plot of cell–cell interactions through CCL signalling. Interactions to and from microfoldTECs are visualized. Line thickness indicates interaction strength. h, Dot plot visualizing the normalized RNA expression of selected marker genes involved in BAFF–APRIL signalling. The colour of the dot and its size correspond to the average expression level and proportion of expressing cells, respectively. d,e, n refers to the number of biologically independent mice. Data shown as mean ± s.e.m. Data were analysed using unpaired one-tailed t-tests, examined over two independent experiments and calculated as a percentage of the average frequency or count of all Spib+/– chimeric mice within a given experiment.
Extended Data Fig. 9 Graphical summary of the developmental stages of mTECs and the functional roles of microfoldTECs and endoTECs.
Fate mapping, as well as developmental trajectories, indicate that mTEC mimetics succeed the mTEC-II stage—with the exception of myoTEC, which seem to bifurcate from mTEC-I. Our findings indicate that, aside from roles in the establishment of central tolerance, mimetic TECs also have functional roles in thymus homeostasis and are involved in complex cellular networks, as exemplified by a specialized niche created by microfoldTECs. Specifically, microfoldTECs create such a ‘hub’ for antigen transfer and cell development by bringing into close proximity thymic B cells, CX3CR1+ APCs and thymic endothelium. The attraction of thymic B cells to microfoldTECs is dependent on the CCL20–CCR6 chemokine axis, and CX3CR1+ APCs then promote the subsequent differentiation of thymic B cells into IgA+ isotype-switched PCs through APRIL signalling. Concurrently, thymic B cells promote the development of microfoldTECs, which, in turn, associate with CD31+ endothelial cells to enable antigen transfer to thymic APCs. Furthermore, microfoldTECs can suppress mTEC development, including their own, through OPG-mediated suppression of RANK signalling. An important influence on thymic size comes from the secretion of metabolic hormones by endoTECs that modulate thymic involution through paracrine secretion of ghrelin.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, the legend for Supplementary Table 1, Supplementary Table 2 and legends for Supplementary Videos 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Givony, T., Leshkowitz, D., Del Castillo, D. et al. Thymic mimetic cells function beyond self-tolerance. Nature 622, 164–172 (2023). https://doi.org/10.1038/s41586-023-06512-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06512-8
This article is cited by
-
AIRE relies on Z-DNA to flag gene targets for thymic T cell tolerization
Nature (2024)
-
Insm1: orchestrating cellular mimicry in the thymus medulla
Cellular & Molecular Immunology (2024)
-
Autoantibodies against type I IFNs in humans with alternative NF-κB pathway deficiency
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.